Biomarkers of Vascular Injury and Type 2 Diabetes: A Prospective Study, Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population (Original Research)
2.2. Ascertainment of Incident Diabetes Mellitus (Original Research)
2.3. Laboratory Methods (Original Research)
2.4. Covariates Assessment
2.5. Systematic Review and Meta-Analysis
2.6. Statistical Analyses
3. Results
3.1. Biomarkers of Vascular Injury and Type 2 Diabetes Risk in the EPIC–Heidelberg
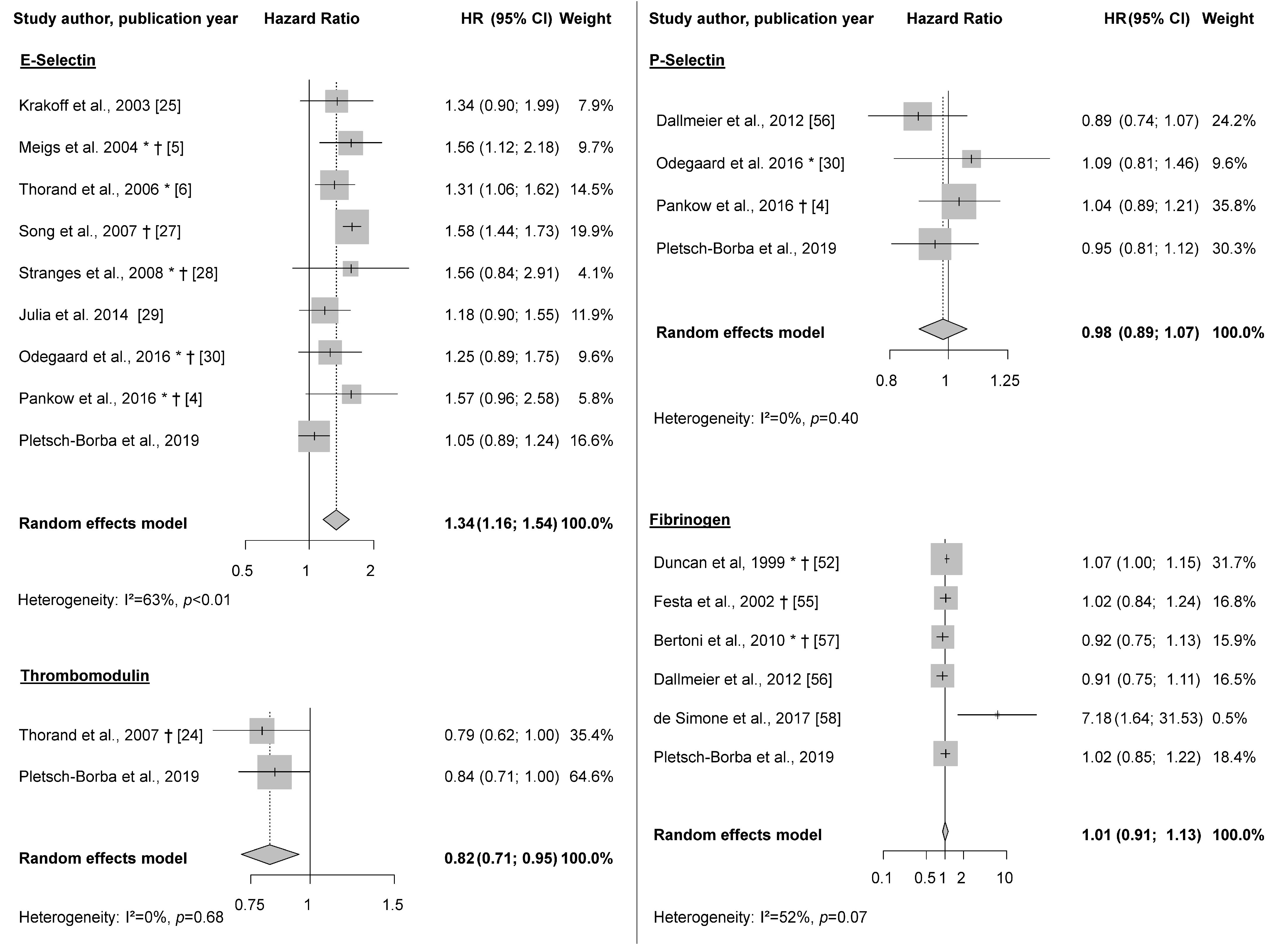
3.2. Systematic Review and Meta-Analysis
3.3. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Dalys, G.B.D.; Collaborators, H. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Muris, D.M.; Houben, A.J.; Schram, M.T.; Stehouwer, C.D. Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: A systematic review and meta-analysis. Arter. Thromb. Vasc. Biol. 2012, 32, 3082–3094. [Google Scholar] [CrossRef] [PubMed]
- Pankow, J.S.; Decker, P.A.; Berardi, C.; Hanson, N.Q.; Sale, M.; Tang, W.; Kanaya, A.M.; Larson, N.B.; Tsai, M.Y.; Wassel, C.L.; et al. Circulating cellular adhesion molecules and risk of diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabet. Med. A J. Br. Diabet. Assoc. 2016, 33, 985–991. [Google Scholar] [CrossRef]
- Meigs, J.B.; Hu, F.B.; Rifai, N.; Manson, J.E. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004, 291, 1978–1986. [Google Scholar] [CrossRef]
- Thorand, B.; Baumert, J.; Chambless, L.; Meisinger, C.; Kolb, H.; Doring, A.; Lowel, H.; Koenig, W.; Group, M.K.S. Elevated markers of endothelial dysfunction predict type 2 diabetes mellitus in middle-aged men and women from the general population. Arter. Thromb. Vasc. Biol. 2006, 26, 398–405. [Google Scholar] [CrossRef]
- Miles, P.D.; Levisetti, M.; Reichart, D.; Khoursheed, M.; Moossa, A.R.; Olefsky, J.M. Kinetics of insulin action in vivo. Identification of rate-limiting steps. Diabetes 1995, 44, 947–953. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelium and haemostasis. Hamostaseologie 2015, 35, 11–16. [Google Scholar] [CrossRef]
- Jones, D.A.; Abbassi, O.; McIntire, L.V.; McEver, R.P.; Smith, C.W. P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys. J. 1993, 65, 1560–1569. [Google Scholar] [CrossRef]
- Bonfanti, R.; Furie, B.C.; Furie, B.; Wagner, D.D. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood 1989, 73, 1109–1112. [Google Scholar] [CrossRef]
- Stenberg, P.E.; McEver, R.P.; Shuman, M.A.; Jacques, Y.V.; Bainton, D.F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J. Cell Biol. 1985, 101, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Campanero, M.R.; Sanchez-Mateos, P.; del Pozo, M.A.; Sanchez-Madrid, F. ICAM-3 regulates lymphocyte morphology and integrin-mediated T cell interaction with endothelial cell and extracellular matrix ligands. J. Cell Biol. 1994, 127, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Torr, E.E.; Gardner, D.H.; Thomas, L.; Goodall, D.M.; Bielemeier, A.; Willetts, R.; Griffiths, H.R.; Marshall, L.J.; Devitt, A. Apoptotic cell-derived ICAM-3 promotes both macrophage chemoattraction to and tethering of apoptotic cells. Cell Death Differ. 2012, 19, 671–679. [Google Scholar] [CrossRef]
- de Fougerolles, A.R.; Springer, T.A. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J. Exp. Med. 1992, 175, 185–190. [Google Scholar] [CrossRef]
- Esmon, C.T.; Fukudome, K.; Mather, T.; Bode, W.; Regan, L.M.; Stearns-Kurosawa, D.J.; Kurosawa, S. Inflammation, sepsis, and coagulation. Haematologica 1999, 84, 254–259. [Google Scholar]
- Gale, A.J. Continuing education course #2: Current understanding of hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar] [CrossRef]
- Hitchcock, I.S.; Kaushansky, K. Thrombopoietin from beginning to end. Br. J. Haematol. 2014, 165, 259–268. [Google Scholar] [CrossRef]
- Kurokawa, T.; Zheng, Y.W.; Ohkohchi, N. Novel functions of platelets in the liver. J. Gastroenterol. Hepatol. 2016, 31, 745–751. [Google Scholar] [CrossRef]
- Lupia, E.; Goffi, A.; Bosco, O.; Montrucchio, G. Thrombopoietin as biomarker and mediator of cardiovascular damage in critical diseases. Mediat. Inflamm. 2012, 2012, 390892. [Google Scholar] [CrossRef]
- Kaushansky, K. Thrombopoietin. N. Engl. J. Med. 1998, 339, 746–754. [Google Scholar] [CrossRef]
- Jackson, S.P. Arterial thrombosis--insidious, unpredictable and deadly. Nat. Med. 2011, 17, 1423–1436. [Google Scholar] [CrossRef]
- Pletsch-Borba, L.; Grafetstätter, M.; Hüsing, A.; González Maldonado, S.; Kloss, M.; Gross, M.L.; Johnson, T.; Sookthai, D.; Bugert, P.; Kaaks, R.; et al. Biomarkers of vascular injury in relation to myocardial infarction risk: A population-based study. Sci. Rep. 2019, 9, 3004. [Google Scholar] [CrossRef]
- Thorand, B.; Baumert, J.; Herder, C.; Meisinger, C.; Koenig, W. Soluble thrombomodulin as a predictor of type 2 diabetes: Results from the MONICA/KORA Augsburg case-cohort study, 1984–1998. Diabetologia 2007, 50, 545–548. [Google Scholar] [CrossRef]
- Krakoff, J.; Funahashi, T.; Stehouwer, C.D.A.; Schalkwijk, C.G.; Tanaka, S.; Matsuzawa, Y.; Kobes, S.; Tataranni, P.A.; Hanson, R.L.; Knowler, W.C.; et al. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care 2003, 26, 1745–1751. [Google Scholar] [CrossRef]
- Thorand, B.; Baumert, J.; Doring, A.; Schneider, A.; Chambless, L.; Lowel, H.; Kolb, H.; Koenig, W. Association of cardiovascular risk factors with markers of endothelial dysfunction in middle-aged men and women. Results from the MONICA/KORA Augsburg Study. Thromb. Haemost. 2006, 95, 134–141. [Google Scholar] [CrossRef]
- Song, Y.; Manson, J.E.; Tinker, L.; Rifai, N.; Cook, N.R.; Hu, F.B.; Hotamisligil, G.S.; Ridker, P.M.; Rodriguez, B.L.; Margolis, K.L.; et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes 2007, 56, 1898–1904. [Google Scholar] [CrossRef]
- Stranges, S.; Rafalson, L.B.; Dmochowski, J.; Rejman, K.; Tracy, R.P.; Trevisan, M.; Donahue, R.P. Additional contribution of emerging risk factors to the prediction of the risk of type 2 diabetes: Evidence from the Western New York Study. Obesity 2008, 16, 1370–1376. [Google Scholar] [CrossRef]
- Julia, C.; Czernichow, S.; Charnaux, N.; Ahluwalia, N.; Andreeva, V.; Touvier, M.; Galan, P.; Fezeu, L. Relationships between adipokines, biomarkers of endothelial function and inflammation and risk of type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 231–238. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef]
- Boeing, H.; Korfmann, A.; Bergmann, M.M. Recruitment procedures of EPIC-Germany. European Investigation into Cancer and Nutrition. Ann. Nutr. Metab. 1999, 43, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Wahrendorf, J.; Becker, N. EPIC-Germany—A source for studies into diet and risk of chronic diseases. European Investigation into Cancer and Nutrition. Ann. Nutr. Metab. 1999, 43, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.E.; Sookthai, D.; Johnson, T.; Schübel, R.; González Maldonado, S.; Pletsch-Borba, L.; Katzke, V.; Bugert, P.; Hoffmeister, M.; Kaaks, R.; et al. Pre-diagnostic plasma concentrations of Fibrinogen, sGPIIb/IIIa, sP-selectin, sThrombomodulin, Thrombopoietin in relation to cancer risk: Findings from a large prospective study. Int. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Grafetstätter, M.; Hüsing, A.; González Maldonado, S.; Sookthai, D.; Johnson, T.; Pletsch-Borba, L.; Katzke, V.A.; Hoffmeister, M.; Bugert, P.; Kaaks, R.; et al. Plasma Fibrinogen and sP-Selectin are Associated with the Risk of Lung Cancer in a Prospective Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.M.; Bussas, U.; Boeing, H. Follow-up procedures in EPIC-Germany—Data quality aspects. European Prospective Investigation into Cancer and Nutrition. Ann. Nutr. Metab. 1999, 43, 225–234. [Google Scholar] [CrossRef]
- Graf, M.E.; Sookthai, D.; Johnson, T.; Schubel, R.; Katzke, V.; Bugert, P.; Hoffmeister, M.; Kaaks, R.; Kühn, T. Biological reproducibility of circulating P-Selectin, Thrombopoietin, GPIIb/IIIa and Thrombomodulin over one year. Clin. Biochem. 2017, 50, 942–946. [Google Scholar] [CrossRef]
- Fibrinogen Studies, C.; Wood, A.M.; White, I.; Thompson, S.G.; Lewington, S.; Danesh, J. Regression dilution methods for meta-analysis: Assessing long-term variability in plasma fibrinogen among 27,247 adults in 15 prospective studies. Int. J. Epidemiol. 2006, 35, 1570–1578. [Google Scholar] [CrossRef]
- Lazar, C.; Meganck, S.; Taminau, J.; Steenhoff, D.; Coletta, A.; Molter, C.; Weiss-Solis, D.Y.; Duque, R.; Bersini, H.; Nowe, A. Batch effect removal methods for microarray gene expression data integration: A survey. Brief. Bioinform. 2013, 14, 469–490. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 24 May 2019).
- Prentice, R.L.; Self, S.G. Aspects of the use of relative risk models in the design and analysis of cohort studies and prevention trials. Stat. Med. 1988, 7, 275–287. [Google Scholar] [CrossRef]
- Pepe, M.S.; Kerr, K.F.; Longton, G.; Wang, Z. Testing for improvement in prediction model performance. Stat. Med. 2013, 32, 1467–1482. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Vevea, J.L. Fixed- and Random-Effects Models in Meta-Analysis. Psychol. Methods 1998, 3, 486–504. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Barzilay, J.I.; Abraham, L.; Heckbert, S.R.; Cushman, M.; Kuller, L.H.; Resnick, H.E.; Tracy, R.P. The relation of markers of inflammation to the development of glucose disorders in the elderly: The Cardiovascular Health Study. Diabetes 2001, 50, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G. Low-grade systemic inflammation and the development of type 2 diabetes—The atherosclerosis risk in communities study. Diabetes 2003, 52, 1799–1805. [Google Scholar] [CrossRef]
- Huang, T.; Glass, K.; Zeleznik, O.A.; Kang, J.H.; Ivey, K.L.; Sonawane, A.R.; Birmann, B.M.; Hersh, C.P.; Hu, F.B.; Tworoger, S.S. A Network Analysis of Biomarkers for Type 2 Diabetes. Diabetes 2019, 68, 281–290. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Georgousopoulou, E.N.; Chrysohoou, C.; Tousoulis, D.; Stefanadis, C.; Pitsavos, C.; Group, A.S. Single and combined effects of inflammatory markers on 10 year diabetes incidence: The mediating role of adiposity-Results from the ATTICA cohort study. Diabetes Metab. Res. Rev. 2018, 34. [Google Scholar] [CrossRef]
- Herder, C.; Baumert, J.; Zierer, A.; Roden, M.; Meisinger, C.; Karakas, M.; Chambless, L.; Rathmann, W.; Peters, A.; Koenig, W.; et al. Immunological and Cardiometabolic Risk Factors in the Prediction of Type 2 Diabetes and Coronary Events: MONICA/KORA Augsburg Case-Cohort Study. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Sharrett, A.R.; Lindberg, G.; Savage, P.J.; Offenbacher, S.; Azambuja, M.I.; Tracy, R.P.; Heiss, G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): A cohort study. Lancet 1999, 353, 1649–1652. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I.; Offenbacher, S.; Wu, K.K.; Savage, P.J.; Heiss, G. Factor VIII and other hemostasis variables are related to incident diabetes in adults - The Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 1999, 22, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Song, Y.; Cook, N.; Tseng, C.-H.; Manson, J.E.; Eaton, C.; Margolis, K.L.; Rodriguez, B.; Phillips, L.S.; Tinker, L.F.; et al. The Lack of Utility of Circulating Biomarkers of Inflammation and Endothelial Dysfunction for Type 2 Diabetes Risk Prediction Among Postmenopausal Women The Women’s Health Initiative Observational Study. Arch. Intern. Med. 2010, 170, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; Williams, K.; Tracy, R.P.; Wagenknecht, L.E.; Haffner, S.M. Progression of plasminogen activator inhibitor-1 and fibrinogen levels in relation to incident type 2 diabetes. Circulation 2006, 113, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; D’Agostino, R.; Tracy, R.P.; Haffner, S.M. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes—The insulin resistance atherosclerosis study. Diabetes 2002, 51, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Dallmeier, D.; Larson, M.G.; Wang, N.; Fontes, J.D.; Benjamin, E.J.; Fox, C.S. Addition of Inflammatory Biomarkers Did Not Improve Diabetes Prediction in the Community: The Framingham Heart Study. J. Am. Heart Assoc. 2012, 1. [Google Scholar] [CrossRef]
- Bertoni, A.G.; Burke, G.L.; Owusu, J.A.; Carnethon, M.R.; Vaidya, D.; Barr, R.G.; Jenny, N.S.; Ouyang, P.; Rotter, J.I. Inflammation and the incidence of type 2 diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2010, 33, 804–810. [Google Scholar] [CrossRef]
- de Simone, G.; Wang, W.; Best, L.G.; Yeh, F.; Izzo, R.; Mancusi, C.; Roman, M.J.; Lee, E.T.; Howard, B.V.; Devereux, R.B. Target organ damage and incident type 2 diabetes mellitus: The Strong Heart Study. Cardiovasc. Diabetol. 2017, 16, 64. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Committee on Diabetes Mellitus: Second report. World Health Organ. Tech. Rep. Ser. 1985, 646, 1–80. [Google Scholar]
- McEver, R.P. Selectins: Lectins that initiate cell adhesion under flow. Curr. Opin. Cell Biol. 2002, 14, 581–586. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Liu, J.; Yang, B.; Zugel, M.; Steinacker, J.M.; Sun, Z.; Schumann, U. Association between circulating cell adhesion molecules and risk of type 2 diabetes: A meta-analysis. Atherosclerosis 2019, 287, 147–154. [Google Scholar] [CrossRef]
- Ito, T.; Kakihana, Y.; Maruyama, I. Thrombomodulin as an intravascular safeguard against inflammatory and thrombotic diseases. Expert Opin. Ther. Targets 2016, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Xie, J.; Zhao, S.; Du, R.; Luo, X.; He, H.; Jiang, S.; Hao, N.; Chen, C.; Guo, C.; et al. ICAM3 mediates inflammatory signaling to promote cancer cell stemness. Cancer Lett. 2018, 422, 29–43. [Google Scholar] [CrossRef] [PubMed]

| Type 2 Diabetes Cases (n = 163) | Non-Cases (n = 2061) | p-Value | |
|---|---|---|---|
| Age at recruitment (years) * | 54.2 (47.4; 58.7) | 50.34 (43.0; 57.2) | 0.09 |
| Women † | 62 (38.0%) | 1155 (56.0%) | <0.001 |
| Hypertension (yes) † | 94 (57.7%) | 700 (34.0%) | <0.001 |
| BMI (kg/m2) * | 29.2 (27.6; 31.8) | 24.9 (22.5; 27.6) | 0.05 |
| Height (cm) * | 170.2 (163.6; 176.0) | 169.0 (162.8; 175.7) | 0.25 |
| Weight (kg) * | 85.3 (77.0; 94.0) | 72.2 (63.0; 82.0) | 0.33 |
| Waist circumference (cm) * | 99.6 (92.7; 106.0) | 86.0 (76.0; 94.5) | 0.10 |
| Alcohol intake at baseline (g/day) * | 11.1 (3.1; 24.5) | 10.3 (2.9; 24.4) | <0.01 |
| Education level † | <0.001 | ||
| Primary School † | 67 (41.1%) | 526 (25.5%) | |
| Secondary School † | 65 (39.9%) | 851 (41.3%) | |
| University Degree † | 31 (19.0%) | 684 (33.2%) | |
| Smoking Status † | 0.35 | ||
| Never † | 68 (41.7%) | 906 (44.0%) | |
| Former, quit <10yrs ago † | 29 (17.8%) | 456 (22.1%) | |
| Former, quit ≥ 10yrs ago † | 23 (14.1%) | 226 (11.0%) | |
| Current <15 cigarettes/day † | 21 (12.9%) | 261 (12.7%) | |
| Current ≥ 15 cigarettes/day † | 22 (13.5%) | 207 (10.0%) | |
| Aspirin use (yes) † | 4 (2.5%) | 57 (2.8%) | 0.81 |
| Antithrombotic drug use (yes) † | 1 (0.6%) | 18 (0.9%) | 0.72 |
| Physical Activity b (Cambridge index) † | 0.03 | ||
| Inactive/moderately inactive † | 89 (54.6%) | 946 (45.9%) | |
| Moderately active/active † | 74 (45.4%) | 1115 (54.1%) | |
| CRP (mg/l) * | 2.0 (0.9; 3.6) | 0.91 (0,47; 2,29) | 0.03 |
| LDL (mmol/l) * | 4.2 (3.7; 4.9) | 3.9 (3,3; 4,6) | 0.90 |
| Triglycerides (mmol/l) * | 2.2 (1.6; 3.4) | 1.5 (1.0; 2.2) | <0.001 |
| HDL (mmol/l) * | 1.2 (1.0; 1.5) | 1.4 (1.2; 1.8) | <0.01 |
| Total Cholesterol (mmol/l) * | 5.9 (5.4; 6.9) | 5.8 (5.1; 6.5) | 0.72 |
| HbA1c (mmol/mol, %) * | 38.0 (35.0; 41.0), 5.6% (5.4%; 5.9%) | 34.0 (32.0; 36.0), 5.3% (5.1%; 5.4%) | <0.001 |
| E-Selectin (ng/mL) * | 11.4 (8.9; 14.9) | 9.6 (6.9; 13.2) | 0.32 |
| P-Selectin (ng/mL) * | 29.6 (24.0; 38.0) | 27.2 (21.5; 33.9) | 0.08 |
| ICAM3 (ng/mL) * | 0.42 (0.36; 0.55) | 0.44 (0.36; 0.55) | <0.01 |
| Thrombomodulin (ng/mL) * | 3.0 (2.6; 3.5) | 2.9 (2.4; 3.38) | 0.32 |
| Thrombopoietin (pg/mL) * | 334.2 (294.4; 398.4) | 342.8 (287.6; 409.8) | <0.001 |
| Glycoprotein IIb/IIIa (ng/mL) * | 402.9 (324.8; 525.6) | 382.1 (313.4; 490.7) | 0.84 |
| Fibrinogen (µg/mL) * | 3,998 (3643; 4471) | 3,731 (3350; 4204) | 0.32 |
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Case Number | HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| E-Selectin (ng/mL) | |||||
| Tertile 1 | 32 | Ref. | Ref. | ||
| Tertile 2 | 63 | 2.05 (1.34, 3.14) | <0.01 | 2.13 (1.38, 3.29) | <0.01 |
| Tertile 3 | 67 | 2.14 (1.40, 3.26) | <0.001 | 1.44 (0.93, 2.22) | 0.10 |
| Continuous (per SD) | 162 | 1.39 (1.17, 1.64) | <0.001 | 1.05 (0.90, 1.24) | 0.54 |
| P-Selectin (ng/mL) | |||||
| Tertile 1 | 46 | Ref. | Ref. | ||
| Tertile 2 | 51 | 1.08 (0.72, 1.61) | 0.71 | 0.82 (0.55, 1.24) | 0.36 |
| Tertile 3 | 65 | 1.37 (0.94, 2.00) | 0.10 | 0.81 (0.54, 1.21) | 0.30 |
| Continuous (per SD) | 162 | 1.20 (1.02, 1.42) | 0.03 | 0.95 (0.81, 1.12) | 0.57 |
| ICAM3 (ng/mL) | |||||
| Tertile 1 | 66 | Ref. | Ref. | ||
| Tertile 2 | 46 | 0.67 (0.46, 0.98) | 0.04 | 0.66 (0.45, 0.98) | 0.04 |
| Tertile 3 | 50 | 0.74 (0.51, 1.07) | 0.11 | 0.62 (0.43, 0.91) | 0.01 |
| Continuous (per SD) | 162 | 0.92 (0.79, 1.08) | 0.32 | 0.89 (0.76, 1.03) | 0.13 |
| Thrombomodulin (ng/mL) | |||||
| Tertile 1 | 49 | Ref. | Ref. | ||
| Tertile 2 | 54 | 1.07 (0.73, 1.58) | 0.73 | 0.93 (0.63, 1.39) | 0.74 |
| Tertile 3 | 59 | 1.12 (0.76, 1.64) | 0.56 | 0.94 (0.63, 1.40) | 0.74 |
| Continuous (per SD) | 162 | 0.97 (0.82, 1.14) | 0.69 | 0.84 (0.71, 1.00) | 0.06 |
| Thrombopoietin (pg/mL) | |||||
| Tertile 1 | 54 | Ref. | Ref. | ||
| Tertile 2 | 60 | 1.13 (0.78, 1.63) | 0.51 | 1.06 (0.72, 1.54) | 0.78 |
| Tertile 3 | 49 | 0.86 (0.59, 1.27) | 0.45 | 0.92 (0.61, 1.37) | 0.67 |
| Continuous (per SD) | 163 | 0.91 (0.78, 1.07) | 0.26 | 0.95 (0.81, 1.12) | 0.56 |
| Glycoprotein IIb/IIIa (ng/mL) | |||||
| Tertile 1 | 55 | Ref. | Ref. | ||
| Tertile 2 | 51 | 0.93 (0.63, 1.36) | 0.70 | 0.79 (0.53, 1.18) | 0.25 |
| Tertile 3 | 56 | 1.02 (0.70, 1.47) | 0.94 | 0.86 (0.59, 1.26) | 0.43 |
| Continuous (per SD) | 162 | 1.01 (0.87, 1.18) | 0.85 | 0.95 (0.82, 1.10) | 0.51 |
| Fibrinogen(µg/mL) | |||||
| Tertile 1 | 33 | Ref. | Ref. | ||
| Tertile 2 | 45 | 1.31 (0.84, 2.06) | 0.23 | 0.96 (0.60, 1.52) | 0.86 |
| Tertile 3 | 85 | 2.46 (1.63, 3.69) | <0.001 | 1.05 (0.67, 1.64) | 0.82 |
| Continuous (per SD) | 163 | 1.46 (1.25, 1.71) | <0.001 | 1.02 (0.85, 1.22) | 0.82 |
| First Author, Year | Study | Ethnicity (%) | Age (Years, Mean) | BMI (kg/m2, Mean) | Hypertension (% Yes) | Current Smokers (% Yes) | Participants without Type 2 Diabetes at Baseline | Incident Type 2 Diabetes Cases | Mean Follow-Up (Years) | Definition of Type 2 Diabetes Diagnosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Developed Type 2 Diabetes | ||||||||||||||
| No | Yes | No | Yes | No | Yes | No | Yes | |||||||
| Duncan, 1999 [52] | ARIC | W: 78, AA: 21 | 54 among all (median) | 26 among all (median) | 30 among all | 25 among all | 12,330 | 1335 | 7 | Reported physician diagnosis, fasting plasma glucose ≥7 mmol/L, casual glucose ≥11.1 mmol/L, or antidiabetic medication use | ||||
| Festa, 2002 [55] | Insulin Resistance Atherosclerosis Study | Cases W: 37, B: 28, H: 35 Non-cases W: 41, B: 26, H:33 | 55 | 56 | 28 | 31 | 30 | 45 | NA | NA | 1,047 | 144 | 5.2 | WHO criteria [59] |
| Krakoff, 2003 [25] | Longitudinal health study in Pima Indians | Pima Indians | 32 | 33 | 36 | 36 | NA | NA | NA | NA | 142 | 71 | 4.6(cases) 6.8(controls) | WHO criteria [59] |
| Meigs, 2004 [5] | Nurses’ Health Study | W: 95, Non-white: 5 | 56 | 56 | 26 | 30 | NA | NA | 13 | 14 | 1,446 | 700 | 10 | Treatment with either insulin or an oral hypoglycaemic agent, at least 1 classic symptom of diabetes plus an elevated plasma glucose level, or an elevated plasma glucose level on 2 occasions. Elevated plasma glucose was defined as at least 140 mg/dL (≥7.8 mmol/L) fasting, at least 200 mg/dL (≥11.1 mmol/L) 2 h after an oral glucose tolerance test for cases diagnosed before 1998; for cases diagnosed in 1998 and later, the fasting plasma glucose threshold was 126 mg/dL (≥7 mmol/L). |
| Thorand, 2006 [6] | MONICA/KORA | White | 51.8 ♂/ 51.7 ♀ | 56.1 ♂/ 56.2 ♀ | 27.1 ♂/ 26.4 ♀ | 29.7 ♂/ 30.9 ♀ | 43.9 ♂/ 35.9 ♀ | 65.8 ♂/ 69.4 ♀ | 29.4 ♂/ 19.2 ♀ | 35.1 ♂/ 15.3 ♀ | 2,244 | 532 | 12 | Physician diagnosis of diabetes, on participants who self-reported diabetes |
| Song, 2007 [27] | Women’s Health Initiative Observational Study | W: 51, B: 29, H: 12, A: 8 | W:64, B:61, H:60, A:64 | W:64, B:61, H:60, A:64 | W:26, B:30, H:28, A:24 | W:33, B: 34, H:31, A:27 | W:6, B:11, H:3, A:4 | W: 7, B:11, H:8, A:3 | NA | NA | 3,782 | 1584 | 5.5 | Self-report of first-time use of hypoglycaemic medication (oral hypoglycaemic agents or insulin) |
| Thorand, 2007 [24] | MONICA/KORA | White | 51 | 56 | 27 | 30 | 40 | 67 | 23 | 27 | 1,204 | 224 | 8 | Self-report of diabetes diagnosed by a physician or intake of antidiabetic medication was validated by physician contact or medical chart review |
| Stranges, 2008 [28] | Western New York Study | W: 90 | 60 | 58 | 29 | 32 | 35 | 56 | 11 | 20 | 219 | 61 | 5.9 | Fasting glucose >125mg/dL or antidiabetic medication intake |
| Bertoni, 2010 [57] | MESA | W: 42, C:12, B: 26, H: 21 | W: 62,C: 61,AA: 62, H: 6 (among all, cases and non-cases) | W: 28, C:24, AA:30, H:29 (among all, cases and non-cases) | W: 37, C: 34, AA: 55, H:37 (among all, cases and non-cases) | W: 11, C: 5 AA: 18, H: 14 (among all, cases and non-cases) | 5,571 | 410 | 4.7 | Use of diabetes drugs or glucose ≥ 7 mmol/L | ||||
| Dallmeier, 2012 [56] | Framingham Heart Study | W: 93, AA: 3, H: 2, A: 2 | 59 | 62 | 28 | 32 | 37 | 68 | 12 | 11 | 2,638 | 162 | 6.6 | Fasting glucose ≥126 mg/dL or the use of insulin or oral hypoglycaemic medication |
| Julia, 2014 [29] | SU. VI. MAX 2 | French | 51 | 52 | 24.38 | 28.24 | NA | NA | NA | NA | 1,263 | 82 | 13 | Fasting glucose ≥126 mg/dL or use of antidiabetic medication |
| Odegaard, 2016 [30] | CARDIA | B: 44 | P-Selectin quartiles (1st to 4th): 40, 40, 40, 40 | E-Selectin quartiles (1st to 4th): 40, 40, 40, 40 | P-Selectin quartiles (1st to 4th): 27, 28, 29, 29 | E-Selectin quartiles (1st to 4th): 26, 27, 29, 31 | NA | NA | P-Selectin quartiles (1st to 4th): 12, 17, 18, 28 | E-Selectin quartiles (1st to 4th): 11, 17, 21, 27 | 2,339 | 222 for E-Selectin and 220 for P-Selectin | 10 | Use of diabetes medication, fasting blood glucose ≥7 mmol/L (126 mg/dL), 2 h post-challenge glucose ≥11.1 mmol/L (200 mg/dL), or a HbA1c ≥ 6.5% (48 mmol/mol) |
| Pankow, 2016 [4] | MESA | W: 38, B: 28, H: 23, A: 11 | P-Selectin quartiles (1st to 4th): 61, 63, 63, 62 | E-Selectin quartiles (1st to 4th): 59, 59, 59, 58 | P-Selectin quartiles (1st to 4th): 27, 27, 27, 28 | E-Selectin quartiles (1st to 4th): 61, 63. 63. 62 | NA | NA | NA | NA | E-Selectin: 826; P-Selectin: 1894 | E-Selectin: 107; P-Selectin: 184 | 10 | Use of insulin or oral diabetes medication or fasting glucose ≥ 7 mmol/L (126 mg/dL). |
| De Simone, 2017 [58] | Strong Heart Study | American Indians | 44 | 47 | 30 | 35 | NA | NA | NA | NA | 2,887 | 297 | 4 | Fasting glucose ≥126 mg/dL or the use of antidiabetic medication |
| Pletsch-Borba, 2019 | EPIC–Heidelberg | White | 50 (median) | 54 (median) | 25 | 29 | 34 | 58 | 23 | 26 | 2,224 | 163 | 16 (median) | Physician’s diagnosis and medical records review |
| Author, Year | Risk Marker | Reduced Model for Type 2 Diabetes Risk (95% CI) | Multivariable-Adjusted Model for Type 2 Diabetes Risk (95% CI) | Quantiles | Reduced Model for Type 2 Diabetes Risk Continuous (per SD) (95% CI) | Multivariable-Adjusted Model for Type 2 Diabetes Risk Continuous (per SD) (95% CI) | Adjustment for Covariates (Multivariable-adj. Model) | Study Quality (max 9) | Study Design |
|---|---|---|---|---|---|---|---|---|---|
| Duncan, 1999 [52] | Fibrinogen | 1.5, p < 0.001 | 1.2 (1.0, 1.5) | quartile | NA | 1.07 (1.00, 1.15) * | 1, 2, 4, 6, 10, 12, 23, 25 | 8 | Cohort |
| Festa, 2002 [55] | Fibrinogen | NA | NA | NA | 1.21 (1.01, 1.44) | 1.02 (0.85, 1.24) | 1, 2, 4, 8, 10 | 8 | Cohort |
| Krakoff, 2003 [25] | E – Selectin | NA | NA | NA | 1.12 (0.82, 1.55) | 1.34 (0.91, 1.99) | 1, 9, 22, 23, 24, 32 | 7 | Nested case – control |
| Meigs, 2004 [5] | E-Selectin | 7.50 (5.05, 11.14) | 4.84 (3.06, 7.67) | Quintile | 1.83 (1.37, 2.45) * | 1.56 (1.12, 2.18) * | 1, 6, 8, 10, 11, 25, 27, 30, 31 | 8 | Nested case-control |
| Thorand, 2006 [6] | E-Selectin | 3.44 (2.46, 4.83) ♂/ 2.79 (1.87, 4.17) ♀ | 2.79 (1.91, 4.09) ♂/ 1.72 (1.07, 2.75) ♀ | Tertile | 1.58 (1.24, 2.02) * ♂/1.44 (1.08, 1.93) * ♀ | 1.42 (1.07, 1.88) * ♂/1.18 (0.85, 1.64) * ♀ | 1, 3, 8, 10, 11, 12, 13, 14, 20, 25, 26 | 9 | Case-cohort |
| Song, 2007 [27] | E-Selectin | 5.48 (4.33, 6.94) | 3.46 (2.56, 4.68) | Quartile | 1.83 (1.70, 1.97) | 1.58 (1.45, 1.73) | 1, 4, 5, 6, 7, 8, 10, 11, 12, 25, 27 | 8 | Nested case-control |
| Thorand, 2007 [24] | Thrombomodulin | NA | NA | NA | 0.92 (0.78, 1.09) | 0.79 (0.62, 1.00) | 1, 2, 3, 8, 10, 11, 12, 13, 20, 25 | 8 | Case-cohort |
| Stranges, 2008 [28] | E-Selectin | 3.18 (1.32, 7.64) | 2.77 (1.13, 6.79) | Tertile | 1.63 (0.88, 3.00) * | 1.56 (0.84, 2.91) * | 1, 2, 5, 6, 8, 23, 25 | 8 | Nested case-control |
| Bertoni, 2010 [57] | Fibrinogen | 1.6 (1.2, 2.1) ** | 1.0 (0.8, 1.3) | Quartile | 1.08 (0.85, 1.36) * | 0.92 (0.75, 1.13) * | 1, 2, 4, 6, 8, 10, 11, 12, 13, 15, 21, 28 | 7 | Cohort |
| Dallmeier, 2012 [56] | P-Selectin and Fibrinogen | NA | NA | NA | P-Selectin 1.11 (0.94, 1.31) and Fibrinogen 1.24 (1.05, 1.47) | P-Selectin 0.89 (0.74, 1.07), Fibrinogen 0.91 (0.75, 1.11) | 1, 2, 3, 8, 10, 13, 18, 19, 23 | 8 | Cohort |
| Julia, 2014 [29] | E-Selectin | 1.18 (0.67, 2.07) | 1.07 (0.60, 1.93) | Tertiles | 1.25 (0.96, 1.62) | 1.18 (0.90, 1.55) | 1, 2, 8, 19, 20, 23, 25, 38 | 8 | Cohort |
| Odegaard, 2016 [30] | E-Selectin, P-Selectin | NA | E-Selectin: 2.48 (1.60, 3.85), P-Selectin: 1.48 (0.98, 2.22) | Quartiles | NA | E-Selectin 1.25 (0.90, 1.75) *, P-Selectin 1.09 (0.82, 1.46) * | 1, 2, 4, 6, 10, 11, 12, 25, 33 | 8 | Cohort |
| Pankow, 2016 [4] | P-Selectin, E-Selectin | E-Selectin 3.76 (1.99, 7.08), P-Selectin 1.62 (1.06, 2.48) | E-Selectin 2.49 (1.26, 4.93), P-Selectin 1.14 (0.73, 1.77) | quartiles | E-Selectin 1.24 (1.04, 1.49), P-Selectin 1.06 (0.91, 1.22) | E-Selectin 1.57 (0.95, 2.58) *, P-Selectin 1.04 (0.89, 1.21) | Cont.: 1, 2, 4, 6, 8, 9, 10, 12, 13, 15, 22, 23, 24, 26, 29. Quantiles: 1, 2, 4, 6, 8, 9, 10, 12, 13, 15, 21, 22 (also 24, 26, and 29 on E-Selectin analyses) | 8 | Cohort |
| De Simone, 2017 [58] | Fibrinogen | NA | NA | NA | NA | 7.18 (1.63, 31.53) | 1, 2, 4, 14, 34, 35, 36, 37 | 7 | Cohort |
| Pletsch-Borba, 2019 | All | see Table 2 | see Table 2 | see Table 2 | see Table 2 | see Table 2 | 1, 2, 8, 10, 11,12, 14, 21, 22, 26 | 9 | Cohort |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pletsch-Borba, L.; Watzinger, C.; Turzanski Fortner, R.; Katzke, V.; Schwingshackl, L.; Sowah, S.A.; Hüsing, A.; Johnson, T.; Groß, M.-L.; González Maldonado, S.; et al. Biomarkers of Vascular Injury and Type 2 Diabetes: A Prospective Study, Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 2075. https://doi.org/10.3390/jcm8122075
Pletsch-Borba L, Watzinger C, Turzanski Fortner R, Katzke V, Schwingshackl L, Sowah SA, Hüsing A, Johnson T, Groß M-L, González Maldonado S, et al. Biomarkers of Vascular Injury and Type 2 Diabetes: A Prospective Study, Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2019; 8(12):2075. https://doi.org/10.3390/jcm8122075
Chicago/Turabian StylePletsch-Borba, Laura, Cora Watzinger, Renée Turzanski Fortner, Verena Katzke, Lukas Schwingshackl, Solomon A. Sowah, Anika Hüsing, Theron Johnson, Marie-Luise Groß, Sandra González Maldonado, and et al. 2019. "Biomarkers of Vascular Injury and Type 2 Diabetes: A Prospective Study, Systematic Review and Meta-Analysis" Journal of Clinical Medicine 8, no. 12: 2075. https://doi.org/10.3390/jcm8122075
APA StylePletsch-Borba, L., Watzinger, C., Turzanski Fortner, R., Katzke, V., Schwingshackl, L., Sowah, S. A., Hüsing, A., Johnson, T., Groß, M.-L., González Maldonado, S., Hoffmeister, M., Bugert, P., Kaaks, R., Grafetstätter, M., & Kühn, T. (2019). Biomarkers of Vascular Injury and Type 2 Diabetes: A Prospective Study, Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 8(12), 2075. https://doi.org/10.3390/jcm8122075






