Comparison between Adult Patients with Sickle Cell Disease of Sub-Saharan African Origin Born in Metropolitan France and in Sub-Saharan Africa
Abstract
1. Introduction
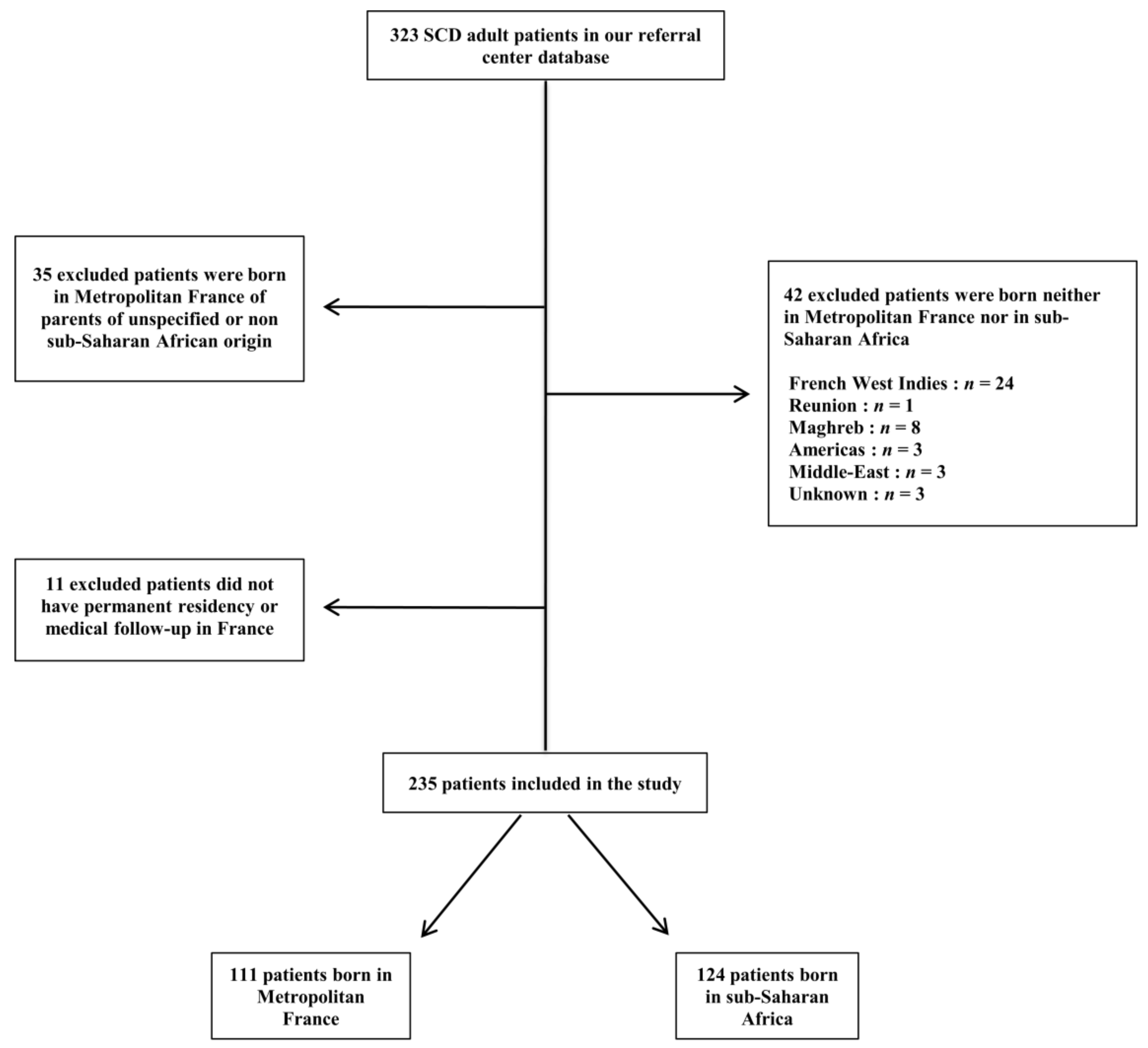
2. Patients and Methods
2.1. Study Design
2.2. Specific Data Collection
2.3. Statistical Analysis
3. Results
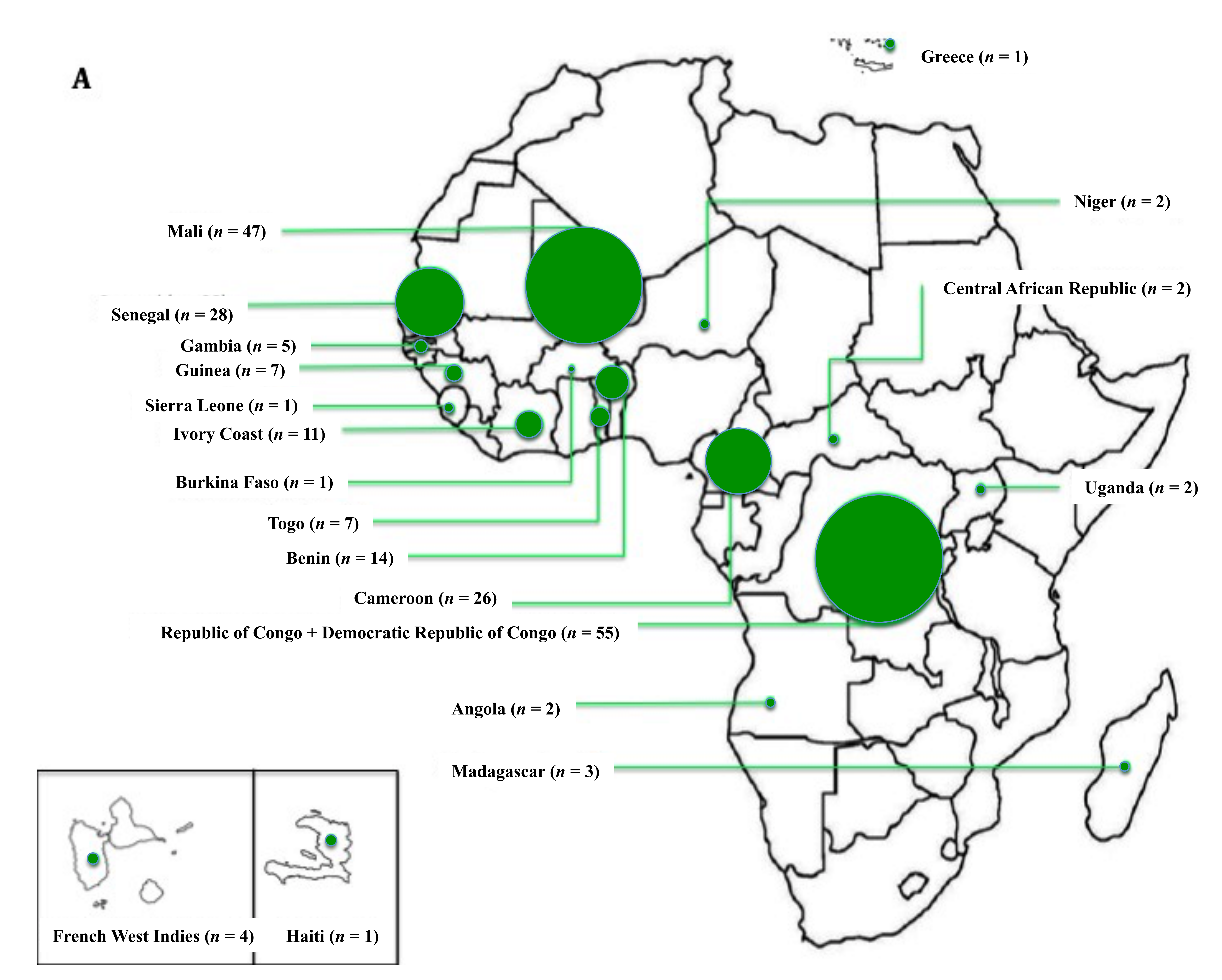
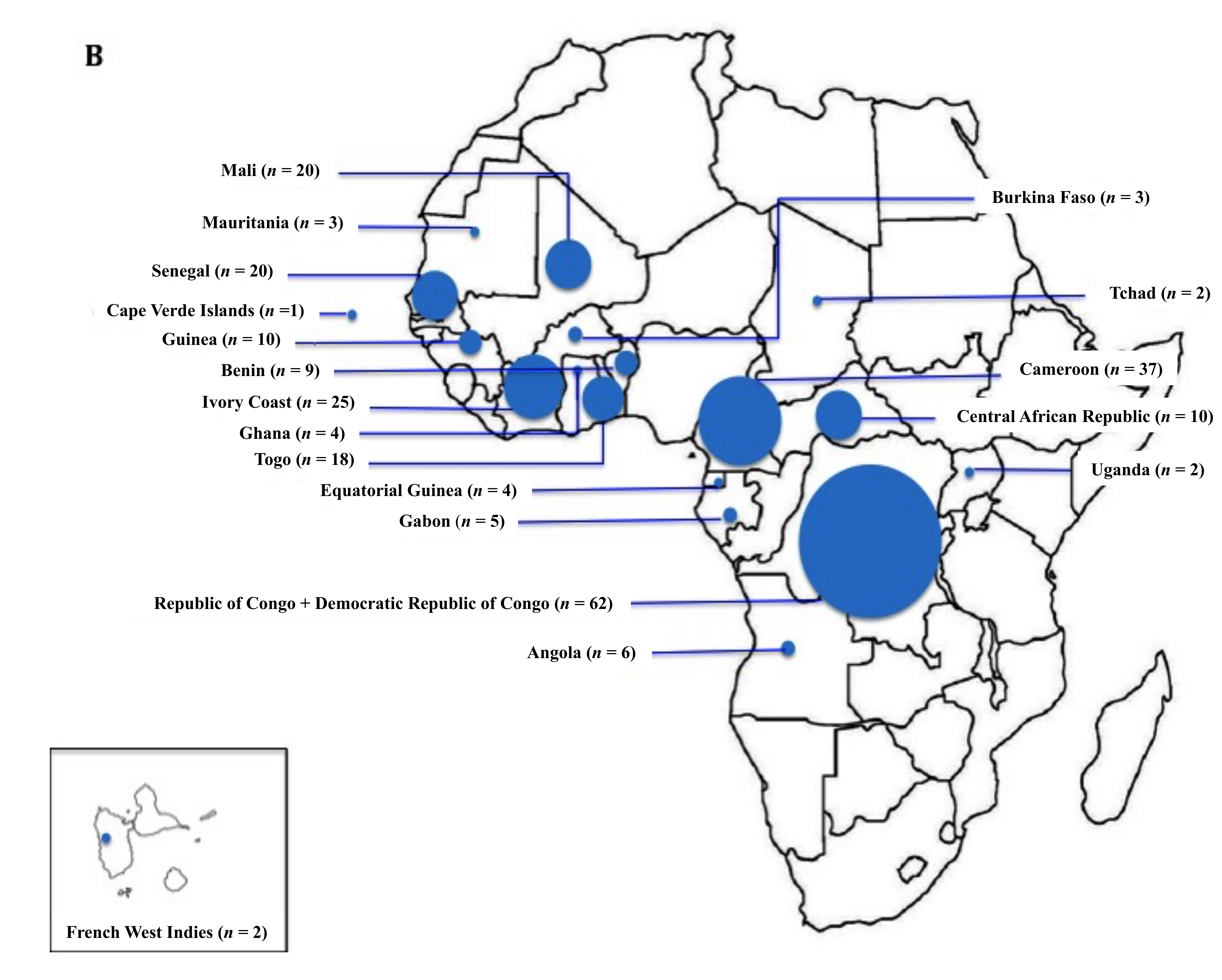
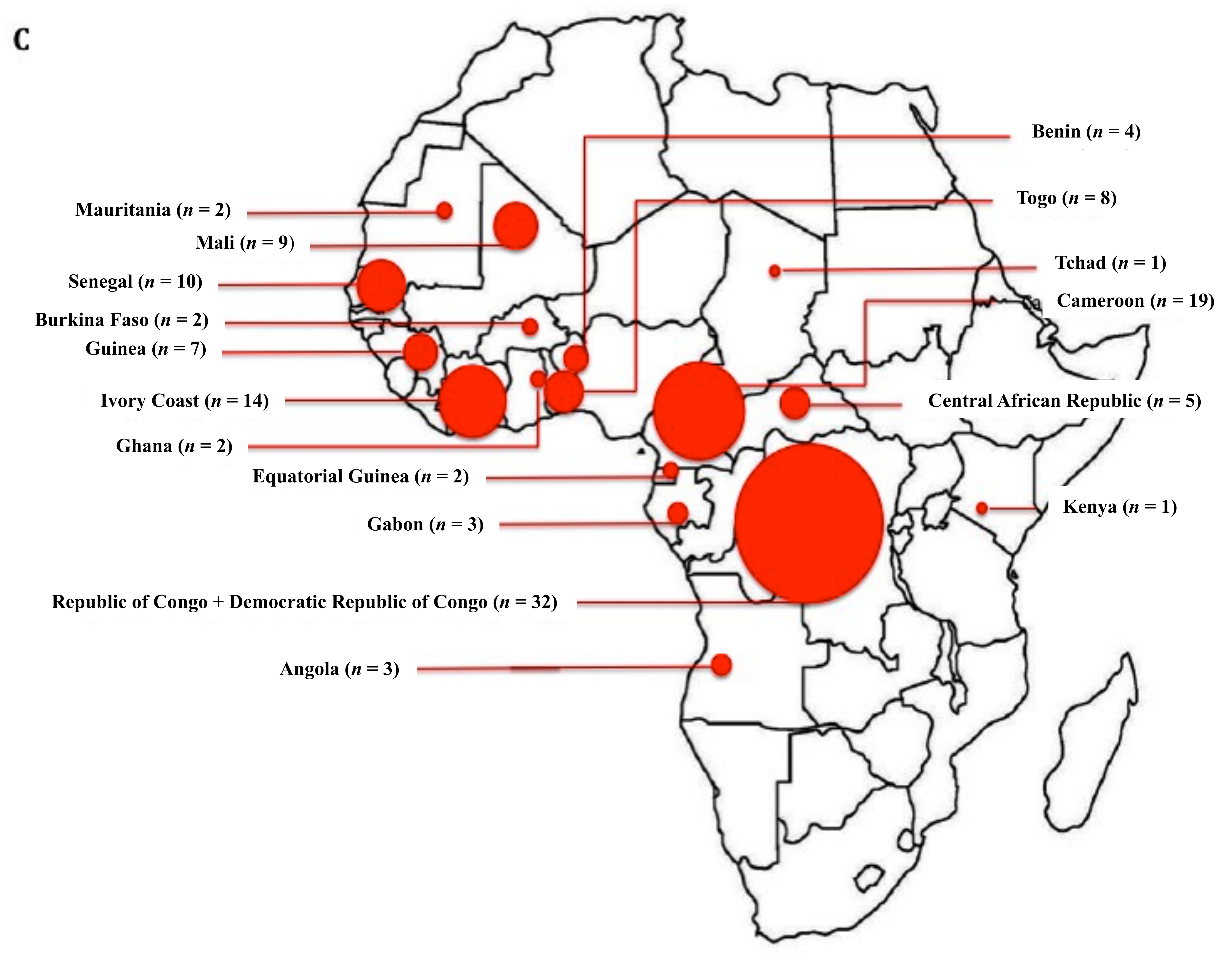
3.1. Demographic Data
3.2. Acute Clinical Events and Chronic Complications
3.3. Therapeutic Management
3.4. Maternal and Fetal Complications
3.5. Comorbidities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef]
- Ranque, B.; Menet, A.; Diop, I.B.; Thiam, M.M.; Diallo, D.; Diop, S.; Diagne, I.; Sanogo, I.; Kingue, S.; Chelo, D.; et al. Early renal damage in patients with sickle cell disease in sub-Saharan Africa: A multinational, prospective, cross-sectional study. Lancet Haematol. 2014, 1, e64–e73. [Google Scholar] [CrossRef]
- Shriner, D.; Rotimi, C.N. Whole-Genome-Sequence-Based Haplotypes Reveal Single Origin of the Sickle Allele during the Holocene Wet Phase. Am. J. Hum. Genet. 2018, 102, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Lainé, A.; Tchernia, G. “L’émergence” d’une maladie multimillénaire.: Circulations de savoirs et production d’inégalités face à la drépanocytose. Virginie Chasles. In Santé et Mondialisation; Université Jean-Moulin-Lyon 3: Lyon, France, 2010; pp. 238–261. [Google Scholar]
- Arlet, J.-B. Drépanocytose—Tout praticien aura à assumer des soins de proximité. Concours Med. 2016, 138, 279–308. [Google Scholar]
- Institut National de Veille Sanitaire. L’état de Santé de la Population en France—Suivi des Objectifs Annexés à la loi de Santé Publique—Rapport 2011. Available online: https://solidarites-sante.gouv.fr/IMG/pdf/Etat_sante-population_2011.pdf (accessed on 21 July 2019).
- Dormandy, E.; James, J.; Inusa, B.; Rees, D. How many people have sickle cell disease in the UK? J. Public Health 2018, 40, e291–e295. [Google Scholar] [CrossRef]
- Williams, T.N.; Uyoga, S.; Macharia, A.; Ndila, C.; McAuley, C.F.; Opi, D.H.; Mwarumba, S.; Makani, J.; Komba, A.; Ndiritu, M.N.; et al. Bacteraemia in Kenyan children with sickle-cell anaemia: A retrospective cohort and case–control study. Lancet 2009, 374, 1364–1370. [Google Scholar] [CrossRef]
- McAuley, C.F.; Webb, C.; Makani, J.; Macharia, A.; Uyoga, S.; Opi, D.H.; Ndila, C.; Ngatia, A.; Scott, J.A.G.; Marsh, K.; et al. High mortality from Plasmodium falciparum malaria in children living with sickle cell anemia on the coast of Kenya. Blood 2010, 116, 1663–1668. [Google Scholar] [CrossRef]
- Platt, O.S.; Brambilla, D.J.; Rosse, W.F.; Milner, P.F.; Castro, O.; Steinberg, M.H.; Klug, P.P. Mortality in sickle cell disease—Life Expectancy and Risk Factors for Early Death. N. Engl. J. Med. 1994, 330, 1639–1644. [Google Scholar] [CrossRef]
- Elmariah, H.; Garrett, M.E.; De Castro, L.M.; Jonassaint, J.C.; Ataga, K.I.; Eckman, J.R.; Ashley-Koch, A.E.; Telen, M.J. Factors associated with survival in a contemporary adult sickle cell disease cohort: Factors Associated with Survival in Sickle Cell Disease. Am. J. Hematol. 2014, 89, 530–535. [Google Scholar] [CrossRef]
- Gomes, E.; Castetbon, K.; Goulet, V. Mortalité liée à la drépanocytose en France: Âge de décès et causes associées (1979–2010). Bull. Epidémiol. Hebd. 2015, 8, 142–150. [Google Scholar]
- Arlet, J.-B.; Ribeil, J.-A.; Chatellier, G.; Eladari, D.; De Seigneux, S.; Souberbielle, J.-C.; Friedlander, G.; de Montalembert, M.; Pouchot, J.; Prié, D.; et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: A prospective observational cohort study. BMC Nephrol. 2012, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, P.; Habibi, A.; Stehlé, T.; Di Liberto, G.; Rakotoson, M.G.; Gellen-Dautremer, J.; Loric, S.; Moutereau, S.; Sahali, D.; Wagner-Ballon, O.; et al. Six Months of Hydroxyurea Reduces Albuminuria in Patients with Sickle Cell Disease. J. Am. Soc. Nephrol. 2016, 27, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Bodez, D.; Habibi, A.; Guellich, A.; Rappeneau, S.; Inamo, J.; Guendouz, S.; Gellen-Dautremer, J.; Pissard, S.; Loric, S.; et al. Haematological determinants of cardiac involvement in adults with sickle cell disease. Eur. Heart J. 2016, 37, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Barst, R.J.; Gibbs, J.S.R.; Hildesheim, M.; Sachdev, V.; Nouraie, M.; Hassell, K.L.; Little, J.A.; Schraufnagel, D.E.; Krishnamurti, L.; et al. Risk Factors for Death in 632 Patients with Sickle Cell Disease in the United States and United Kingdom. PLoS ONE 2014, 9, e99489. [Google Scholar] [CrossRef]
- Grosse, S.D.; Odame, I.; Atrash, H.K.; Amendah, D.D.; Piel, F.B.; Williams, T.N. Sickle Cell Disease in Africa. Am. J. Prev. Med. 2011, 41, S398–S405. [Google Scholar] [CrossRef]
- Makani, J.; Williams, T.N.; Marsh, K. Sickle cell disease in Africa: Burden and research priorities. Ann. Trop. Med. Parasitol. 2007, 101, 3–14. [Google Scholar] [CrossRef]
- Dembélé, A.K.; Toure, B.A.; Sarro, Y.S.; Guindo, A.; Fané, B.; Offredo, L.; Kené, S.; Conaré, I.; Tessougué, O.; Traoré, Y.; et al. Prévalence et facteurs de risque de la rétinopathie drépanocytaire dans un centre de suivi drépanocytaire d’Afrique subsaharienne. Rev. Méd. Interne 2017, 38, 572–577. [Google Scholar] [CrossRef]
- Serjeant, G.R.; Serjeant, B.E.; Mohan, J.S.; Clare, A. Leg Ulceration in Sickle Cell Disease: Medieval Medicine in a Modern World. Hematol. Oncol. Clin. 2005, 19, 943–956. [Google Scholar] [CrossRef]
- Arlet, J.-B.; Comarmond, C.; Habibi, A.; Stankovic, K.; Ribeil, J.-A.; Lopez Sublet, M.; Affo, L.; Chantalat Auger, C.; Bouldouyre, M.-A.; Gellen-Dautremer, J.; et al. Prevalence and Characteristics of Hepatitis C Virus Infection in Adult Sickle Cell Disease Patients Living in France. J. Infect. Dis. Epidemiol. 2016, 2, 020. [Google Scholar] [CrossRef]
- Al-Saqladi, A.-W.M.; Cipolotti, R.; Fijnvandraat, K.; Brabin, B. Growth and nutritional status of children with homozygous sickle cell disease. Ann. Trop. Paediatr. 2008, 28, 165–189. [Google Scholar] [CrossRef]
- Wang, W.C.; Helms, R.W.; Lynn, H.S.; Redding-Lallinger, R.; Gee, B.E.; Ohene-Frempong, K.; Smith-Whitley, K.; Waclawiw, M.A.; Vichinsky, E.P.; Styles, L.A.; et al. Effect of hydroxyurea on growth in children with sickle cell anemia: Results of the HUG-KIDS study. J. Pediatr. 2002, 140, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Morales, K.H.; Scher, C.D.; Styles, L.; Olivieri, N.; Adams, R.; Brambilla, D. Effect of Long-term Transfusion on Growth in Children with Sickle Cell Anemia: Results of the Stop Trial. J. Pediatr. 2005, 147, 244–247. [Google Scholar] [CrossRef]
- Hankins, J.S.; Aygun, B.; Nottage, K.; Thornburg, C.; Smeltzer, M.P.; Ware, R.E.; Wang, W.C. From Infancy to Adolescence: Fifteen Years of Continuous Treatment with Hydroxyurea in Sickle Cell Anemia. Medicine 2014, 93, e215. [Google Scholar] [CrossRef] [PubMed]
- Platt, O.S.; Rosenstock, W.; Espeland, M.A. Influence of Sickle Hemoglobinopathies on Growth and Development. N. Engl. J. Med. 1984, 311, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Alexandre-Heymann, L.; Dubert, M.; Diallo, D.A.; Diop, S.; Tolo, A.; Belinga, S.; Sanogo, I.; Diagne, I.; Wamba, G.; Boidy, K.; et al. Prevalence and correlates of growth failure in young African patients with sickle cell disease. Br. J. Haematol. 2019, 184, 253–262. [Google Scholar] [CrossRef]
- Risk Factor Collaboration. Evolution of Adult Height over Time. Rank. People Born 1896–1996. Available online: http://www.ncdrisc.org/height-mean-ranking.html (accessed on 21 July 2019).
| Demographic and Biological Parameters | Whole Population n = 235 | Patients Born in France n = 111 | Patients Born in Sub-Saharan Africa n = 124 | p Value * | p Value ** |
|---|---|---|---|---|---|
| Male, n (%) | 112 (47.7) | 63 (56.8) | 49 (39.5) | 0.008 | 0.02 |
| Age at last follow-up (years) | 29.2 (23–35.1) | 25.6 (22.1–30.5) | 32.1 (24.4–39) | <0.001 | <0.001 |
| Age at SCD diagnosis (years) | 1 (0–5) | 0 (0–2) | 3.0 (1–9.5) | <0.001 | 0.001 |
| Neonatal screening, n (%) | 50/170 (29.4) | 42/83 (50.6) | 8/87 (9.2) | <0.001 | <0.001 |
| Age at arrival in metropolitan France (years) | - | - | 18 (13–23) | - | - |
| Duration of residency in France (years) | - | - | 12.6 (8.3–19.2) | - | - |
| Height (cm) | 171.8 (165–178) | 174.5 (168–179) | 169 (163–175) | 0.001 | 0.001 |
| Male (cm) | 176 (173.3–182) | 176.6 (171.8–180) | |||
| Female (cm) | 169 (164.3–173.8) | 165 (161–169) | |||
| Weight (kg) | 66.7 ± 12.4 | 67.6 ± 13.2 | 65.9 ± 11.8 | 0.02 | 0.007 |
| Male (kg) | 68.9 ± 11.6 | 68 ± 10.4 | |||
| Female (kg) | 65.9 ± 14.9 | 64.5 ± 12.4 | |||
| BMI (kg/m2) | 22.1 (20–24.6) | 21.6 (19.9–24.2) | 22.4 (20.3–24.9) | 0.3 | 0.1 |
| Genotypes | |||||
| S/S, n (%) | 180 (76.6) | 92 (82.9) | 88 (71) | 0.6 | - |
| S/C, n (%) | 40 (17) | 16 (14.4) | 24 (19.4) | ||
| S/β+, n (%) | 9 (3.8) | 2 (1.8) | 7 (5.6) | ||
| S/β°, n (%) | 6 (2.6) | 1 (0.9) | 5 (4) | ||
| Hb (g/dL) | 9 (8–10.7) | 8.6 (7.6–10.2) | 9.3 (8.2–11) | 0.2 | 0.3 |
| MCV (fL) | 79 (73–86) | 79 (73.1–86) | 80 (72–87) | 0.4 | 0.5 |
| Reticulocytes (g/L) | 214.0 (136–318) | 217.5 (144.5–316) | 201(132–319) | 0.3 | 0.2 |
| LDH (UI/L) | 405 (305–484) | 383 (305–489) | 407 (299.5–477.5) | 0.1 | 0.1 |
| Total bilirubin (μmol/L) | 35 (22–58) | 35.5 (24.5–67) | 33 (19–54) | 0.5 | 0.6 |
| HbA2 (%) | 3.4 (3–3.9) | 3.4 (3–3.6) | 3.5 (3–4) | 0.7 | 0.4 |
| HbF (%) | 4.8 (2.3–8.2) | 4.2 (1.7–8) | 5.6 (2.9–8.2) | 0.2 | 0.4 |
| Acute Complications | Whole Population n = 235 | Patients Born in France n = 111 | Patients Born in Sub-Saharan Africa n = 124 | pa | pb |
|---|---|---|---|---|---|
| Vaso-occlusive crisis | |||||
| Number of admissions for VOC in the last 12 months | 1 (0–2) | 1 (1–2) | 1 (0–2) | 0.7 | 0.8 |
| Acute chest syndrome, n (%) | 162/231 (70.1) | 86/110 (78.2) | 76/121 (62.8) | 0.01 | 0.01 |
| Number of episodes over lifetime | 1 (0–3) | 2 (1–4) | 1 (0–3) | <0.001 | 0.002 |
| Age at first episode (years) | 21 (16.4–26) | 19 (11.6–22.3) | 24 (18.4–29.5) | <0.001 | 0.007 |
| ≥ 1 ICU admission for ACS over lifetime | 95/216 (44) | 57/107 (53.3) | 38/109 (34.9) | 0.006 | 0.006 |
| Priapism, n (%) | 36/99 (36.4) | 20/56 (35.7) | 16/43 (37.2) | 0.9 | 0.5 |
| Age at 1st priapism episode (years) | 16.8 (12.3–22.5) | 17 (12.2–22.7) | 15.4 (12.3–18.6) | 0.8 | 0.8 |
| Stroke, n (%) | 19/224 (8.5) | 6/107 (5.6) | 13/117 (11.1) | 0.1 | 0.2 |
| Age at first stroke (years) | 20.3 (11.1–33.5) | 17.1 (12.6–21.5) | 23.2 (11.1–33.9) | 0.3 | 0.1 |
| Splenic complications | |||||
| Splenic sequestration, n (%) | 15/223 (6.7) | 8/105 (7.6) | 7/118 (5.9) | 0.6 | 0.4 |
| Splenectomy, n (%) | 15/223 (6.7) | 8/105 (7.6) | 7/118 (5.9) | 0.6 | 0.4 |
| Age at splenectomy (years) | 11.3 (9–14.8) | 12.1 (6.9–16) | 10.9 (9.5–13.5) | 0.9 | 0.3 |
| Cholecystectomy, n (%) | 134/224 (59.6) | 73/109 (67) | 61/116 (52.6) | 0.03 | 0.04 |
| Age at cholecystectomy (years) | 17 (12.6–23.8) | 14.8 (10.9–20.8) | 21.7 (15.5–30.5) | <0.001 | 0.5 |
| Thrombo-embolic events, n (%) | 20/230 (8.7) | 9/107 (8.4) | 11/123 (8.9) | 0.9 | 0.1 |
| Pulmonary embolism, n (%) | 15/229 (6.6) | 7/107 (6.5) | 8/122 (6.6) | 1 | 0.3 |
| Age at 1st thrombo-embolic event (years) | 28.2 (24.1–32.7) | 29.2 (23.8–30.7) | 26.9 (24.4–35.1) | 0.7 | 0.2 |
| Chronic Complications | Whole Population n = 235 | Patients Born in France n = 111 | Patients Born in Sub-Saharan Africa n = 124 | pa | pb |
|---|---|---|---|---|---|
| Retinopathy, n (%) | 103/207 (49.8) | 51/101 (50.5) | 52/106 (49.1) | 0.8 | 0.4 |
| Age at diagnosis of retinopathy (years) | 24.7 (20.3–30.5) | 21.2 (16.7–25.7) | 28.9 (24.2–34.3) | <0.001 | 0.05 |
| Laser photocoagulation, n (%) | 52/185 (28.1) | 25/90 (27.8) | 27/95 (28.4) | 0.9 | 0.1 |
| Cardiac involvement, n (%) | 67/177 (37.9) | 36/85 (42.4) | 31/92 (33.7) | 0.3 | 0.6 |
| Age at diagnosis of cardiopathy (years) | 26.3 (20.6–30.4) | 24.5 (20.5–28.7) | 27.7 (21.2–31) | 0.1 | 0.6 |
| LV systolic dysfunction, n (%) | 14/228 (6.1) | 8/107 (7.5) | 6/121 (5) | 0.5 | 0.1 |
| LV and/or LA dilatation, n (%) | 56/161 (34.8) | 31/82 (37.8) | 25/79 (31.6) | 0.4 | 0.7 |
| Age at diagnosis of LV dilatation (years) | 25.2 (20.4–30.1) | 24.5 (18.7–28.7) | 27.7 (21–30.8) | 0.09 | 0.4 |
| TRV ≥ 2,5 m/s, n (%) | 33/86 (38.4) | 14/41 (34.1) | 19/45 (42.2) | 0.4 | 0.9 |
| Cerebral vasculopathy, n (%) | 33/102 (32.3) | 13/52 (25) | 20/50 (40) | 0.1 | 0.2 |
| Brain aneurysms, n (%) | 14/98 (14.3) | 4/52 (7.7) | 10/46 (21.7) | 0.08 | 0.08 |
| Silent cerebral infarcts, n (%) | 10/85 (11.8) | 6/45 (13.3) | 4/40 (10) | 0.6 | 0.4 |
| Moyamoya, n (%) | 2/97 (2.1) | 1/54 (1.9) | 1/43 (2.3) | 0.9 | 0.9 |
| Vessel stenosis, n (%) | 7/98 (7.1) | 2/52 (3.8) | 5/46 (10.9) | 0.2 | 0.3 |
| Nephropathy, n (%) | 64/164 (39) | 31/84 (36.9) | 33/80 (41.3) | 0.6 | 0.4 |
| eGFR (mL/mn/1.73 m2) | 124 (109–134) | 127 (116–136) | 119 (99–131) | 0.02 | 0.1 |
| Hyperfiltration §, n (%) | 60/220 (27.3) | 30/105 (28.6) | 30/115 (26.1) | 0.8 | 0.5 |
| Chronic kidney insufficiency, n (%) | 7/220 (3.2) | 0/105 (0) | 7/108 (6.1) | 0.01 | 1 |
| ACR > 3 mg/mmol, n (%) | 53/144 (36.8) | 27/73 (37) | 26/71 (36.6) | 0.9 | 0.9 |
| ACR (mg/mmol) | 1.8 (0.8–6.8) | 1.5 (0.7–4.8) | 2 (0.8–10) | 0.5 | 0.4 |
| Bone complications | |||||
| Avascular osteonecrosis (AON), n (%) | 70/217 (32.3) | 33/105 (31.4) | 37/112 (33) | 0.8 | 0.7 |
| Age at diagnosis of AON (years) | 19.8 (16.7–28.1) | 18.9 (16.5–25.7) | 21.2 (16.9–31.1) | 0.06 | 0.4 |
| H-shaped vertebrae, n (%) | 47/88 (53.4) | 20/42 (47.6) | 27/46 (58.7) | 0.3 | 0.2 |
| Fracture, n (%) | 57/181 (31.5) | 32/85 (37.6) | 25/96 (26) | 0.09 | 0.1 |
| Osteomyelitis, n (%) | 45/214 (21) | 25/96 (26) | 20/118 (16.9) | 0.1 | 0.06 |
| Age at 1st osteomyelitis episode (years) | 10.8 (6.6–19.4) | 8.2 (2.8–19.9) | 12 (8–18.3) | 0.3 | 0.4 |
| Skin ulcers, n (%) | 24/188 (12.8) | 6/95 (6.3) | 18/93 (19.4) | 0.007 | 0.03 |
| Age at first episode (years) | 18.6 (16.4–26.8) | 23.2 (18.6–29.6) | 18.5 (13.7–26.7) | 0.3 | 0.01 |
| Treatments | Whole population n = 235 | Patients Born in France n = 111 | Patients Born in Sub-Saharan Africa n = 124 | pa | pb |
|---|---|---|---|---|---|
| Hydroxyurea | |||||
| Lifetime exposure, n (%) | 135 (57.4) | 70 (63.1) | 65 (52.4) | 0.1 | 0.2 |
| Current treatment, n (%) | 108 (46) | 55 (49.5) | 53 (42.7) | 0.3 | 0.8 |
| Age at introduction (years) | 19.6 (15.4–25.3) | 18.6 (14.4–23.5) | 22.3 (16.4–30) | 0.0007 | 0.002 |
| Cumulative lifetime dose (g) | 1299 (485–2453) | 1299 (540–2748) | 1306 (451–1954) | 0.6 | 0.4 |
| Cumulative lifetime duration (years) | 4 (1.4–8) | 3.8 (1.7–8.2) | 4.1 (1.4–8) | 0.7 | 0.002 |
| Blood transfusions | |||||
| ≥ 1 transfusion over lifetime, n (%) | 190/216 (88) | 94/104 (90.4) | 96/112 (85.7) | 0.3 | 0.9 |
| ≥ 1 transfusion in Africa, n (%) | 20/54 (37) | 1/13 (7.7) | 19/41 (46.3) | 0.02 | 0.01 |
| Chronic blood transfusion program | |||||
| Previous, n (%) | 63/222 (28.4) | 29/106 (27.4) | 34/116 (29.3%) | 0.7 | 0.9 |
| Current, n (%) | 29/227 (12.8) | 17/111 (15.3%) | 12/116 (10.3%) | 0.3 | 0.3 |
| Blood transfusion complications, n (%) | 44/195 (22.6) | 24/96 (25) | 20/99 (20.2) | 0.4 | 0.3 |
| Types of transfusion complications * | 0.9 | 0.3 | |||
| Antibodies without hemolytic reaction, n (%) | 33/43 (76.7) | 18/24 (75) | 15/19 (78.9) | ||
| DHTR with antibodies, n (%) | 6/43 (14) | 4/24 (16.7) | 2/19 (10.5) | ||
| DHTR without antibodies, n (%) | 2/43 (4.7) | 1/24 (4.2) | 1/19 (5.3) | ||
| Acute hemolytic transfusion reaction, n (%) | 2/43 (4.7) | 1/24 (4.2) | 1/19 (5.3) | ||
| Proven hemochromatosis (liver biopsy or MRI), n (%) | 28/212 (13.2) | 13/104 (12.5) | 15/108 (13.9) | 0.8 | 0.4 |
| Ferritin (μg/L) | 109 (40–371) | 99.0 (44–288) | 116.5 (39.5–432.5) | 0.4 | 0.3 |
| Age at diagnosis of hemochromatosis (years) | 18.8 (16.2–28.3) | 16.9 (13.8–20.4) | 25.3 (18.3–31.2) | 0.03 | 0.006 |
| Morphine | |||||
| Pruritus to intravenous morphine, n (%) | 40/126 (31.7) | 22/67 (32.8) | 18/59 (30.5) | 0.8 | 0.6 |
| Chronic dependence on opioids, n (%) | 22/150 (14.7) | 14/79 (17.7) | 8/71 (11.3) | 0.3 | 0.1 |
| Infection Status | Whole Population n = 235 | Patients Born in France n = 111 | Patients Born in Sub-Saharan Africa n = 124 | pa | pb |
|---|---|---|---|---|---|
| Active or resolved HCV infection, n (%) | 16/174 (9.2) | 3/84 (3.6) | 13/90 (14.4) | 0.02 | 0.1 |
| Active or resolved HBV infection, n (%) | 21/179 (11.7) | 1/85 (1.2) | 20/94 (21.3) | <0.0001 | 0.004 |
| Positive HIV serology, n (%) | 3/174 (1.7) | 0/81 (0) | 3/93 (3.2) | 0.2 | 1 |
| Positive PVB19 serology, n (%) | 52/75 (69.3) | 30/42 (71.4) | 22/33 (66.7) | 0.7 | 1 |
| Positive HTLV1 serology, n (%) | 1/68 (1.5) | 0/31 (0) | 1/37 (2.7) | 1 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honsel, V.; Khimoud, D.; Ranque, B.; Offredo, L.; Joseph, L.; Pouchot, J.; Arlet, J.-B. Comparison between Adult Patients with Sickle Cell Disease of Sub-Saharan African Origin Born in Metropolitan France and in Sub-Saharan Africa. J. Clin. Med. 2019, 8, 2173. https://doi.org/10.3390/jcm8122173
Honsel V, Khimoud D, Ranque B, Offredo L, Joseph L, Pouchot J, Arlet J-B. Comparison between Adult Patients with Sickle Cell Disease of Sub-Saharan African Origin Born in Metropolitan France and in Sub-Saharan Africa. Journal of Clinical Medicine. 2019; 8(12):2173. https://doi.org/10.3390/jcm8122173
Chicago/Turabian StyleHonsel, Vasco, Djamal Khimoud, Brigitte Ranque, Lucile Offredo, Laure Joseph, Jacques Pouchot, and Jean-Benoît Arlet. 2019. "Comparison between Adult Patients with Sickle Cell Disease of Sub-Saharan African Origin Born in Metropolitan France and in Sub-Saharan Africa" Journal of Clinical Medicine 8, no. 12: 2173. https://doi.org/10.3390/jcm8122173
APA StyleHonsel, V., Khimoud, D., Ranque, B., Offredo, L., Joseph, L., Pouchot, J., & Arlet, J.-B. (2019). Comparison between Adult Patients with Sickle Cell Disease of Sub-Saharan African Origin Born in Metropolitan France and in Sub-Saharan Africa. Journal of Clinical Medicine, 8(12), 2173. https://doi.org/10.3390/jcm8122173




