Methotrexate Restores CD73 Expression on Th1.17 in Rheumatoid Arthritis and Psoriatic Arthritis Patients and May Contribute to Its Anti-Inflammatory Effect through Ado Production
Abstract
1. Introduction
2. Experimental Section
2.1. Patients
2.2. Peripheral Blood Mononuclear Cells (PBMC) and Synovial Fluid Mononuclear Cells (SFMC) Isolation
2.3. Flow Cytometry Analyses
2.4. Analysis of Cytokines Production Capacity after Reactivation
2.5. CD73+ Teff Sorting and In Vitro Activation to Asses CD73 Dynamic Expression
2.6. Th1.17, Th1 and Th17 Sorting for In Vitro AMP Degradation Assay
2.7. Nucleotides and Nucleosides Quantification by Liquid Chromatography Coupled with Tandem Mass Spectrometry (LC-MS/MS)
2.8. Statistical Analysis
3. Results
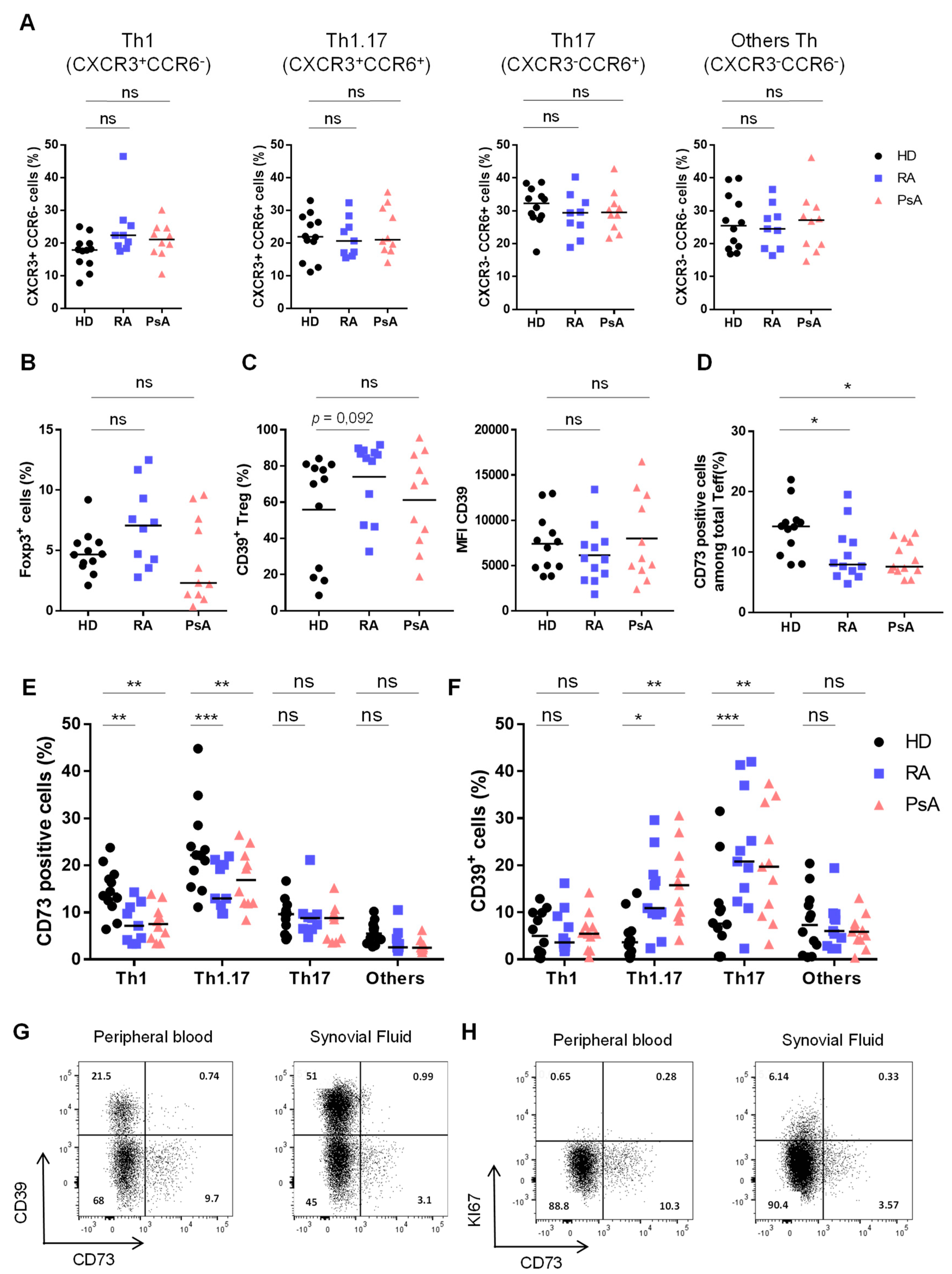
3.1. Activated Th1.17 from Peripheral Blood of Untreated RA and PsA Patients Express Low Levels of CD73
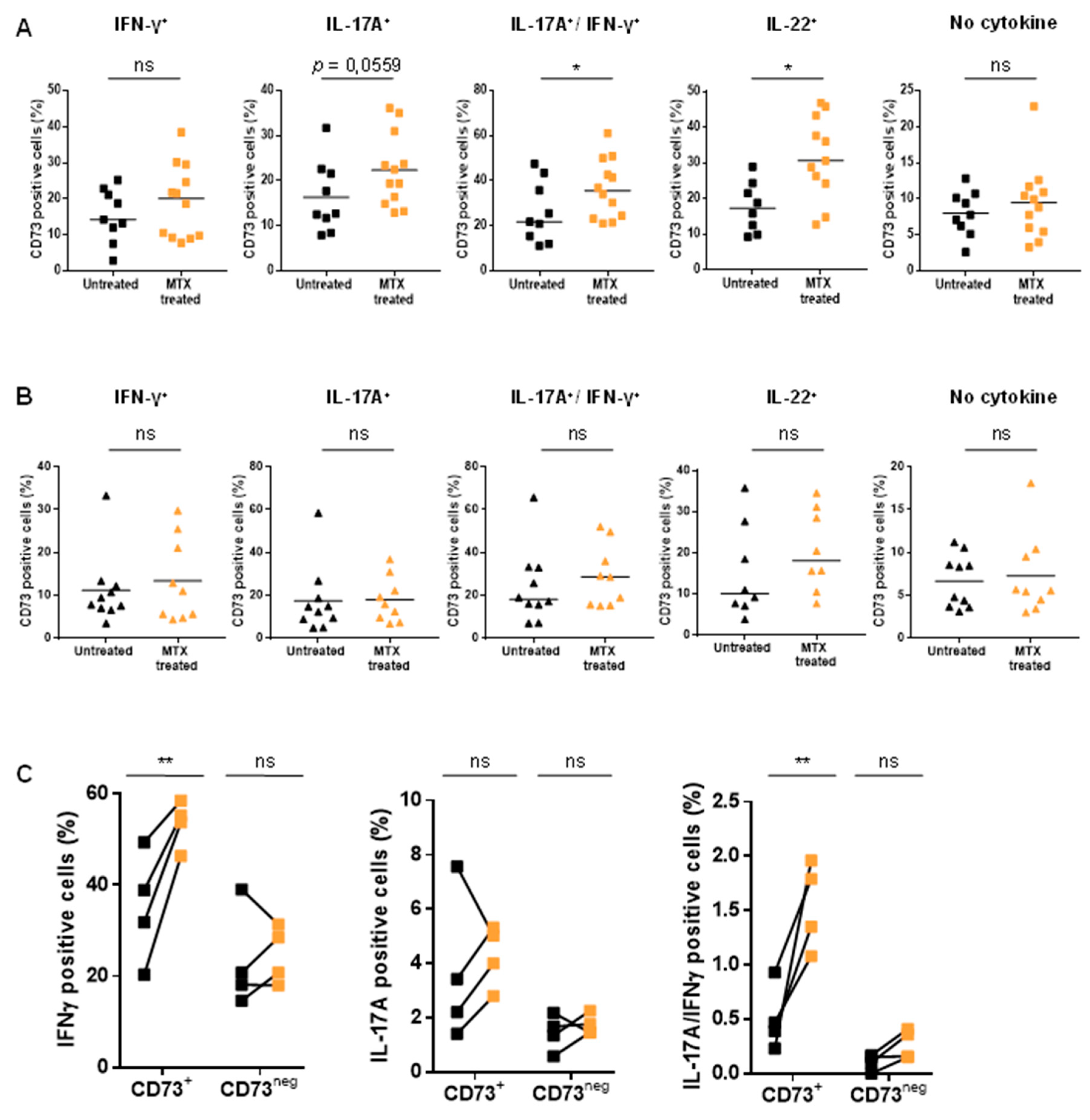
3.2. Untreated RA and PsA Patients’ Blood Teff are Polyfunctional but Express Lower Levels of CD73 among IFN-γ/IL-17A Expressing Cells
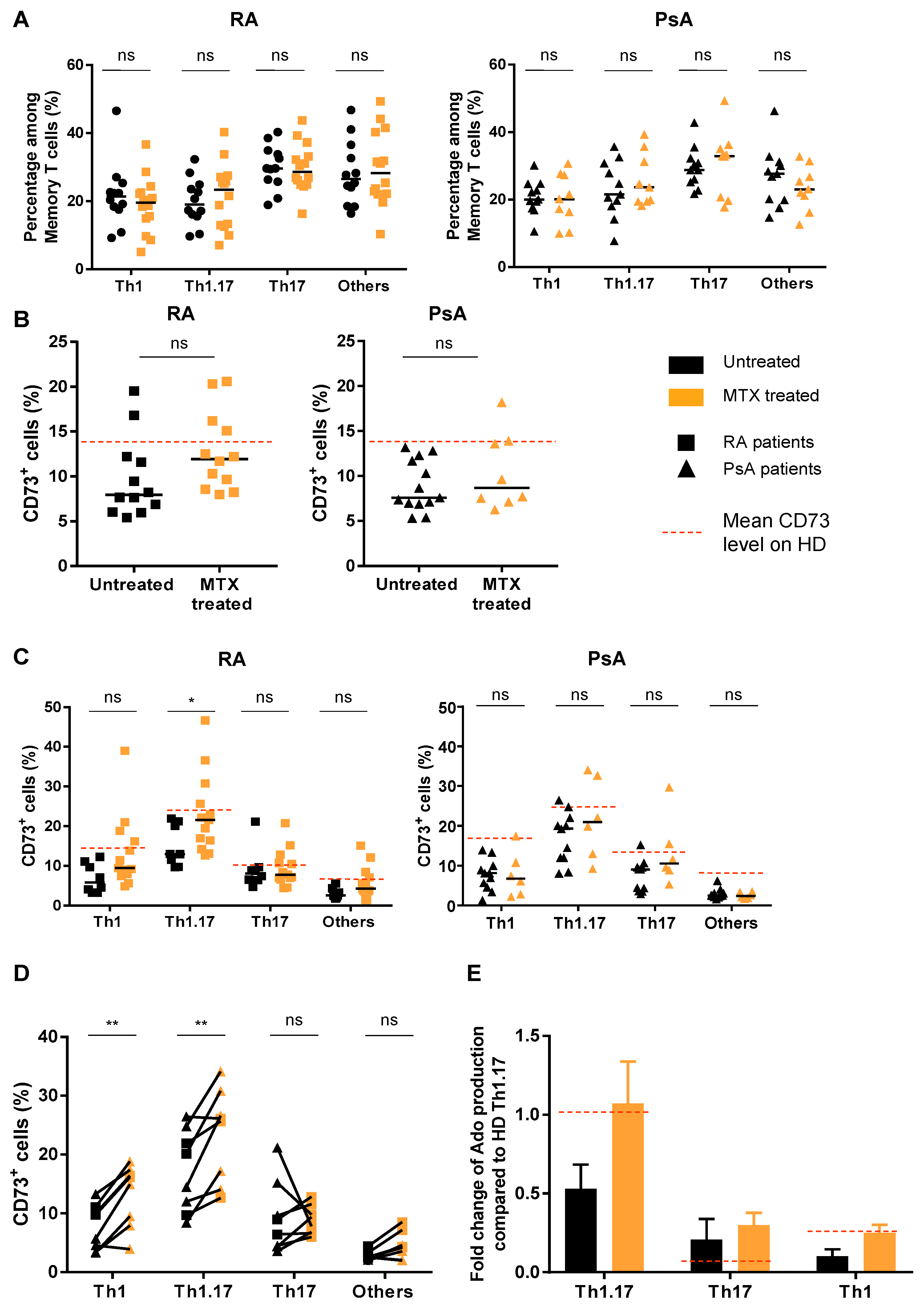
3.3. Treatment of Patients with MTX Partially Restores CD73 Expression on Teff Resulting in an Enhanced Ado Production
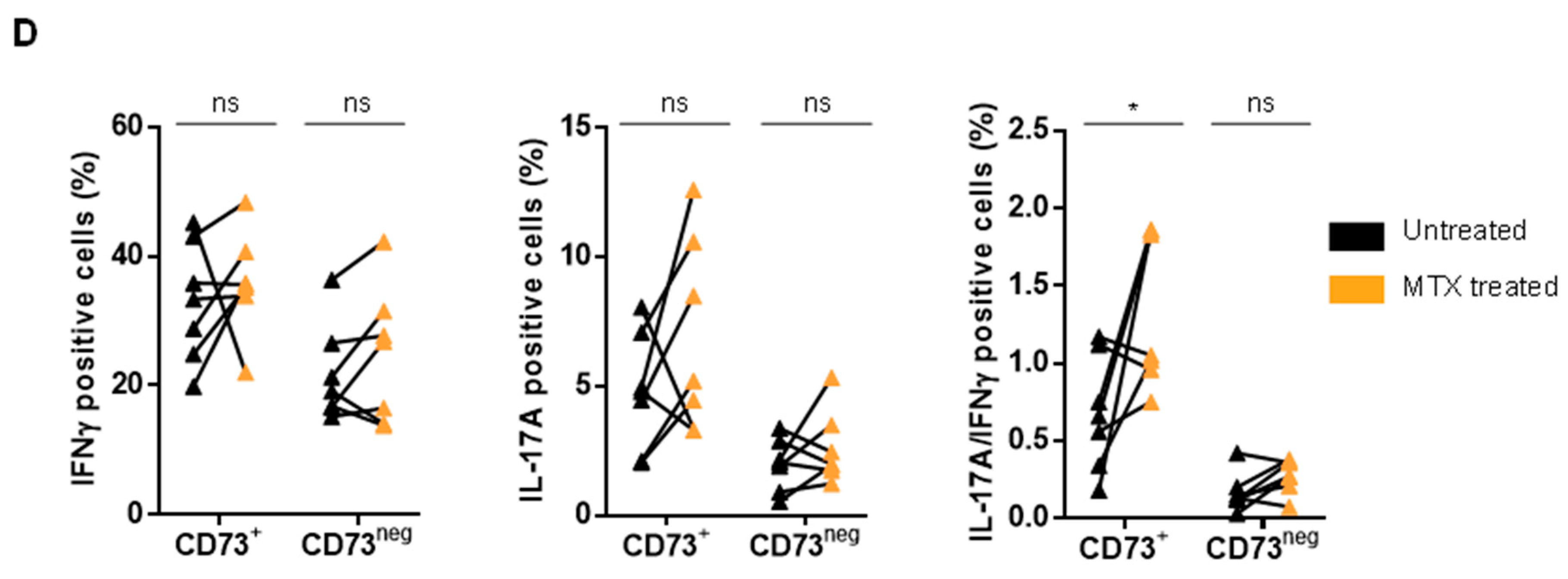
3.4. MTX Treatment Impacts Teff Polyfunctionality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64, ii14–ii17. [Google Scholar] [CrossRef] [PubMed]
- Basdeo, S.A.; Cluxton, D.; Sulaimani, J.; Moran, B.; Canavan, M.; Orr, C.; Veale, D.J.; Fearon, U.; Fletcher, J.M. Ex-Th17 (Nonclassical Th1) Cells Are Functionally Distinct from Classical Th1 and Th17 Cells and Are Not Constrained by Regulatory T Cells. J. Immunol. 2017, 198, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Nistala, K.; Adams, S.; Cambrook, H.; Ursu, S.; Olivito, B.; de Jager, W.; Evans, J.G.; Cimaz, R.; Bajaj-Elliott, M.; Wedderburn, L.R. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl. Acad. Sci. USA 2010, 107, 14751–14756. [Google Scholar] [CrossRef]
- Raychaudhuri, S.P.; Raychaudhuri, S.K.; Genovese, M.C. IL-17 receptor and its functional significance in psoriatic arthritis. Mol. Cell Biochem. 2012, 359, 419–429. [Google Scholar] [CrossRef]
- Burman, A.; Haworth, O.; Bradfield, P.; Parsonage, G.; Filer, A.; Thomas, A.M.; Amft, N.; Salmon, M.; Buckley, C.D. The role of leukocyte-stromal interactions in chronic inflammatory joint disease. Jt. Bone Spine 2005, 72, 10–16. [Google Scholar] [CrossRef][Green Version]
- Hot, A.; Zrioual, S.; Lenief, V.; Miossec, P. IL-17 and tumour necrosis factor α combination induces a HIF-1α-dependent invasive phenotype in synoviocytes. Ann. Rheum. Dis. 2012, 71, 1393–1401. [Google Scholar] [CrossRef]
- Raychaudhuri, S.K.; Saxena, A.; Raychaudhuri, S.P. Role of IL-17 in the pathogenesis of psoriatic arthritis and axial spondyloarthritis. Clin. Rheumatol. 2015, 34, 1019–1023. [Google Scholar] [CrossRef]
- van Hamburg, J.P.; Asmawidjaja, P.S.; Davelaar, N.; Mus, A.M.C.; Colin, E.M.; Hazes, J.M.W.; Dolhain, R.J.; Lubberts, E. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011, 63, 73–83. [Google Scholar] [CrossRef]
- Kotake, S.; Udagawa, N.; Takahashi, N.; Matsuzaki, K.; Itoh, K.; Ishiyama, S.; Saito, S.; Inoue, K.; Kamatani, N.; Gillespie, M.T.; et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 1999, 103, 1345–1352. [Google Scholar] [CrossRef]
- Ménétrier-Caux, C.; Curiel, T.; Faget, J.; Manuel, M.; Caux, C.; Zou, W. Targeting regulatory T cells. Target. Oncol. 2012, 7, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Gourdin, N.; Bossennec, M.; Rodriguez, C.; Vigano, S.; Machon, C.; Jandus, C.; Faget, J.; Durand, I.; Chopin, N.; Bauché, D.; et al. Autocrine Adenosine regulates tumor polyfunctional CD73+CD4+ effector T cells devoid of immune checkpoints. Cancer Res. 2018, 78, 3604–3618. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.M.; Pratt, A.G.; Isaacs, J.D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 2016, 12, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Criado, A.B.; Mongey, A.B.; Avouac, J.; Marotte, H.; Mueller, R.B. How to Get the Most from Methotrexate (MTX) Treatment for Your Rheumatoid Arthritis Patient?-MTX in the Treat-to-Target Strategy. J. Clin. Med. 2019, 8, 515. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Takedachi, M.; Thompson, L.F.; Wilder, T.F.; Fernández, P.; Cronstein, B.N. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: Findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 2007, 56, 1440–1445. [Google Scholar] [CrossRef]
- Mirabet, M.; Herrera, C.; Cordero, O.J.; Mallol, J.; Lluis, C.; Franco, R. Expression of A2B adenosine receptors in human lymphocytes: Their role in T cell activation. J. Cell Sci. 1999, 112, 491–502. [Google Scholar]
- Zarek, P.E.; Huang, C.-T.; Lutz, E.R.; Kowalski, J.; Horton, M.R.; Linden, J.; Drake, C.G.; Powell, J.D. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 2008, 111, 251–259. [Google Scholar] [CrossRef]
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012, 3, 190. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2005, 54, 2665–2673. [Google Scholar] [CrossRef]
- Wells, G.; Becker, J.C.; Teng, J.; Dougados, M.; Schiff, M.; Smolen, J.; Aletaha, D.; van Riel, P.L. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann. Rheum. Dis. 2009, 68, 954–960. [Google Scholar]
- Sallusto, F.; Lanzavecchia, A. Heterogeneity of CD4+ memory T cells: Functional modules for tailored immunity. Eur. J. Immunol. 2009, 39, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Machon, C.; Jordheim, L.P.; Puy, J.-Y.; Lefebvre, I.; Dumontet, C.; Guitton, J. Fully validated assay for the quantification of endogenous nucleoside mono- and triphosphates using online extraction coupled with liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2925–2941. [Google Scholar] [CrossRef] [PubMed]
- Moncrieffe, H.; Nistala, K.; Kamhieh, Y.; Evans, J.; Eddaoudi, A.; Eaton, S.; Wedderburn, L.R. High Expression of the Ectonucleotidase CD39 on T Cells from the Inflamed Site Identifies Two Distinct Populations, One Regulatory and One Memory T Cell Population. J. Immunol. 2010, 185, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yan, J.; Putheti, P.; Wu, Y.; Sun, X.; Toxavidis, V.; Tigges, J.; Kassam, N.; Enjyoji, K.; Robson, S.C.; et al. Isolated CD39 Expression on CD4+ T Cells Denotes both Regulatory and Memory Populations. Am. J. Transpl. 2009, 9, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Sitkovsky, M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Cooney, L.A.; Fox, D.A. The role of T helper type 17 cells in inflammatory arthritis. Clin. Exp. Immunol. 2010, 159, 225–237. [Google Scholar] [CrossRef]
- Kotake, S.; Yago, T.; Kobashigawa, T.; Nanke, Y. The Plasticity of Th17 Cells in the Pathogenesis of Rheumatoid Arthritis. J. Clin. Med. 2017, 6, 67. [Google Scholar] [CrossRef]
- Walter, G.J.; Fleskens, V.; Frederiksen, K.S.; Rajasekhar, M.; Menon, B.; Gerwien, J.G.; Evans, H.G.; Taams, L.S. Phenotypic, Functional, and Gene Expression Profiling of Peripheral CD45RA+ and CD45RO+ CD4+CD25+CD127low Treg Cells in Patients with Chronic Rheumatoid Arthritis. Arthritis Rheum. 2016, 68, 103–116. [Google Scholar] [CrossRef]
- Herrath, J.; Chemin, K.; Albrecht, I.; Catrina, A.I.; Malmström, V. Surface expression of CD39 identifies an enriched Treg-cell subset in the rheumatic joint, which does not suppress IL-17A secretion. Eur. J. Immunol. 2014, 44, 2979–2989. [Google Scholar] [CrossRef]
- Botta Gordon-Smith, S.; Ursu, S.; Eaton, S.; Moncrieffe, H.; Wedderburn, L.R. Correlation of Low CD73 Expression on Synovial Lymphocytes with Reduced Adenosine Generation and Higher Disease Severity in Juvenile Idiopathic Arthritis. Arthritis Rheum. 2015, 67, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Prakken, B.; Wehrens, E.; van Wijk, F. Editorial: Quality or quantity? Unraveling the role of Treg cells in rheumatoid arthritis. Arthritis Rheum. 2013, 65, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Santarlasci, V.; Capone, M.; Rossi, M.C.; Querci, V.; Mazzoni, A.; Cimaz, R.; De Palma, R.; Liotta, F.; Maggi, E.; et al. Distinctive features of classic and nonclassic (Th17 derived) human Th1 cells. Eur. J. Immunol. 2012, 42, 3180–3188. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Bioley, G.; Rivals, J.-P.; Dudler, J.; Speiser, D.; Romero, P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008, 58, 2307–2317. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Li, Y.; Qi, L.; Liu, X.; Yuan, C.; Hu, N.W.; Ma, D.X.; Li, Z.F.; Yang, Q.; et al. Increased Frequencies of Th22 Cells as well as Th17 Cells in the Peripheral Blood of Patients with Ankylosing Spondylitis and Rheumatoid Arthritis. PLoS ONE 2012, 7, e31000. [Google Scholar] [CrossRef]
- Benham, H.; Norris, P.; Goodall, J.; Wechalekar, M.D.; FitzGerald, O.; Szentpetery, A.; Smith, M.; Thomas, R.; Gaston, H. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res. Ther. 2013, 15, R136. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, E.; Warszawska, K.; Schulze-Tanzil, G.; Witte, K.; Philipp, S.; Kunz, S.; Döcke, W.D.; Asadullah, K.; Volk, H.D.; et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: A novel immunological cascade with potential relevance in psoriasis. Eur. J. Immunol. 2009, 39, 3570–3581. [Google Scholar] [CrossRef]
- Ezeonyeji, A.; Baldwin, H.; Vukmanovic-Stejic, M.; Ehrenstein, M.R. CD4 T-Cell Dysregulation in Psoriatic Arthritis Reveals a Regulatory Role for IL-22. Front. Immunol. 2017, 8, 1403. [Google Scholar] [CrossRef]
- van Hamburg, J.P.; Corneth, O.B.J.; Paulissen, S.M.J.; Davelaar, N.; Asmawidjaja, P.S.; Mus, A.M.; Lubberts, E. IL-17/Th17 mediated synovial inflammation is IL-22 independent. Ann. Rheum. Dis. 2013, 72, 1700–1707. [Google Scholar] [CrossRef]
- Peres, R.S.; Liew, F.Y.; Talbot, J.; Carregaro, V.; Oliveira, R.D.; Almeida, S.L.; França, R.F.; Donate, P.B.; Pinto, L.G.; Ferreira, F.I.; et al. Low expression of CD39 on regulatory T cells as a biomarker for resistance to methotrexate therapy in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2015, 112, 2509–2514. [Google Scholar] [CrossRef]
- Dwyer, K.M.; Hanidziar, D.; Putheti, P.; Hill, P.A.; Pommey, S.; McRae, J.L.; Winterhalter, A.; Doherty, G.; Deaglio, S.; Koulmanda, M.; et al. Expression of CD39 by human peripheral blood CD4+CD25+ T cells denotes a regulatory memory phenotype. Am. J. Transplant. 2010, 10, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Katiyar, S.; Singh, A.; Misra, R.; Aggarwal, A. CD39 positive regulatory T cell frequency as a biomarker of treatment response to methotrexate in rheumatoid arthritis. Int. J. Rheum. Dis. 2018, 21, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Fagerli, K.M.; Lie, E.; van der Heijde, D.; Heiberg, M.S.; Lexberg, A.S.; Rødevand, E.; Kalstad, S.; Mikkelsen, K.; Kvien, T.K. The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: Results from 440 patients included in the NOR-DMARD study. Ann. Rheum. Dis. 2014, 73, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Dougados, M.; Soubrier, M.; Antunez, A.; Balint, P.; Balsa, A.; Buch, M.; Kalstad, S.; Mikkelsen, K.; Kvien, T.K. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: Results of an international, cross-sectional study (COMORA). Ann. Rheum. Dis. 2013, 73, 62–68. [Google Scholar] [CrossRef]


| RA Patients n = 26 | PsA Patients n = 15 | Healthy Controls n = 12 | |
|---|---|---|---|
| Gender ratio (f/m, n) | 19/7 | 9/6 | 8/4 |
| Age (years) | 56 (22–82) | 53 (31–79) | 49 (25–69) |
| DAS28 Untreated MTX-treated | 4.6 (1.6–6.4) 3.4 (1.8–7.2) | - | |
| CRP (mg/mL) Untreated MTX-treated | 17.9 (0.6–60) 5.2 (0.5–29) | 16.4 (2.6–88) 4.3 (1.3–10) | |
| Mean MTX treatment duration (months) | 9.3 (3–22) | 6 (3–22) | - |
| MTX doses (mg/week) | (15–20) | (15–20) | - |
| RF detection (yes/no, n) | 22/4 | Not detected | - |
| ACPA detection (yes/no, n) | 24/2 | Not detected | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossennec, M.; Rodriguez, C.; Hubert, M.; Di-Roio, A.; Machon, C.; Guitton, J.; Battiston-Montagne, P.; Couturier, M.; Marotte, H.; Caux, C.; et al. Methotrexate Restores CD73 Expression on Th1.17 in Rheumatoid Arthritis and Psoriatic Arthritis Patients and May Contribute to Its Anti-Inflammatory Effect through Ado Production. J. Clin. Med. 2019, 8, 1859. https://doi.org/10.3390/jcm8111859
Bossennec M, Rodriguez C, Hubert M, Di-Roio A, Machon C, Guitton J, Battiston-Montagne P, Couturier M, Marotte H, Caux C, et al. Methotrexate Restores CD73 Expression on Th1.17 in Rheumatoid Arthritis and Psoriatic Arthritis Patients and May Contribute to Its Anti-Inflammatory Effect through Ado Production. Journal of Clinical Medicine. 2019; 8(11):1859. https://doi.org/10.3390/jcm8111859
Chicago/Turabian StyleBossennec, Marion, Céline Rodriguez, Margaux Hubert, Anthony Di-Roio, Christelle Machon, Jérôme Guitton, Priscilla Battiston-Montagne, Mathilde Couturier, Hubert Marotte, Christophe Caux, and et al. 2019. "Methotrexate Restores CD73 Expression on Th1.17 in Rheumatoid Arthritis and Psoriatic Arthritis Patients and May Contribute to Its Anti-Inflammatory Effect through Ado Production" Journal of Clinical Medicine 8, no. 11: 1859. https://doi.org/10.3390/jcm8111859
APA StyleBossennec, M., Rodriguez, C., Hubert, M., Di-Roio, A., Machon, C., Guitton, J., Battiston-Montagne, P., Couturier, M., Marotte, H., Caux, C., Coury, F., & Ménétrier-Caux, C. (2019). Methotrexate Restores CD73 Expression on Th1.17 in Rheumatoid Arthritis and Psoriatic Arthritis Patients and May Contribute to Its Anti-Inflammatory Effect through Ado Production. Journal of Clinical Medicine, 8(11), 1859. https://doi.org/10.3390/jcm8111859







