1. Introduction
Pollen allergies and hay fever, typically manifesting as allergic rhinitis and allergic conjunctivitis, represent seasonal allergic reactions that can limit the ordinary lives of individuals [
1,
2,
3]. Patients with pollen allergies experience various nasal symptoms such as sneezing, runny nose, and stuffy nose, in addition to eye-related conditions such as redness, watery eyes, and itching [
1,
2,
3]. In Japan, the number of people with allergic responses to Japanese cedar pollen has been increasing over the last two decades [
4,
5,
6]. Additionally, some patients with pollen allergies can have adverse reactions to as many as 50 types of plants including cypress, rice, ragweed, and mugwort. The treatment of pollen allergy is generally limited to suppressing symptoms, with oral antihistamines, nasal drops, or eye drops typically being used as therapeutic strategies [
7,
8,
9]. Although desensitization therapy has been tried [
9,
10,
11], it has not been fully effective, and patients just wait for the pollen season to pass. Precautions can be taken to avoid or minimize exposure to pollen through decreasing outdoor activities, closing windows, operating central air conditioning systems with filter attachments, bathing and shampooing the body and hair well to remove attached pollens, and drying clothes well. Notwithstanding these precautions, patients with pollen allergies continue to suffer with symptoms, even in their own homes, during the pollen season [
4,
5,
6].
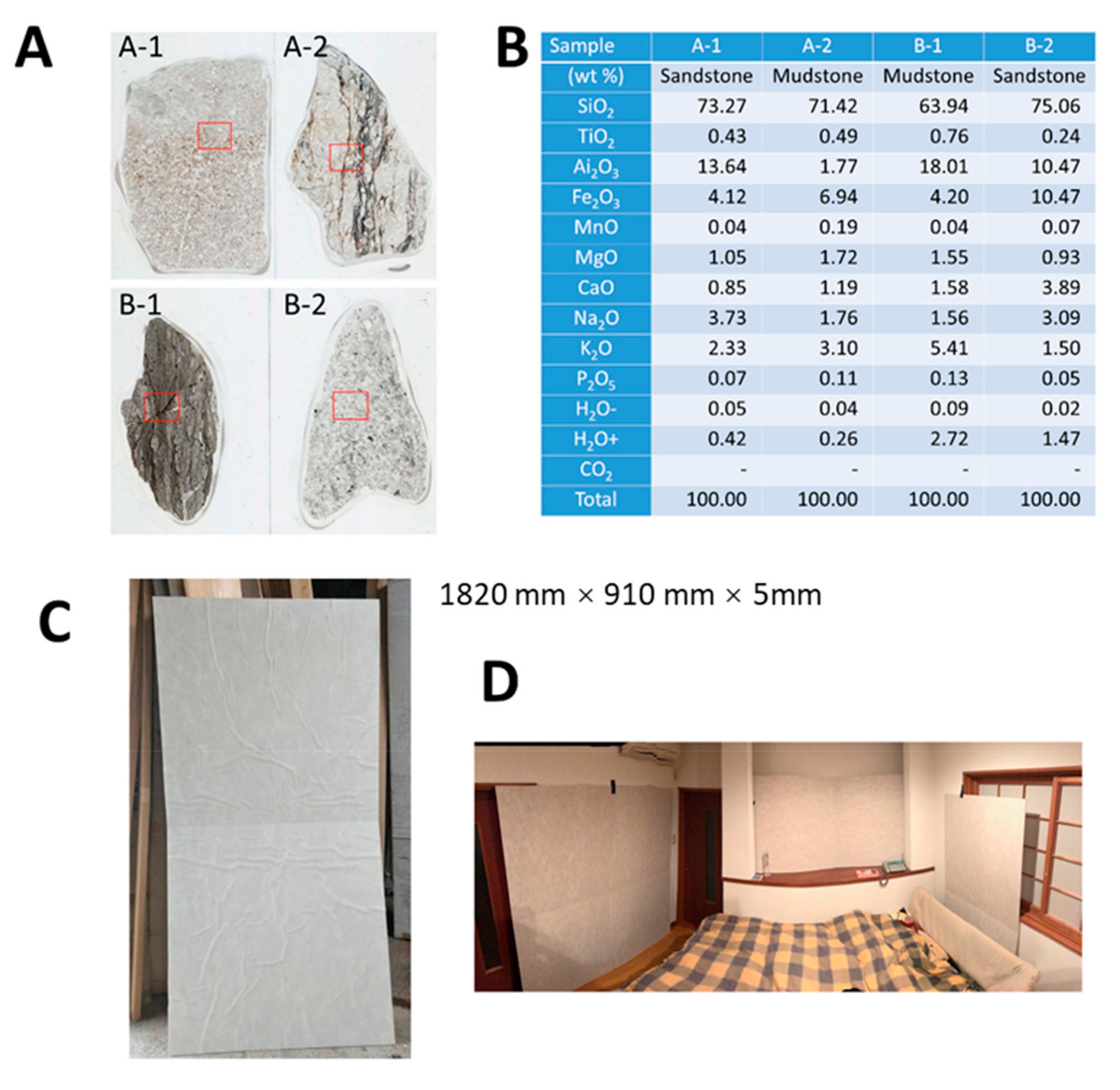
The Cosmic Garden Co. Ltd. is a custom-home building company located in Okayama City, Japan. Some of the features of their homes include a modified “2 × 4” construction method for aseismic ability and the avoidance of chemical substances and smells when possible, as well as the construction of well-sealed, super-insulated, durable homes. As we previously reported, this company uses specific natural ore powder within wall materials when constructing homes. This natural ore is obtained near Aso Mountain, Kumamoto Prefecture, Kyushu Island, Japan, and is known to release far-infrared rays. The appearance of this natural ore is shown in
Figure 1A and the chemical analyses of four main parts (shown in
Figure 1A) of this natural ore examined by an X-ray fluorescent (KrF) method are shown in
Figure 1B, as previously reported. The analyses revealed no remarkable characteristics. Additionally, the Cosmic Garden Co. Ltd. has incorporated this natural ore into the wall material of more than 200 homes. Home occupants have expressed improvements in allergic symptoms such as pollen allergy, bronchial asthma, and atopic dermatitis. Additionally, many occupants reported improved sleep quality. Moreover, the office space of this company includes this specific ore powder in the walls, and many customers have claimed immediate improvement of symptoms related to pollen allergy after entering this office space.
In our previous study [
12], we investigated the biological effects of cloth containing a specific natural ore powder (CCSNOP) on patients with pollen allergy in terms of symptoms, biological markers, mood state via a questionnaire referred to as Profile of Mood Status 2 (POMS2), stress marker using salivary amylase (sAmy), serum immunoglobulin (Ig) E specific to 33 antigens, peripheral blood counts including percentage of white blood cells, and 29 serum cytokines under conditions when subjects were exposed to CCSNOP for 60 min. For the control, subjects were exposed to non-woven cloth (NWC). From this previous study, some symptoms such as nasal obstruction and lacrimation improved, and POMS2 evaluations showed that patients were calmer following their stay with CCSNOP. However, relative eosinophil percentage, non-specific Ig E, epidermal growth factor (EGF), monocyte chemotactic protein-1 (MCP-1) and tumor necrosis factor-α levels were higher in subjects exposed to CCSNOP compared with NWC. However, in that previous study, it was not confirmed whether exposure to CCSNOP did indeed improve symptoms of pollen allergy, or whether the indoor air circumstances using CCSNOP affected the human immune system [
12].
The present study was executed after Wadakohsan Corp. (a real estate business) began selling detached homes containing CCSNOP provided by Cosmic Garden Co. Ltd. In this study, CCSNOP or NWC (
Figure 1C) panels (of indistinguishable appearance) were placed in the sleeping rooms of subjects (
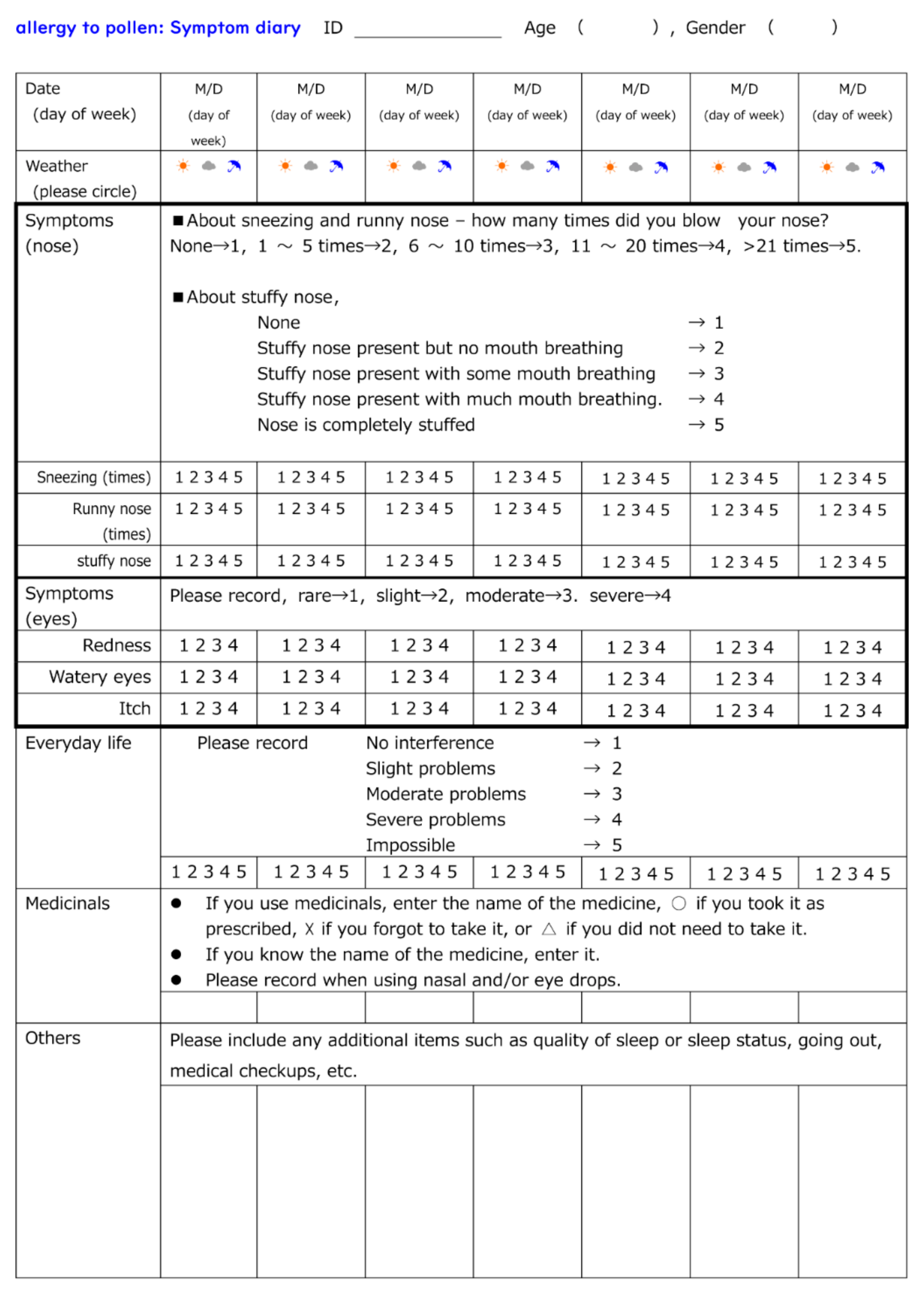
Figure 1D) with pollen allergy (self-reported) for two weeks at least one or more weeks after the beginning of the Japanese cedar pollen dispersal season. Subjects were required to keep a “pollen allergy diary” that included details of symptoms, medication employed, and other comments (
Figure 2). Additionally, biological monitoring was performed one month prior to the anticipated pollen dispersal, immediately prior to and following panel placement, and two months after Japanese cedar pollen dispersal had ceased.
2. Experimental Section
2.1. Subjects
All 31 subjects were Japanese. Subjects were recruited following public announcement of the intended study. The subjects comprised 14 males (average age (AA) ± standard deviations (SD): 45.1 ± 14.1 years old) and 17 females (37.1 ± 11.0 years old), with the AA of the 31 subjects being 41.2 ± 12.8 years old. Eight subjects were living near Kobe City, Hyogo Prefecture, Japan. For them, the POMS2 questionnaire, sAmy sampling, and venous blood collection were done within rooms of Wadakohsan Corp. in Kobe City (Kobe venue). The other 23 subjects were living in Okayama Prefecture. Thus, sampling was performed in rooms at Okayama University in Okayama City, and rooms at Kawasaki Medical School in Kurashiki City, with both cities being in Okayama Prefecture (ca. 10 km apart; Okayama venues). The cities of Kobe, Okayama, and Kurashiki are in the western region of Japan and are ca. 120 km apart. However, dispersal of Japanese cedar pollens does not differ markedly between these regions according to weather forecast data and the Japanese Ministry of the Environment. The Kobe venue included eight subjects (Male:Female = 4:4), while the Okayama venues included 23 subjects (M:F = 10:13). All subjects self-reported pollen allergy to Japanese cedar with various typical symptoms. Subjects with well-controlled lifestyles possessing diseases such as hypertension, diabetes mellitus, respiratory diseases, and liver and kidney diseases were included in the study; however, patients with cancers, collagen diseases, and other severe diseases were omitted from the study. All subjects continued with their regular daily lives during the study without modification.
The study was designed as a double-blind study. Thus, all researchers measuring POMS2, sAmy, and blood sampling were unaware of which panel (CCSNOP or NWC) each subject was being exposed to. Following the final sampling in June, 2018, the distribution of subjects within the CCSNOP and NWC groups was revealed. The Kobe venue had 2 males and 2 females with CCSNOP and 2 males and 1 female with NWC. The Okayama venues included 6 males and 10 females with CCSNOP and 4 males and 3 females with NWC. In total, 8 males and 13 females (21 subjects) comprised the CCSONOP group and 6 males and 4 females (10 subjects) comprised the NWC group. CCSNOP and NWC groups differed since we designed this study not only to compare the two groups but also to analyze changes in biological markers among subjects of the CCSNOP group.
2.2. Ethical Matters
This study was approved by the Kawasaki Medical School Ethics Committee (Issue No. 2576, date of approval December, 12th, 2016). All subjects of this study were approached verbally and in writing, and those from whom written consent was obtained were recruited as subjects in this study. All methods used in this study were performed in accordance with the relevant guidelines and regulations shown by Kawasaki Medical School Ethics Committee as well as the Declaration of Helsinki. Additionally, the monitoring and audit for this study were performed by the Academic Research Organization (ARO), Center for Innovative Clinical Medicine, Okayama University, since favorable results may be used in the advertising of Cosmic Garden Co. Ltd. and Wadakohsan Corp. There were no problems with the monitoring and auditing of this study.
2.3. Study Design
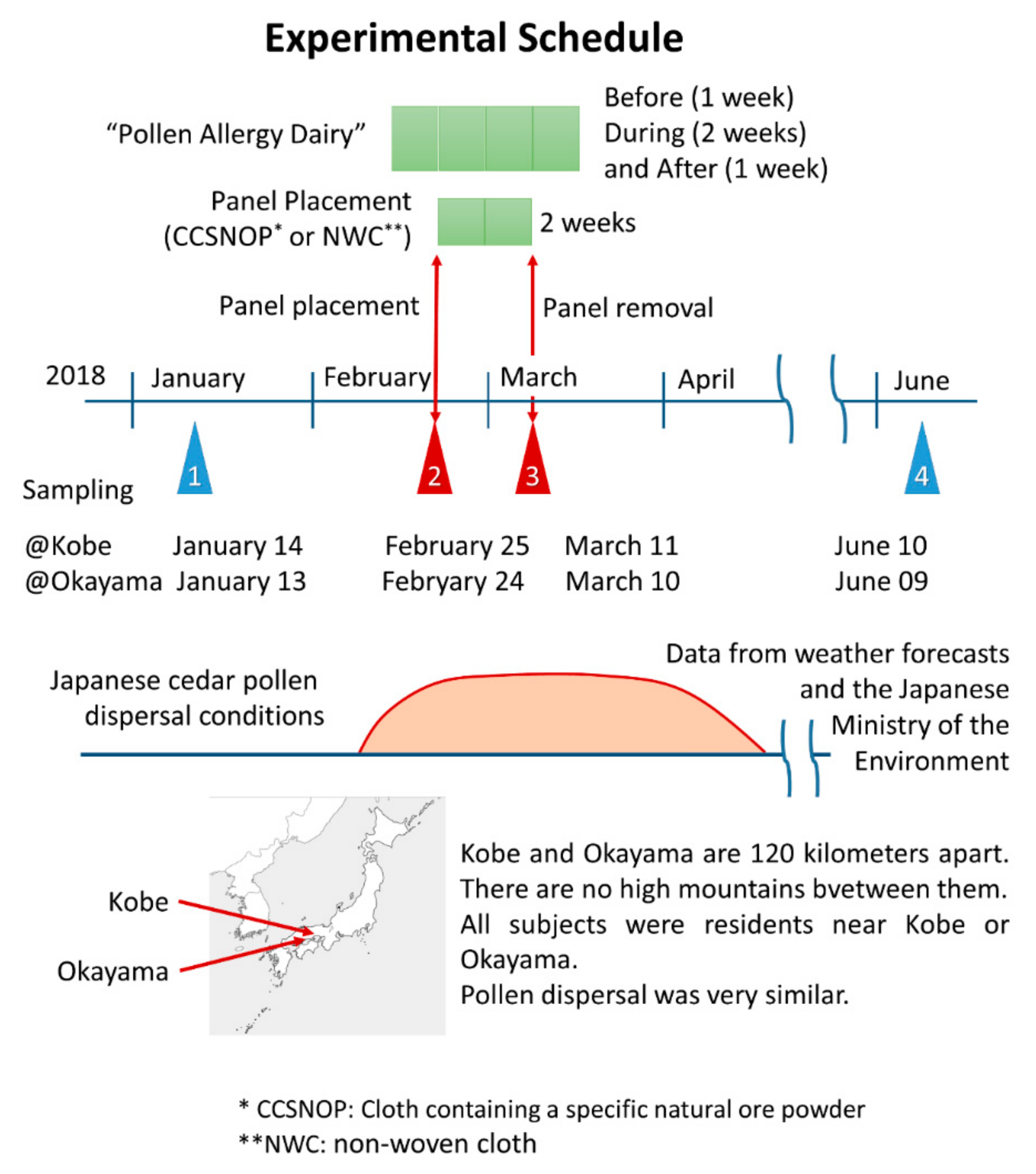
As shown in
Figure 3, the study was performed from January to June, 2018. The samplings (POMS2, aAmy, and blood collection) were performed four times. The first was early January, one month before pollen dispersal. The second was at the end of February, one or more weeks after the commencement of dispersal. The third was two weeks after the second time. During these two weeks each subject was exposed to CCSNOP or NWC panels that had been placed in their bedroom (
Figure 1D). The last sampling was performed in early June, almost two months following the cessation of pollen dispersal. All dates are listed in
Figure 3. One week prior to panel placement and during the 2-week period of panel exposure, all subjects kept a “pollen allergy diary” as shown in
Figure 3.
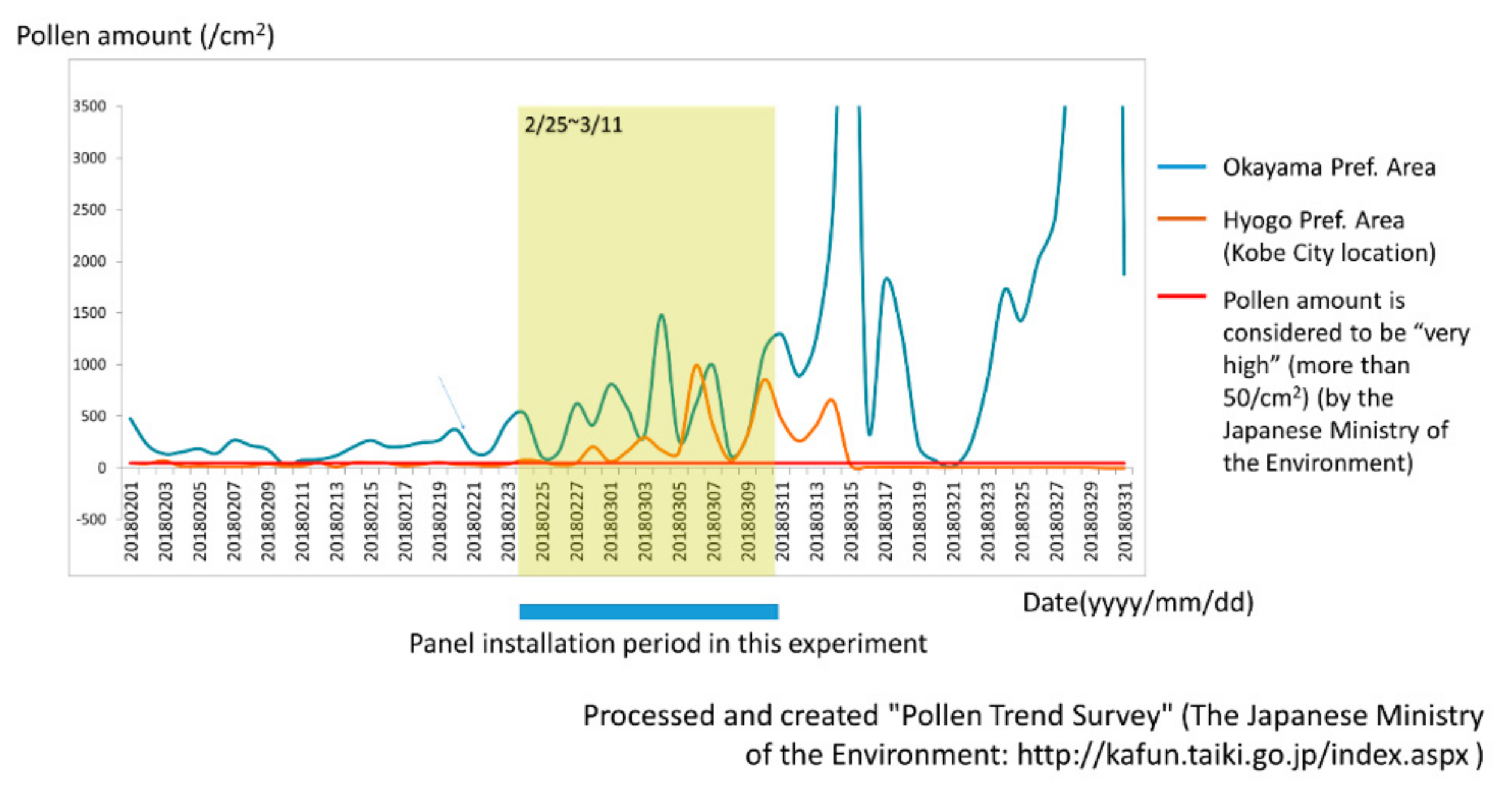
The degree of Japanese cedar pollen dispersal was similar in the Kobe and Okayama areas according to weather forecast data and the Japanese Ministry of the Environment. As shown in
Figure 4, the amount of pollen during the period of CCSNOP or control panel installation did not differ significantly in either Okayama City (Okayama Prefecture) or Kobe City (Hyogo Prefecture) where the subjects lived. However, it greatly exceeded the 50/cm
2 that the Ministry of the Environment of Japan stated as representing a “very high” pollen level.
2.4. Pollen Allergy Diary
As shown in
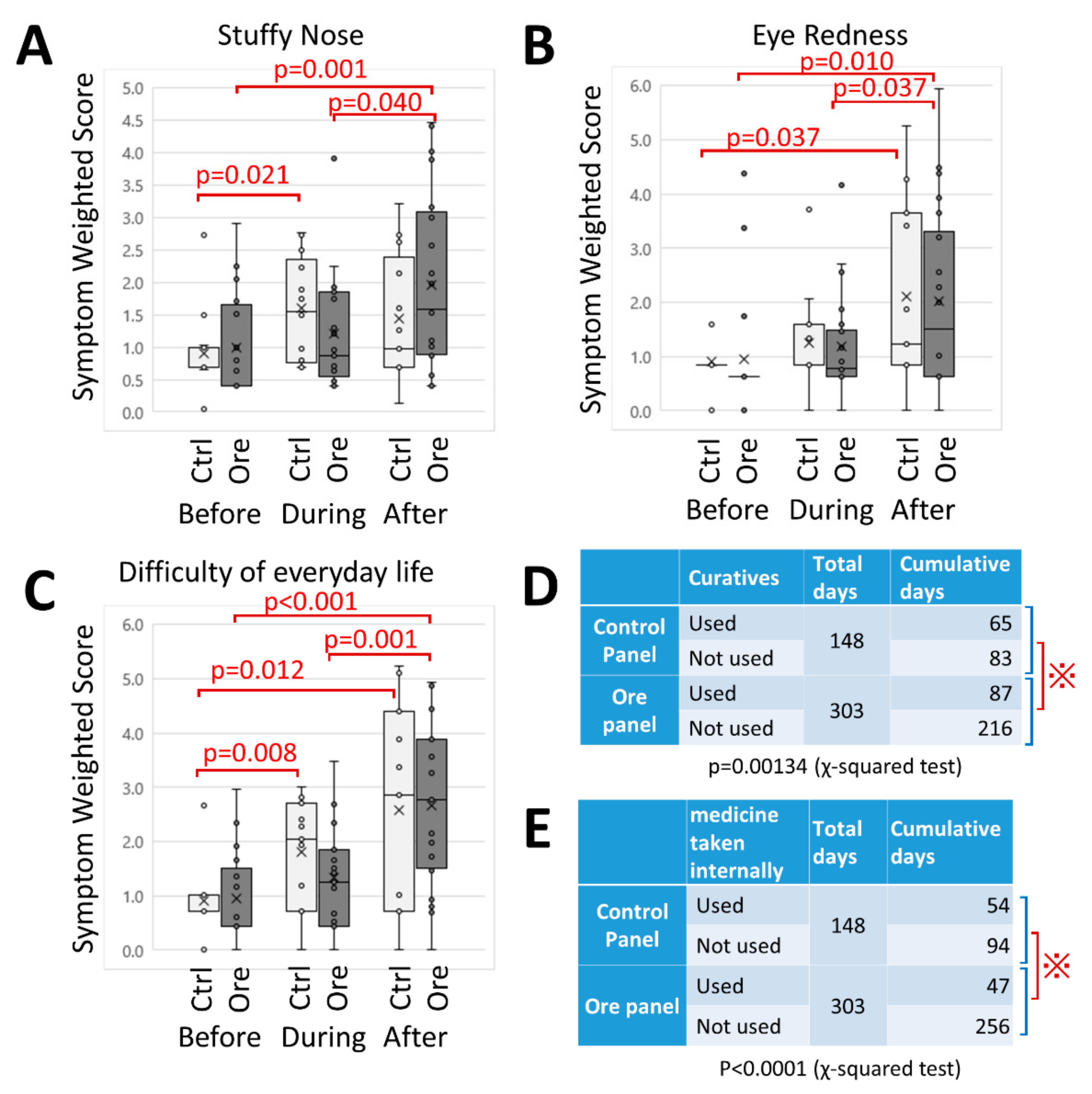
Figure 3, all subjects kept a “pollen allergy diary” for 4 weeks. An ID number was assigned to subjects by the personal information manager, and was unknown to researchers. Diary entries contained the date, day of the week, and an assessment of the weather. Subjects also recorded any symptoms related to the nose or eye on a scale of 1 (none) to 5 (most severe). Furthermore, problems of daily life caused by pollen allergy was also ranked as 1 (none) to 5 (incomplete). Moreover, the use of medication (oral drugs, nasal drops and eye drops) was described. Other matters related to pollen allergy such as physical and mental condition were recorded in the “Others” category. To analyze symptoms “Before”, “During”, and “After” periods of panel exposure, as well as to compare CCSNOP and NWC groups, recorded scores of 1 to 5 were squared to clarify the differences (symptom-weighted score).
2.5. Survey of Mood (POMS2)
Investigation of mood was examined using POMS2 [
14,
15]. Although POMS2 is a descriptive questionnaire that conventionally determines the state of mood for the previous two weeks or so, in our study, subjects were asked to assess their mood state for that present moment.
The questionnaire comprised 30 questions, with answers that ranged in five stages from “not at all” to “very much”. The “feeling condition” was measured according to the following six scales:
- T-A:
Tension-Anxiety (Tension-Anxiety), “Feeling tight/under tension” comprised five stages. The higher the score, the more nervous a subject feels.
- D:
Depression (Depression), comprised five stages such as “feeling down or dark.” Higher scores indicate increased loss of confidence.
- A-H:
Five stages such as Anger-Hostility and “Bad Mood”. A higher score indicates increased anger.
- F:
Comprised five stages such as “Fatigue” and “I get tired”. A higher score indicates an increased feeling of being tired.
- C:
Confusion, five items such as “Confusion”. A higher score indicates increased confusion and decreased lucidity.
- V:
Vigor (Vigor), five items such as “Vivid”. As this item is a positive item, unlike the other five scales, a lower score indicates that the activity is lost.
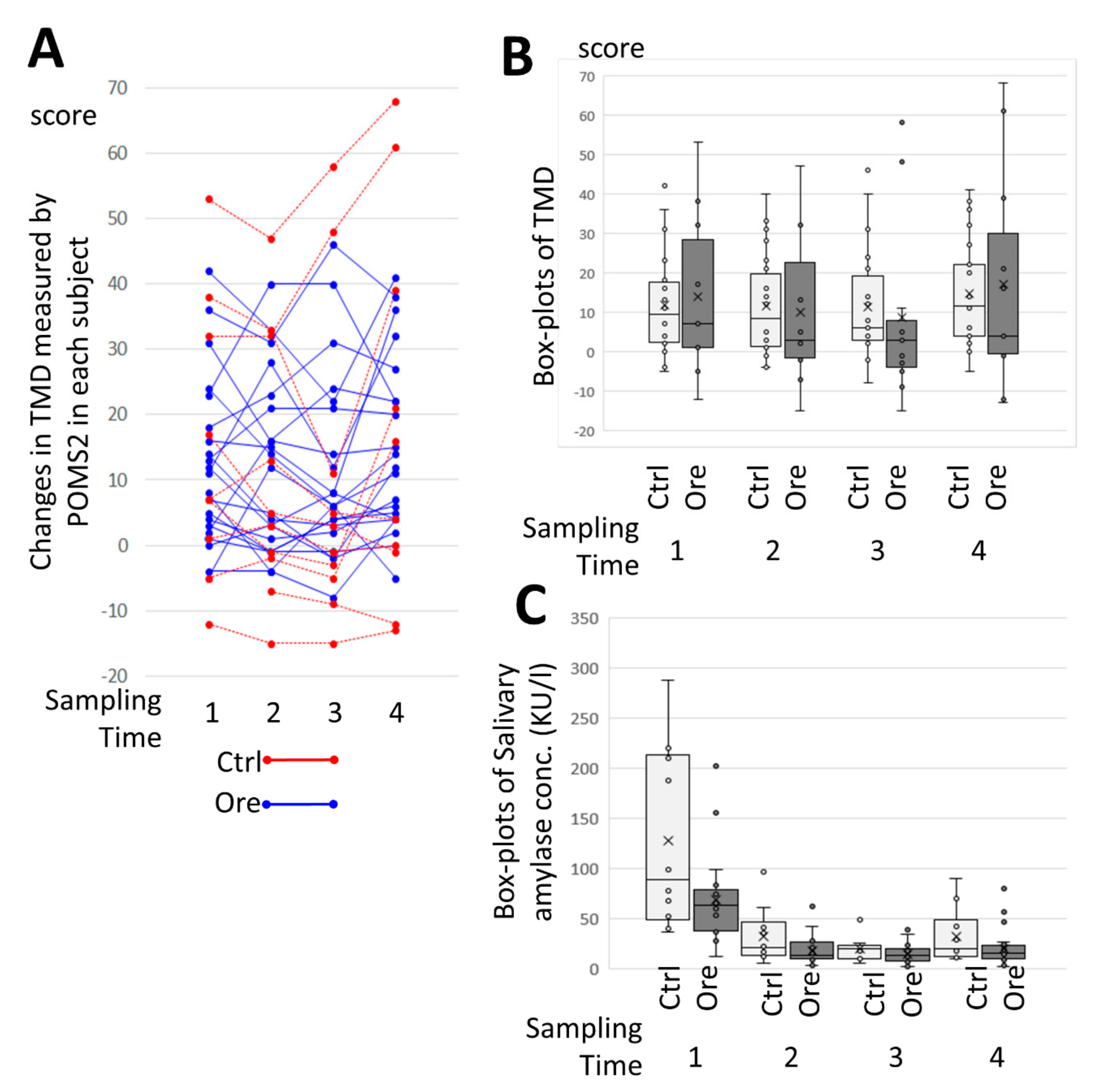
Total Mood Disturbance (TMD) was also calculated. This is a comprehensive expression of negative mood state (formula for calculation shown below). The higher the main score, the more negative the mood; it was determined as follows:
2.6. Saliva Amylase
Subjects first rinsed their mouths before sampling, completed the POMS2 questionnaire, and then sAmy measurements were undertaken [
16,
17]. After placement of the dedicated stick (Nipro saliva amylase monitor chip Product Code 59-010) under the tongue for 30 seconds, the concentration of amylase was measured using a Salivary Amylase Monitor® (Nipro Corporation, Osaka, Japan) according to the manufacturer’s instructions. The results were recorded on a special form.
2.7. Blood Sampling
Fifteen milliliters of peripheral venous blood was taken from subjects and the following parameters were measured:
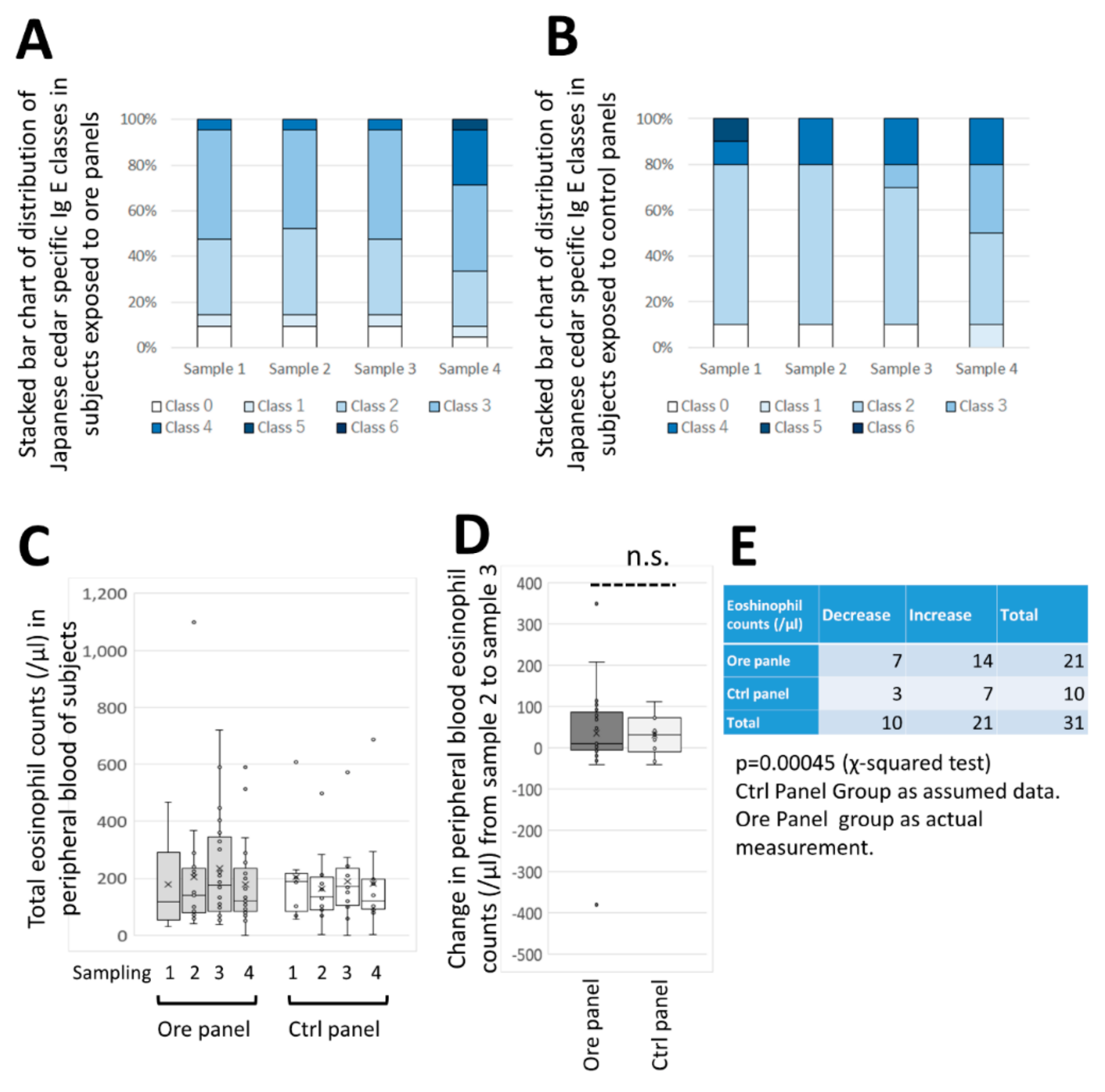
General condition: blood chemistry including liver (AST, ALT, and γGT) and kidney (BUN and creatinine) functions, blood sugar, HbA1c and lipids (including LDL and HDL cholesterols and triglyceride), and peripheral blood counts including white blood cell fraction (granulocytes, eosinophil, basophil, monocytes, lymphocytes, and others) were recorded.
Immunoglobulins (Igs): Ig G, A, M, and non-specific Ig E, as well as Ig G4 since Ig G4 is related to food allergy. More recently, Ig G4 has been considered to be associated with IG G4-related diseases, which are systemic diseases characterized by elevated serum IgG4, IgG4-positive plasma cell infiltration into the affected tissue, and fibrosis [
18,
19,
20].
Multi-antigen specific Ig E (33 types): This measurement kit typically included antigens for cedar, cypress, house dust, dermatophagoides pteronyssinus, and others. Measurements 1 to 3 above were performed in a clinical laboratory testing company (BML Inc., Shibuya-ku, Tokyo, Japan).
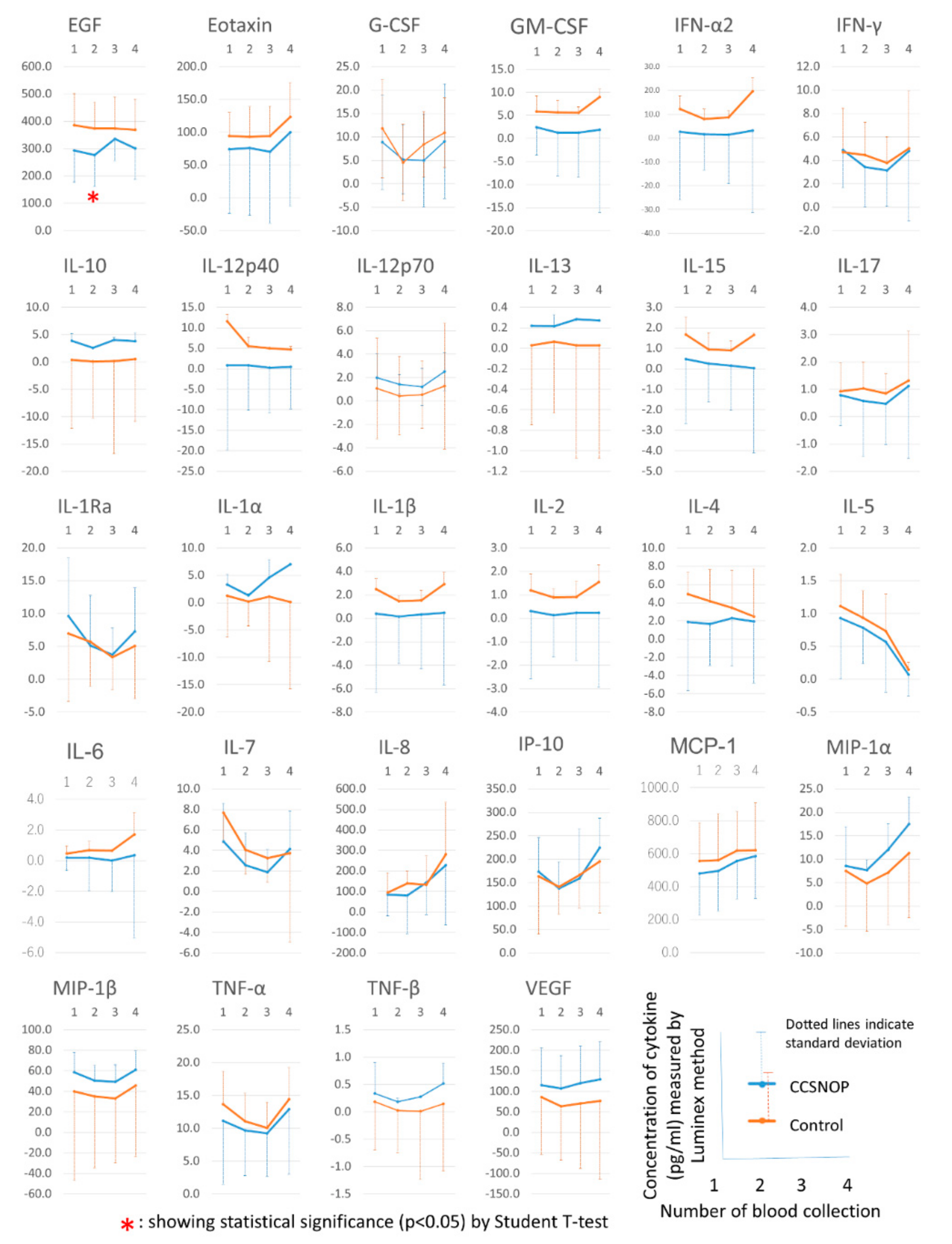
Cytokines: Twenty-nine types of cytokines were measured using a Luminex Cytokine 29-Plex Human Cytokine/Chemokine Panel (HCYTMAG-60K-PX29, Merck Millipore, Billerica, MA). The cytokines included in this kit were EGF, eotaxin, granulocyte-colony stimulating factor (G-CSF), monocyte/macrophage-CSF (M-CSF), interferon (INF)-α2, IFN-γ, interleukin (IL)-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IL-1 receptor antagonist (IL = 1ra), IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, interferon γ-induced protein 10 (IP-10, also known as C-X-C motif chemokine 10 (CXCL10)), MCP-1, macrophage inflammatory protein ((MIP)-1α, also known as chemokine (C-C motif) ligand 3 (CCL3)), MIP-1β (also known as CCL4), tumor necrosis factor (TNF)-α, TNF-β, and vascular endothelial growth factor (VEGF). Some cytokines showed less than the measurement limitation. In these cases, for convenience, a value of one tenth of the lower limit of the measurement was substituted and utilized for statistical analyses [
12,
21,
22].
2.8. Statistical Analyses
Statistical analyses were performed using SPSS version 22 (IBM, Chicago, IL, USA) or Microsoft Office Excel 2013 (Microsoft Japan, Tokyo, Japan). A significant difference was shown if the degree of risk was less than 5% (p < 0.05). To compare the symptom-weighted scores of CCSNOP (ore) and NWC (control; ctrl), POMS2 score analyses, sAmy concentrations, and absolute eosinophil counts, and for a comparison of values of the panel-prediction formula in subjects with CCSNOP and NWC, two-way analysis of variance (two-way ANOVA) and Student’s t-test (bilateral) were applied. Additionally, the use of allergy-related medicines by subjects and changes in absolute eosinophil counts were assessed by a χ-squared test. Regarding generation of the formula used to predict subjects who were exposed to CCSNOP or NWC, multiple regression analysis was performed. After obtaining the formula, a receiver operating characteristic (ROC) curve was also generated.
4. Discussion
Pollen allergies continue to be an issue for those affected in contemporary societies [
1,
2,
3]. Although various strategies have been employed to deal with this medical issue, such as desensitization therapy [
9,
10,
11], many patients simply endure by taking medicines that help to relieve the symptoms until the end of the pollen dispersal season. Most of the drugs only provide symptomatic relief, and in the end, the only recourse available to many patients is to try and avoid exposure [
4,
5,
6].
We investigated whether the residential environment could induce some kind of pollen allergy relief. Cosmic Garden Co., Ltd. has been selling detached houses in which powder derived from ore collected near Aso Mountain, Kumamoto Prefecture, Kyushu Island, Japan, is mixed with interior wall materials. Anecdotal evidence suggested that home occupants experienced relief of symptoms related to pollen allergy. Therefore, in our previous study, we investigated the effects of mineral-containing (CCSNOP) or control (NWC) panels on pollen allergy patients every two weeks for one hour. The severity of symptoms, changes in eosinophils, cytokines, mood (measured by POMS2), stress markers measured by sAmy, and blood samples were investigated [
12]. It was found that the severity of symptoms improved with CCSNOP. Furthermore, eosinophils increased slightly but significantly in the CCSNOP group [
12]. Although changes in certain cytokine levels differed between the CSNOP and NWC groups, the biological significance of this finding remains to be determined [
12]. Since it seemed that CCSNOP had a demonstrably positive effect on pollen allergy patients, it was thought that an investigation comprising longer-term exposure to CCSNOP, including during the pollen dispersal season, would be instructive.
Therefore, in the present study, we decided to examine various biological indicators along with symptoms in subjects with Japanese cedar pollen allergy who were exposed to CCSNOP or NWC panels in their bedroom.
The results of this study showed that CCSNOP alleviated the symptoms of pollen allergy and that the use of medicines decreased in the CCSNOP group. Inspection of the “pollen allergy diaries” revealed that even difficulties of daily life were reduced in the CCSNOP group, although the TMD (measured by POMS2) and the degree of stress (measured by sAmy) showed no differences between the CCSNOP and NWC groups.
However, blood sample analyses indicated that the absolute number of eosinophils tended to be lower in the CCSNOP group compared with the NWC group during the pollen dispersal season.
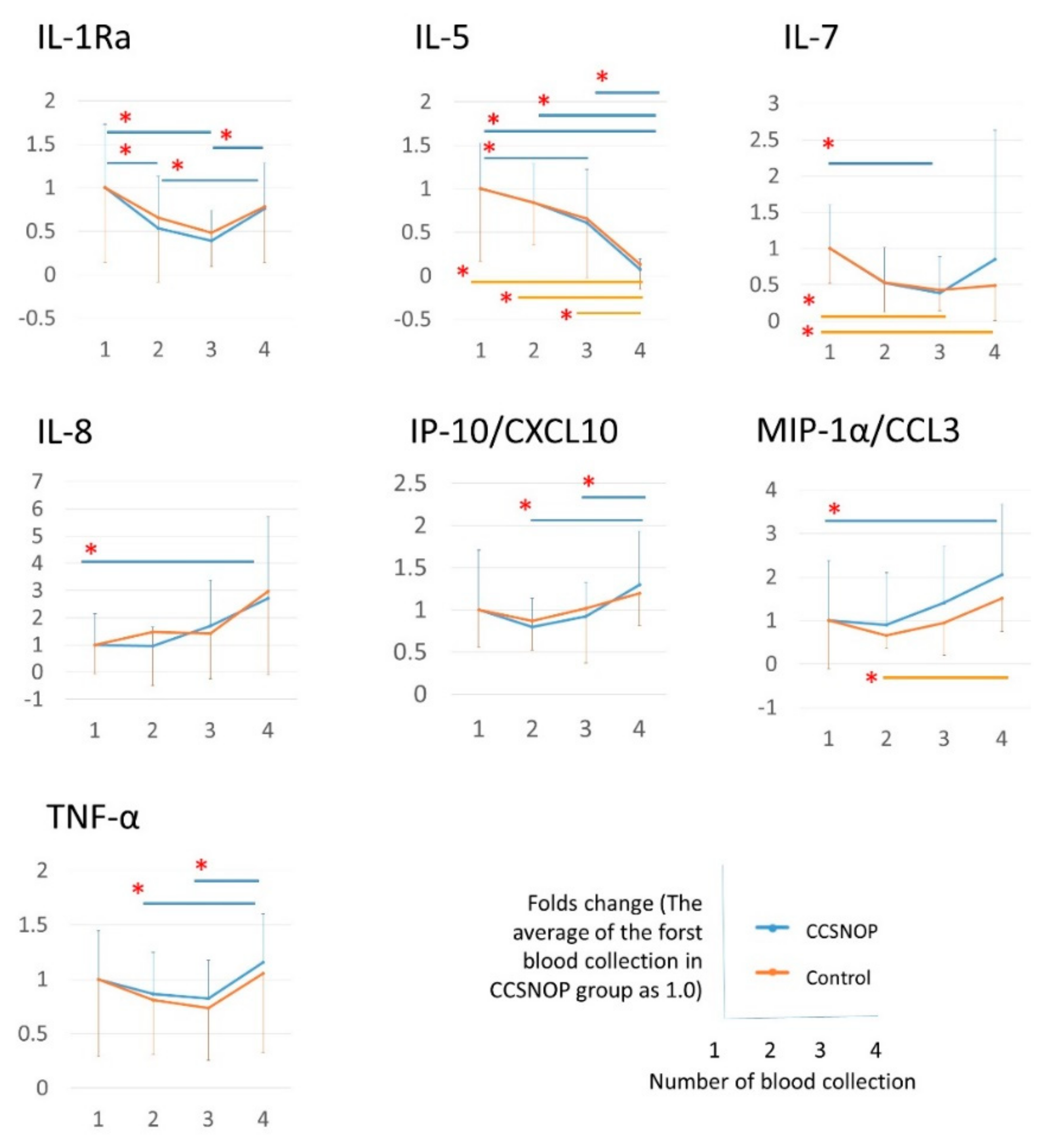
Cytokines, especially MCP1, IP10, CXCL10/IP-10, CCL4, and CCL3, which are related to the onset of hay fever and the mechanism of symptom appearance, are shown in
Figure 8. CCSNOP and control groups did not show significant changes. As discussed below, a formula was established to extract and detect subjects belonging to the CCSNOP group on the basis of these cytokines. These results suggest that this mineral powder panel may not have a direct effect on the pathogenesis of hay fever allergies, but may have an effect on secondary parts such as the appearance of symptoms.
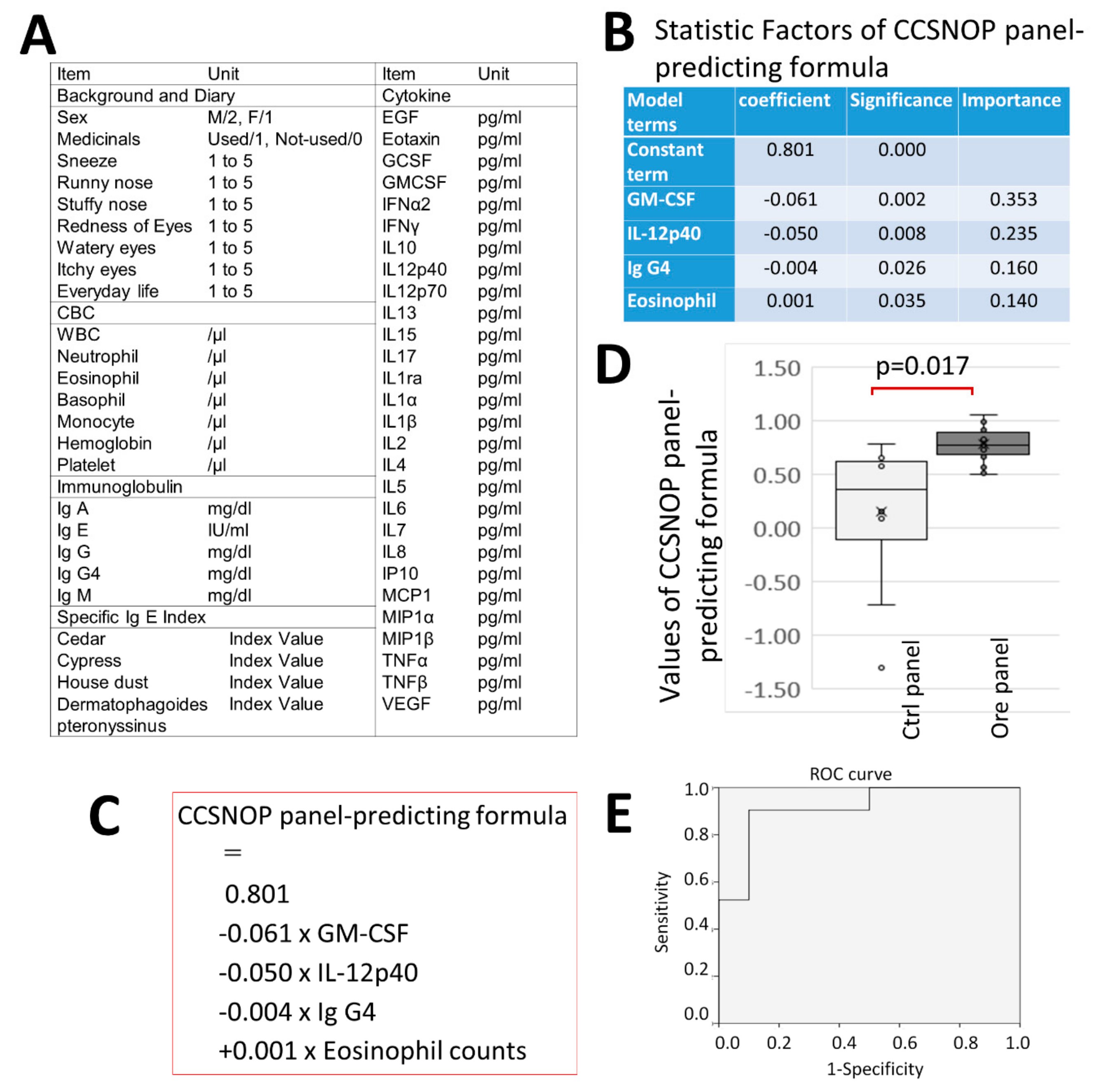
Furthermore, when many cytokines were measured and a formula for detecting CCSNOP subjects was generated along with other indicators, GM-SCF and IL-12p40 were extracted along with eosinophil count and Ig G4 values. These two cytokines are of greater importance. Additionally, when using this formula, it was revealed that blood sampling detected a significant difference in subjects exposed to CCSNOP panels for two weeks.
GM-CSF is known as a cytokine that induces differentiation of pluripotent hematopoietic stem cells into granulocyte-monocyte cells [
23,
24]. In relation to pollen allergy and GM-SCF, this cytokine has been reported to increase with IL-33 and IL-25 in animal models, and following the activation of neutrophils and antigen-specific T cells [
25]. Additionally, one report has shown that IL-33 is activated by GM-CSF and IL-8 in the nasal mucosal epithelium of allergic rhinitis [
26]. Moreover, some reports have indicated that production of GM-CSF was activated in patients with seasonal allergic rhinitis (possibly caused by pollen) [
27].
Considering these reports and the prediction formula generated in this study, GM-CSF was extracted with a negative coefficient in the prediction formula, and could be utilized to detect a person staying under CCSNOP conditions. Therefore, CCSNOP may act on subjects by improving the pathophysiology of pollen allergy even at the cytokine level.
IL-12p40, along with IL-12p70, is a component of IL-12. IL-12 is well-known as a cytokine responsible for induction into Th1 cells together with IFN-γ [
28,
29]. Therefore, since a state with high IL-12 induces differentiation to Th1 rather than Th2, it might be that patients with pollen allergy possess reduced IL-12 production. However, IL-12p40 was also extracted with a negative coefficient in our formula. Although interpretation of this finding seems difficult, it may be that subjects exposed to CCSNOP experience decreasing levels of IL-12 over the 2-week period of stay, since they showed a reduction in the severity of symptoms during that period.
The formula generated in this study also indicated that IgG4 was extracted with a negative coefficient, even though the importance was less than that of the cytokines. Recently, IgG4 has been implicated to play a role in various diseases that have shown elevated serum IgG4, IgG4-positive plasma cell infiltration into affected tissue, and fibrosis [
18,
19,
20]. However, IgG4 was initially considered in relation to the allergic condition. Production of IgG4 is induced by IL4 and IL-13, which are Th2-type cytokines mainly involved in allergic reactions under antigen stimulation. Thus, the negative coefficient of IgG4 in the generated formula may indicate a reduction in allergic reactions in subjects exposed to CCSNOP conditions.
In our previous observational study, we were able to confirm that the symptoms of hay fever improved in the 1-hour CCSNOP environment, but that was a unique environment of only 1 hour. While the subjects were living in a normal period, the effect was observed by setting up a CCSNOP environment in the bedroom at home, and as a result, symptoms were alleviated and medication was avoided. In addition, although there was no direct effect on individual cytokines allegedly associated with allergies, it is almost certain that it has an effect and that it alleviates allergic symptoms, and the mechanism is almost unknown at present, probably due to the decrease in pollen itself in the air. Whether the changes in the immune system that are shown in the current study are direct effects of the of CCSNOP or whether relaxation resulted in changes in such symptoms is, at present, unknown.
There were no adverse effects in any of the subjects exposed to CCSNOP conditions in this study. Additionally, Cosmic Garden Co. Ltd. has sold more than 200 homes with powder or with this specific ore and no adverse effects have been reported.
Originally, it would be best if the effects of CCSNOP could be verified using animal models. However, it is very difficult to create a situation where the animal model is exposed to the environment by CCSNOP or NWC while building and using an animal model of hay fever in our laboratory. In future, it will be necessary to observe such a situation through some joint research. However, occupants of CCSNOP-containing detached homes sold by Cosmic Garden Co., Ltd. have already provided anecdotal reports of hay fever symptom relief. Consequently, an impact following the use of this specific ore could be expected.
The limitation of this study was that it comprised a small number of subjects and all results were extracted by comparing the CCSNOP group with the NWC group. The changes over time in this group did not yield a very definitive finding. However, we believe that exposure to CCSNOP reduced allergy-related symptoms in addition to affecting certain biological reactions in pollen allergy patients. Long-term monitoring of individuals living in homes containing this ore powder as part of the inner wall material may yield more information regarding the effects of CCSNOP on patients with pollen allergies.