Abstract
The effectiveness and safety of shoulder arthroplasties in the general context of a Spanish patient population remains unclear. The aim of this study was to ascertain both the effectiveness and safety of primary shoulder arthroplasties and the prosthesis types used in Spain. A systematic review of all the available literature evaluating the effectiveness and safety of primary shoulder arthroplasties in Spain was performed. A narrative synthesis was performed, and evidence tables were created in four dimensions: study design, arthroplasty characteristics, safety, and effectiveness. Orthopaedic Data Evaluation Panel (ODEP) scores were used to evaluate prosthesis types. Twenty-one studies were selected that included a total of 1293 arthroplasties. The most common indication was fractures, while the prosthesis most frequently used was the Delta Xtend (ODEP 10A). The most common complication was scapular notching. Prosthesis revision rate was approximately 6% for follow-ups between 12 and 79 months. In addition, significant improvements were observed in the Constant–Murley test score after the intervention. Currently in Spain, shoulder arthroplasty can be considered a safe and effective procedure with functional recovery and pain reduction for eligible patients with humeral fracture, rotator cuff arthropathy, fracture sequelae and malunion of the proximal humerus, and degenerative disease. Future longitudinal research and population-based studies could serve to confirm these results and identify points of improvement.
1. Introduction
Shoulder arthroplasty is currently considered to be an established therapeutic option and an effective and efficient procedure to improve physical function, pain and quality of life in patients [1,2,3,4,5]. As a result of continual technological progress and emerging indications for shoulder arthroplasty, including proximal humeral fractures, osteoarthritis and massive rotator cuff tears [6], the utilization of this procedure has increased throughout the world and in some countries it has tripled in the last decade [7,8,9].
The use of shoulder arthroplasty has increased significantly in Spain in recent years [10,11], but remains a lower-volume procedure compared to knee and hip replacement. Given the increasing indication for these types of procedures and their potential to improve the health of patients, it is vitally important for both patients and clinicians that the complications associated with these interventions are well understood. Previous research has proposed that the three most common complications are instability, periprosthetic fracture and infection [12,13,14]. Understanding this information within the context of the effectiveness of different types of prostheses and models, and in certain population groups, is likely to be highly relevant [1,7,14]. However, as far as we know, no studies have evaluated the effectiveness and safety of these procedures in the general context of a Spanish population. Furthermore, there is no arthroplasty register that can be directly assessed. Both analysing the results of these procedures by population and establishing a registry could be useful in evaluating the results of shoulder arthroplasties more precisely in a specific healthcare context such as Spain, facilitating a comparison to other international contexts.
Regarding the safety and effectiveness of primary shoulder arthroplasties, evidence suggests that some of the most frequent complications associated with this procedure are scapular notching, dislocation, periprosthetic fracture, and infection [14,15,16]. Furthermore, the revision rate of shoulder arthroplasties is estimated to be approximately 5% and 10% at 5 and 10 years respectively, and may be lower in reverse arthroplasties compared to hemiarthroplasty and total shoulder arthroplasty [15,16,17]. However, in the Spanish context, to date no population-level studies have been conducted to evaluate these outcomes, or aimed to quantify the safety and effectiveness of primary shoulder arthroplasties. Furthermore, the evidence found in specific studies that have already been carried out is divergent, which may be due to a focus on specific models or types of prostheses, or certain pathologies and clinical populations [18,19,20].
In this context, the objective of this study was to describe the scientific evidence available on the effectiveness and safety of primary shoulder arthroplasties in Spain and the types of prosthesis used in this population.
2. Materials and Methods
A systematic review was conducted on the results of shoulder arthroplasties performed in public hospitals in Spain, and the results are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [21]. The review has been registered in PROSPERO (CRD42019115342). The following databases were used as sources of information: EMBASE, PubMed, Scielo, Cochrane Reviews and Center for Reviews and Dissemination. The search range was restricted from January 2003 to December 2018. Given the continuous improvement in shoulder arthroplasty results and the advancement in surgical techniques, the lower limit was set at 2003. This limit was fixed in order to maximize the study period and overcome any potential limitations related to underestimating the current results after pooling long-term retrospective data.
A search filter was developed specifically designed for PubMed/Medline to achieve the objectives of this study (Appendix A), and was adapted to other databases. The search strategy was based on previous studies in an attempt to maximize the number of documents identified [22,23,24]. Keywords for procedure as well as anatomical and territorial location were used. In addition, the references found in systematic reviews and meta-analyses were used to identify primary studies, a grey literature search was conducted, and key authors were contacted.
2.1. Inclusion and Exclusion Criteria and the Revision Process
The PICO criteria (population, intervention, comparison, outcome) were used to identify studies. We included documents in English and Spanish that focused on the evaluation of effectiveness and safety in primary shoulder arthroplasties performed in public hospitals in Spain. Due to limitations related to the robustness of the data and possible biases when making inferences in the population, only studies with a sample size of 20 or more primary interventions were included. Documents that included patients under 18 years of age, studies that evaluated revision implants or those indicated for tumours or congenital diseases, and studies aimed at evaluating surgical techniques were excluded. Additionally, studies evaluating complications, adverse effects and/or effectiveness based on certain patient characteristics were excluded due to the difficulty in generalizing and comparing their results.
A screening of the title, summary and full text was carried out independently by two expert reviewers (JAT and XGC), while possible discrepancies were resolved by a third reviewer (KS). After study selection, a narrative synthesis of the evidence obtained was carried out. Given the variability in study characteristics and outcome variable presentation, a meta-analysis was deemed unfeasible. Therefore, the information was extracted in various tables of evidence with four dimensions: study design, arthroplasty characteristics, safety and effectiveness of primary shoulder arthroplasties. To evaluate the prosthesis models, the Orthopaedic Data Evaluation Panel (ODEP) scores were used [25]. ODEP is a panel of independent experts that publishes reference indexes to assess the effectiveness of different models of anatomical and reverse prostheses. The criteria used were based on implant survival, follow-up time and the size of the cohort analysed. Based on these criteria, implants were assigned to categories in terms of the evidence supporting their use.
2.2. Quality of Studies Included
The quality of the studies and their design were considered according to the Scottish Intercollegiate Guidelines Network (SIGN) levels of evidence hierarchy [26]. Furthermore, the Risk of Bias tool (RoB 2.0) was used for randomized trials to assess the quality of evidence [27], while for non-randomized trials, the Risk of Bias in Non-Randomized Studies of Interventions tool (ROBINS-I) was used [28]. Finally, for single-cohort study designs, the scale of evidence assessment for case series studies from the Institute of Health Economics was used [29].
SIGN levels were assigned according to the quality of the study evidence based on their design from the 1 ++ level of higher evidence reserved for high-quality meta-analyses, systematic reviews of randomized clinical trials, and randomized clinical trials with very low risk of bias, to level 4, which includes expert opinion. To compare studies, the risk of bias for each reference was calculated using the RoB 2.0 and ROBINS-I tools [27,28], assigning 4 points to “critical” risk assessments; 3 points to “high” risk; 2 points to “moderate” risk, “some considerations” or cases assessed with “insufficient information”; and 1 point for “low” risk assessment. Next, the percentage of points obtained over the total points possible for all categories was calculated. For single-cohort studies, the percentage of positive responses was obtained from the assessment scale.
3. Results
A total of 360 references were identified (259 from EMBASE, 84 from PubMed and 17 from SCIELO) (Figure 1). After eliminating duplicates, 323 were screened by title and abstract. After screening, 277 documents were excluded, thus including 46 for full-text review. Of these 46 references, 25 were excluded and 21 studies were included in the final evidence tables [18,19,20,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47].
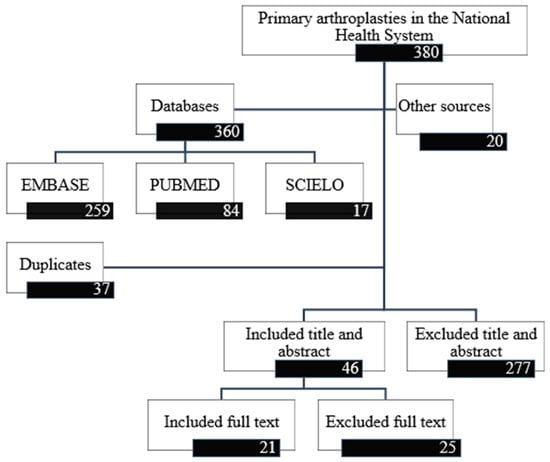
Figure 1.
Study selection flow diagram.
Table 1 shows the level of evidence of the selected studies. Two randomized clinical trials had a SIGN 1+ level and two non-randomized clinical trials a SIGN 2 ++. The remaining studies were observational, 11 of which were retrospective. In terms of risk of bias assessment, little variability was observed in the risk attributed to the different studies. The greatest risk of bias came from subjectivity in measuring outcome variables, followed by the possible impact of uncontrolled confounding variables. The average range for patient follow-up was between 12 and 79 months, and included the results of 1293 arthroplasties. The number of cases per study ranged from 21 to 163.

Table 1.
Primary shoulder arthroplasties. Evidence, risk of bias, and study design.
Table 2 shows that the average age of patients included was over 70 years, approximately 70% of whom were women. In addition, two studies documented low comorbidity in their patients (Charlson Comorbidity Index less than 2) [18,19]. There were four main indications for undertaking primary arthroplasty: acute fractures and fracture-dislocations of the proximal humerus, rotator cuff arthropathy, fracture sequelae and malunion of the proximal humerus, and degenerative diseases. About half of the interventions were performed using a deltopectoral approach, while the rest used a superolateral or anterosuperior approach. In terms of implant characteristics and fixation, 18 studies included reverse prostheses, 8 of which used a cemented fixation and 6 of which were non-cemented. The most frequently used prosthesis models included in these studies were the Delta Xtend, with an ODEP assessment of 10A, and the Delta III, which is not evaluated by ODEP. The third most frequent was the Lima SMR, with an ODEP rating of 10A.

Table 2.
Characteristics of shoulder arthroplasties included in the selected studies.
Table 3 shows that the most frequent complication in reverse shoulder arthroplasties was scapular notching, reported in 14 studies [33,48]. One study found a higher presence in older patients [46]. In terms of complications associated with tuberosities, malunion was documented in six studies, with the maximum rate being 33%. Additionally, resorption of the tuberosities was reported in five studies. Prosthesis infection was documented in seven of the included studies, with one study citing up to 8% [34]. Intraoperative fractures were also documented in seven studies, with the highest values being 7%. Similarly, periprosthetic fracture in five documents and ossification in one were also seen. There was a 1% dislocations rate, and less than 1% were complications relating to fixation, positioning, and movement of the prosthesis. The main neurological vascular and lymphatic system complications were paralysis in three studies and hematoma in three others. In terms of prosthesis survival, 67% of the studies selected cited approximately 6% revision rate between 12 and 78 months. A significant difference was found in the revision rate in two studies comparing partial arthroplasties (hemiarthroplasty) to reverse arthroplasties [32,34]. The revision rate for hemiarthroplasties was far higher, with a difference greater than 15% in both studies.

Table 3.
Reliability of primary shoulder arthroplasties.
In terms of the effectiveness of shoulder arthroplasties, Table 4 shows that in the studies evaluating patients before the intervention, the Constant–Murley test score was approximately 30%. After the intervention, the average score was approximately 65%, with significant improvement reported in four studies [20,30,34,41]. Seven of the eight studies included metrics before and after reported improvement in external rotation, with three studies being statistically significant. In one study, better results in terms of hemiarthroplasties were observed in reverse prostheses [32]. Five of the seven studies that dealt with internal rotation and analysed metrics before and after reported improvements in movement. One of these studies had a significant difference. Better results were observed in patients that had fractures with tuberosity union compared to a group of patients with tuberosity malunion [35]. Sixteen of the 21 studies considered other results, highlighting their frequency on the UCLA Shoulder Rating Scale (UCLA) in two studies, Disabilities of the Arm, Shoulder and Hand (DASH) or QuickDASH in six studies and on Visual Analog Scale (VAS) in four studies.

Table 4.
Effectiveness of primary shoulder arthroplasties.
4. Discussion
The results of the review show that shoulder arthroplasty in Spain can currently be considered an effective and safe procedure, with functional recovery and pain reduction in patients operated on for humeral fracture and rotator cuff arthropathy, fracture sequelae and malunion of the proximal humerus, and degenerative diseases. These results are similar to those found in other countries, including Norway, Germany, the Netherlands and the USA [9,15,49,50,51], with better results observed with reverse-type arthroplasties than hemiarthroplasties.
Regarding the safety of shoulder arthroplasty, the overall complication rate in this procedure appears to be centred around 15%, with the most commonly observed complications being instability, periprosthetic fracture or infection [13,14]. The results obtained from this study show that the most frequent complications in Spain were of the same profile as other countries, with similar rates also reported [9,15,52,53,54]. In addition, increased safety has been observed in recent years worldwide as reported by the Nordic or the Kaiser Permanente registers [15,55]. These improved results could be for various reasons, with progress in prosthesis design being particularly relevant. The presence of reverse prostheses should be noted as they have become one of the treatments of choice for pathologies like proximal humeral fractures or rotator cuff arthropathy [56,57,58,59]. The results obtained when assessing reverse prostheses suggest that, while their rate of complications might be slightly higher than those observed in other contexts [9,15,52,53,54], their results could be better when compared to other types of prostheses [32,33,36,48]. This facilitates the hypothesis that reduced incidence of the aforementioned complications could be largely due to an increased use of these types of implants.
Currently, the evidence for implant survival in Spain at the population level is limited. However, the results of the reviewed studies are similar to data from international registries, which estimate an implant revision rate of approximately 90%–95% at 5 and 10 years [9,15,49,50,52]. In addition, most of the prosthesis models identified in this review are commonly used internationally, and the ODEP assessment of most of those included in the selected studies was acceptable [9,25]. Similar to our results, the 10-year cumulative revision rate after primary reverse shoulder arthroplasty in the Nordic countries was between 90% to 95% and the model most frequently used was Delta Xtend [55]. Moreover, the results from other European shoulder arthroplasty registers, including the National Joint Registry (NJR) in the United Kingdom and the Dutch arthroplasty register (LROI) show that the results in terms of survival rates might be similar across European countries and that the prosthesis models most frequently used are usually the same [51,60]. However, considering the results for shoulder arthroplasty effectiveness, in terms of functionality, pain and impact on the patient’s life, there is some difference in calculating scores, which hinders synthesis [61,62]. Regardless of the calculation differences, especially for the Constant–Murley test, improved scores after the intervention were observed in the results of the studies reviewed. The results show that an improvement in pain could be relevant, even at short-term follow-ups after arthroplasty, which is contrary to results suggested by a previous study proposing that improvement in the short-term may not be as evident as in longer-term follow-ups [63].
The authors accept that a significant limitation of the present study is the search strategy. Given its focus on the Spanish population, extrapolating and generalizing its results to other populations is challenging. However, we believe that delimiting the safety and effectiveness of shoulder arthroplasties in a specific healthcare context may be useful in encouraging and improving results at all levels: surgical, management, and patient. In addition, the results shown can be useful and relevant at the international level when making comparisons and establishing common standards of reference. It is also important to mention the limitation related to the inclusion criteria, given that patients under 18 years old and patients with tumours were excluded. These criteria restrict the capacity to extrapolate the results obtained to the whole population. Despite this limitation, the results found could be applicable to most of patients eligible for a shoulder arthroplasty, and thus it is reasonable to assume that they could be at least approaching the true results of these procedures in Spain. Another limitation is related to the heterogeneity of the studies in terms of their design and presentation of results, which makes a meta-analysis impossible. However, the evidence presented was synthesized as much as possible to be able to serve both as a reference in assessing the safety and effectiveness of shoulder arthroplasty, and as a starting point for new studies on the subject. Lastly, these studies were considered without stratifying by patient diagnosis or the severity of their symptoms. As such, it is possible that some of the results described correspond to selected samples of patients with certain diagnoses. Despite this, most of the studies focus on the two main reasons for intervention in shoulder arthroplasty: fracture of the humerus and rotator cuff arthropathy, which is why we believe the results shown can be widely generalized to the population susceptible to receiving a shoulder arthroplasty and not only to those with one of the two diagnoses. However, further epidemiological research stratified by these indications both in Spain and in other countries could be valuable to obtain a more precise representation of the safety and effectiveness of shoulder arthroplasties at the population level.
5. Conclusions
In Spain, primary shoulder arthroplasties, for those who are able to receive them, are an effective and safe procedure that allow functional recovery and pain reduction in patients with humeral fracture, rotator cuff arthropathy, fracture sequelae and malunion of the proximal humerus, and degenerative diseases. The prosthesis type with the best survival is the reverse prosthesis. Future longitudinal population-based studies, particularly randomized controlled trials, as well as the establishment of a shoulder arthroplasty registry could confirm these results and identify areas of improvement, including the recommendation of specific types of prostheses or models with preferable results.
Author Contributions
All authors conceived the study design, participated in writing the manuscript, and have critically reviewed and agreed on the final version of this article.
Funding
This study was funded by the partnership agreement signed by the Carlos III Health Institute, an autonomous body of the Ministry of Economy and Competitiveness, and the Agency for Health Quality and Assessment of Catalonia, within the activity development framework of the Spanish Network of Health Technology and Performance Evaluation Agencies of the National Health System, funded by the Ministry of Health, Social Services and Equality.
Acknowledgments
We acknowledge Carlos III Health Institute, CIBER Epidemiology and Public Health (CIBERESP), Antoni Parada and Olga Martinez for their support.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. PubMed Filter
#1 shoulder[MeSH] OR shoulder[ti] OR humeral[ti] OR glenohumeral[ti] OR glenoid[ti]
#2 arthroplasty[MeSH] OR arthroplast*[ti] OR replacement[ti] OR “prosthesis implantation”[MeSH] OR prosthes*[ti] OR implant* OR “prostheses and implants”[MeSH]
#3 Spain[tiab] OR Spanish[tiab] OR España[tiab] OR Espana[tiab] OR Espan*[tiab] OR Andalusia*[tiab] OR Catalan*[tiab] OR Catalonian[tiab] OR Madrid[tiab] OR Madrilen*[tiab] OR Madrileñ*[tiab] OR Valencian*[tiab] OR Galicia[tiab] OR Galego[tiab] OR Galleg*[tiab] OR Galaic[tiab] OR “Castilla y Leon”[tiab] OR “Castilla and Leon”[tiab] OR Leones*[tiab] OR Basque[tiab] OR basc*[tiab] OR “Castilla-La Mancha”[tiab] OR Canary[tiab] OR Canarian[tiab] OR Canari*[tiab] OR Murcia[tiab] OR Murcian*[tiab] OR Aragon*[tiab] OR Extremadura[tiab] OR Extremeno[tiab] OR Extremena[tiab] OR Extremeño[tiab] OR Extremeña[tiab] OR Balear[tiab] OR Asturias[tiab] OR Asturian*[tiab] OR Navarra[tiab] OR Navarre[tiab] OR Cantabria[tiab] OR Cantabric*[tiab] OR “La Rioja”[tiab] OR Riojan*[tiab]
#4 spain[MeSH] OR spain OR espagne OR espana OR spain[ad] OR espagne[ad] OR espana[ad] OR osasunbidea[ad] OR osakidetza[ad] OR insalud[ad] OR sergas[ad] OR catalunya[ad] OR catalonia[ad] OR catalogne[ad] OR cataluna[ad] OR catala[ad] OR barcelon[ad] OR barcelona[ad] OR barcelones[ad] OR barceloneta[ad] OR barcelonia[ad] OR tarragona[ad] OR lleida[ad] OR lerida[ad] OR girona[ad] OR gerona[ad] OR sabadell[ad] OR hospitalet[ad] OR l’hospitalet[ad] OR valencia[ad] OR castello[ad] OR castellon[ad] OR alacant[ad] OR alicant[ad] OR alicante[ad] OR murcia[ad] OR murcian OR murciana[ad] OR murciano[ad] OR andaluci[ad] OR andalucia[ad] OR andaluciajunta[ad] OR andalusi[ad] OR andalusia[ad] OR andalusian[ad] OR andaluz[ad] OR andaluza[ad] OR sevill[ad] OR sevilla[ad] OR seville[ad] OR granada[ad] OR huelva[ad] OR almeria[ad] OR cadiz[ad] OR jaen[ad] OR malaga[ad] OR extremadura[ad] OR caceres[ad] OR badajoz[ad] OR madrid[ad] OR galicia[ad] OR gallego[ad] OR compostela[ad] OR vigo[ad] OR coruna[ad] OR ferrol[ad] OR orense[ad] OR ourense[ad] OR pontevedra[ad] OR oviedo[ad] OR gijon[ad] OR asturia[ad] OR asturiano[ad] OR asturias[ad] OR asturias[ad] OR cantabria[ad] OR cantabrico[ad] OR cantabro[ad] OR santander[ad] OR vasco[ad] OR euskadi[ad] OR basque[ad] OR bilbao[ad] OR bilbo[ad] OR donosti[ad] OR donostia[ad] OR vizcaya[ad] OR guipuzcoa[ad] OR gipuzkoa[ad] OR alava[ad] OR alaba[ad] OR vitoria[ad] OR vitoria[ad] OR vitoria-gasteiz[ad] OR bizkaia[ad] OR navarra[ad] OR pamplona[ad] OR irunea[ad] OR aragon[ad] OR aragones[ad] OR zaragoza[ad] OR teruel[ad] OR huesca[ad] OR mancha[ad] OR “ciudad real”[ad] OR albacete[ad] OR cuenca[ad] OR balear[ad] OR baleares[ad] OR balearic[ad] OR balears[ad] OR mallorca[ad] OR menorca[ad] OR ibiza[ad] OR eivissa[ad] OR palmas[ad] OR lanzarote[ad] OR canaria[ad] OR canarian[ad] OR canarias[ad] OR canario[ad] OR tenerife[ad] OR castilla[ad] OR salamanca[ad] OR zamora[ad] OR valladolid[ad] OR segovia[ad] OR soria[ad] OR palencia[ad] OR avila[ad] OR burgos[ad] OR (leon[ad] NOT (france[ad] OR clermont[ad] OR rennes[ad] OR lyon[ad] OR USA[ad] OR mexic[ad] OR mexica[ad])) OR (cordoba[ad] NOT (argentin[ad] OR argentina[ad])) OR (toledo[ad] NOT (ohio[ad] OR us[ad] OR usa[ad] OR OH[ad])) OR (guadalajara[ad] NOT (mexic[ad] OR mexica[ad] OR mexicali[ad] OR mexican[ad] OR mexicana[ad] OR mexicano[ad] OR mexicanos[ad]))
#5 #1 AND #2 AND (#3 OR #4) Filters: Publication date from 2003/01/01
References
- Boileau, P.; Sinnerton, R.J.; Chuinard, C.; Walch, G. Arthroplasty of the shoulder. J. Bone Jt. Surg. Br. 2006, 88, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Rangan, A.; Handoll, H.; Brealey, S.; Jefferson, L.; Keding, A.; Martin, B.; Goodchild, L.; Chuang, L.; Hewitt, C.; Torgerson, D. Surgical vs. nonsurgical treatment of adults with displaced fractures of the proximal humerus. Ned. Tijdschr. Traumachir. 2016, 24, 18. [Google Scholar] [CrossRef]
- Handoll, H.; Brealey, S.; Rangan, A.; Keding, A.; Corbacho, B.; Jefferson, L.; Chuang, L.-H.; Goodchild, L.; Hewitt, C.; Torgerson, D. The ProFHer (PROximal fracture of the humerus: Evaluation by randomisation) trial—A pragmatic multicentre randomized controlled trial evaluating the clinical effectiveness and cost-effectiveness of surgical compared with non-surgical treatment for proxi. Health Technol. Assess. 2015, 19, 1–279. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Sperling, J.; Buchbinder, R.; McMaken, K. Surgery for shoulder osteoarthritis. Cochrane Database Syst. Rev. 2010, 6, CD008089. [Google Scholar] [CrossRef] [PubMed]
- Sumrein, B.O.; Huttunen, T.T.; Launonen, A.P.; Berg, H.E.; Fellander-Tsai, L.; Mattila, V.M. Proximal humeral fractures in Sweden—A registry-based study. Osteoporos. Int. 2017, 28, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ryösä, A.; Laimi, K.; Äärimaa, V.; Lehtimäki, K.; Kukkonen, J.; Saltychev, M. Surgery or conservative treatment for rotator cuff tear: A meta-analysis. Disabil. Rehabil. 2017, 39, 1357–1363. [Google Scholar] [CrossRef]
- Sharma, S.; Dreghorn, C.R. Registry of shoulder arthroplasty—The Scottish experience. Ann. R. Coll. Surg. Engl. 2006, 88, 122–126. [Google Scholar] [CrossRef]
- Handoll, H.H.; Brorson, S. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst. Rev. 2015, 11, CD000434. [Google Scholar] [CrossRef]
- Lubbeke, A.; Rees, J.L.; Barea, C.; Combescure, C.; Carr, A.J.; Silman, A.J. International variation in shoulder arthroplasty. Acta Orthop. 2017, 88, 592–599. [Google Scholar] [CrossRef]
- Arias-de la Torre, J.; Capdevila, A.; Martínez, O.; Domingo, L.; Marinelli, M.; Robles, N.; Nardi, J.; Puig-Verdié, L.; Pallisó, F.; Espallargues, M.; et al. A decade of the Catalonian Arthroplasty Register (RACat): Variability, exhaustivity, and survival of prostheses between 2005 and 2014. Rev. Esp. Cir. Ortop. Traumatol. 2017, 61, 70–81. [Google Scholar] [CrossRef]
- Portal estadístico Ministerio de Sanidad, Servicios Sociales e Igualdad Conjunto Mínimo Básico de Datos al Alta Hospitalria.
- Familiari, F.; Rojas, J.; Doral, M.N.; Huri, G.; McFarland, E.G. Reverse total shoulder arthroplasty. EFORT Open Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gauci, M.-O.; Cavalier, M.; Gonzalez, J.-F.; Holzer, N.; Baring, T.; Walch, G.; Boileau, P. Revision of failed shoulder arthroplasty: Epidemiology, etiology, and surgical options. J. Shoulder Elb. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bohsali, K.I.; Bois, A.J.; Wirth, M.A. Complications of shoulder arthroplasty. J. Bone Jt. Surg. Am. 2017, 1, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.T.; Ake, C.F.; Burke, M.F.; Singh, A.; Yian, E.H.; Paxton, E.W.; Navarro, R.A. The kaiser permanente shoulder arthroplasty registry: Results from 6336 primary shoulder arthroplasties. Acta Orthop. 2015, 86, 286–292. [Google Scholar] [CrossRef]
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Annual Report 2016; AOANJRR: Adelaide, Australia, 2016. [Google Scholar]
- Hernandez, N.M.; Chalmers, B.P.; Wagner, E.R.; Sperling, J.W.; Cofield, R.H.; Sanchez-Sotelo, J. Revision to reverse total shoulder arthroplasty restores stability for patients with unstable shoulder prostheses. Clin. Orthop. Relat. Res. 2017, 475, 2716–2722. [Google Scholar] [CrossRef]
- Alcobia-Diaz, B.; Lopiz, Y.; Garcia-Fernandez, C.; Rizo de Alvaro, B.; Marco, F. Patient reported activities after reverse total shoulder arthroplasty in rotator cuff arthropathy patients. Rev. Esp. Cir. Ortop. Traumatol. 2017, 61, 273–280. [Google Scholar] [CrossRef]
- Andrés-Cano, P.; Galán, A.; Arenas, J.; Del Águila, B.; Guerado, E. Results of uncemented hemiarthroplasty as primary treatment of severe proximal humerus fractures in the elderly. Eur. J. Orthop. Surg. Traumatol. 2014, 25, 273–280. [Google Scholar] [CrossRef]
- Martinez, A.A.; Calvo, A.; Bejarano, C.; Carbonel, I.; Herrera, A. The use of the Lima reverse shoulder arthroplasty for the treatment of fracture sequelae of the proximal humerus. J. Orthop. Sci. 2012, 17, 141–147. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Arias-de la Torre, J.; Puigdomenech, E.; Valderas, J.M.; Evans, J.P.; Martín, V.; Molina, A.J.; Rodríguez, N.; Espallargues, M. Availability of specific tools to assess patient reported outcomes in hip arthroplasty in Spain. Identifying the best candidates to incorporate in an arthroplasty register: A systematic review and standardized assessment. PLoS ONE 2019, 14, e0214746. [Google Scholar] [CrossRef]
- Valderas, J.M.; Mendivil, J.; Parada, A.; Losada-Yáñez, M.; Alonso, J. Construcción de un filtro geográfico para la identificación en PubMed de estudios realizados en España. Rev. Española Cardiol. 2006, 59, 1244–1251. [Google Scholar] [CrossRef]
- Terwee, C.B.; Jansma, E.P.; Riphagen, I.I.; de Vet, H.C.W. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual. Life Res. 2009, 18, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Northgate Orthopaedic Data Evaluation Panel (ODEP). Available online: http://www.odep.org.uk/ (accessed on 8 October 2019).
- Harbour, R.; Miller, J. A new system for grading recommendations in evidence based guidelines. BMJ 2002, 323, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Sterne, J.A.C. Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2.0). Available online: https://www.bristol.ac.uk/media-library/sites/social-community-medicine/images/centres/cresyda/RoB2-0_indiv_main_guidance.pdf (accessed on 8 October 2019).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016. [Google Scholar] [CrossRef] [PubMed]
- Moga, C.; Guo, B.; Harstall, C. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique; Institute of Health Economics: Edmonton, AB, Canada, 2012. [Google Scholar]
- Torrens, C.; Guirro, P.; Santana, F. The minimal clinically important difference for function and strength in patients undergoing reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2016, 25, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Torrens, C.; Guirro, P.; Miquel, J.; Santana, F. Influence of glenosphere size on the development of scapular notching: A prospective randomized study. J. Shoulder Elb. Surg. 2016, 25, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Sebastia-Forcada, E.; Cebrian-Gomez, R.; Lizaur-Utrila, A.; Gil-Guillen, V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures: A blinded, randomized, controlled, prospective study. J. Shoulder Elb. Surg. 2014, 23, 1419–1426. [Google Scholar] [CrossRef]
- Boyer, E.; Menu, G.; Loisel, F.; Saadnia, R.; Uhring, J.; Adam, A.; Rochet, S.; Clappaz, P.; Baudouin, E.; Lascar, T.; et al. Cementless and locked prosthesis for the treatment of 3-part and 4-part proximal humerus fractures: Prospective clinical evaluation of hemi- and reverse arthroplasty. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 301–308. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Guirro, P.; Santana, F.; Torrens, C. Treatment of fracture sequelae of the proximal humerus: Comparison of hemiarthroplasty and reverse total shoulder arthroplasty. Arch. Orthop. Trauma Surg. 2014, 134, 1545–1550. [Google Scholar] [CrossRef]
- Jorge-Mora, A.; Amhaz-Escanlar, S.; Fernández-Pose, S.; Lope-Del-Teso, C.; Pino-Mínguez, J.; Caeiro-Rey, J.R.; Pretell-Mazzini, J.; Gómez, R. Early outcomes of locked noncemented stems for the management of proximal humeral fractures: A comparative study. J. Shoulder Elb. Surg. 2019, 28, 48–55. [Google Scholar] [CrossRef]
- Sebastia-Forcada, E.; Lizaur-Utrilla, A.; Cebrian-Gomez, R.; Miralles-Muñoz, F.A.; Lopez-Prats, F.A. Outcomes of reverse total shoulder arthroplasty for proximal humeral fractures: Primary arthroplasty versus secondary arthroplasty after failed proximal humeral locking plate fixation. J. Orthop. Trauma 2017, 31, e236–e240. [Google Scholar] [CrossRef] [PubMed]
- Lopiz, Y.; García-Coiradas, J.; Serrano-Mateo, L.; García-Fernández, C.; Marco, F. Reverse shoulder arthroplasty for acute proximal humeral fractures in the geriatric patient: Results, health-related quality of life and complication rates. Int. Orthop. 2016, 40, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.C. Pròtesi Invertida d’Espatlla: Estudi Retrospectiu del Conflicte Escapular en 2 Models Diferents de Pròtesis; Universitat Autònoma de Barcelona, Departament de Cirurgia: Barcelona, Spain, 2012. [Google Scholar]
- Izquierdo-Fernández, A.; Minarro, J.C.; Carpintero-Lluch, R.; Estévez-Torres, E.M.; Carpintero-Benítez, P. Reverse shoulder arthroplasty in obese patients: Analysis of functionality in the medium-term. Arch. Orthop. Trauma Surg. 2017, 138, 5. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Sánchez, L.; Mesa-Mateo, A.; Barrionuevo-Sánchez, F.J.; García-Benítez, B.; Expósito-Triano, S. Total reverse shoulder replacement: Evaluation of the clinical results and complications in a series of 52 cases. Rev. Esp. Cir. Ortop. Traumatol. 2015, 59, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Zafra, M.; Uceda, P.; Flores, M.; Carpintero, P. Reverse total shoulder replacement for nonunion of a fracture of the proximal humerus. Bone Jt. J. 2014, 96, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, C.; Lópiz-Morales, Y.; Rodríguez, A.; López-Durán, L.; Martínez, F.M. Periprosthetic humeral fractures associated with reverse total shoulder arthroplasty: Incidence and management. Int. Orthop. 2015, 39, 1965–1969. [Google Scholar] [CrossRef]
- Torrens, C.; Santana, F.; Marí, R.; Puig, L.; Alier, A. Serum C-reactive protein in patients undergoing elective shoulder arthroplasty: Prospective study. J. Orthop. Sci. 2017, 22, 858–861. [Google Scholar] [CrossRef]
- Torrens, C.; Alentorn-Geli, E.; Mingo, F.; Gamba, C.; Santana, F. Reverse shoulder arthroplasty for the treatment of acute complex proximal humeral fractures: Influence of greater tuberosity healing on the functional outcomes. J. Orthop. Surg. 2018, 26, 2309499018760132. [Google Scholar] [CrossRef]
- Delgado Rodriguez, J.A.; Moreno Palacios, J.A.; Pulido Poma, R.M.; Fernandez Leon, R.A.; Martin Maroto, M.P.; Miranda Vivas, M.T. Functional results of partial shoulder replacement in patients over 65 years. Rev. Esp. Geriatr. Gerontol. 2013, 48, 22–25. [Google Scholar]
- Hernández-Elena, J.; de la Red-Gallego, M.Á.; Garcés-Zarzalejo, C.; Pascual-Carra, M.A.; Pérez-Aguilar, M.D.; Rodríguez-López, T.; Alfonso-Fernández, A.; Pérez-Núñez, M.I. Treatment of proximal humeral fractures by reverse shoulder arthroplasty: Mid-Term evaluation of functional results and Notching. Rev. Esp. Cir. Ortop. Traumatol. 2015, 59, 413–420. [Google Scholar] [CrossRef]
- Villodre-Jiménez, J.; Estrems-Díaz, V.; Diranzo-García, J.; Bru-Pomer, A. Reverse shoulder arthroplasty in 3 and 4 part proximal humeral fractures in patients aged more than 65 years: Results and complications. Rev. Esp. Cir. Ortop. Traumatol. 2016, 61, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sebastià-Forcada, E. Influencia de los Factores Anatómicos en el Resultado de las Prótesis de Hombro para el Tratamiento de las Fracturas Proximales de Húmero en Pacientes de Edad. 2014. Available online: https://dialnet.unirioja.es/servlet/cittes?codigo=68217 (accessed on 8 October 2019).
- Blomquist, J.; Solheim, E.; Liavaag, S.; Schroder, C.P.; Espehaug, B.; Havelin, L.I. Shoulder instability surgery in Norway: The first report from a multicenter register, with 1-year follow-up. Acta Orthop. 2012, 83, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Magosch, P.; Habermeyer, P.; Lichtenberg, S.; Tauber, M.; Gohlke, F.; Mauch, F.; Boehm, D.; Loew, M.; Zeifang, F.; Potzl, W. Results from the German shoulder- and elbow-arthroplasty register (SEPR): Anatomic or reverse shoulder arthroplasty in B2-glenoids? Orthopade 2017, 46, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Dutch Arthroplasty Register (LROI). Annual Repport, 2018; LROI: Hague, The Netherlands, 2018. [Google Scholar]
- Fevang, B.-T.S.; Lie, S.A.; Havelin, L.I.; Skredderstuen, A.; Furnes, O. Risk factors for revision after shoulder arthroplasty: 1825 shoulder arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop. 2009, 80, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.V.; Olsen, B.S.; Fevang, B.-T.S.; Furnes, O.; Skytta, E.T.; Rahme, H.; Salomonsson, B.; Mohammed, K.D.; Page, R.S.; Carr, A.J. A review of national shoulder and elbow joint replacement registries. J. Shoulder Elb. Surg. 2012, 21, 1328–1335. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Clark, N.J.; Assenmacher, A.T.; Samuelsen, B.T.; Sanchez-Sotelo, J.; Cofield, R.H.; Sperling, J.W. What are the complications, survival, and outcomes after revision to reverse shoulder arthroplasty in patients older than 80 years? Clin. Orthop. Relat. Res. 2017, 475, 2744–2751. [Google Scholar] [CrossRef]
- Lehtimaki, K.; Rasmussen, J.V.; Mokka, J.; Salomonsson, B.; Hole, R.; Jensen, S.L.; Aarimaa, V. Risk and risk factors for revision after primary reverse shoulder arthroplasty for cuff tear arthropathy and osteoarthritis: A Nordic Arthroplasty Register Association study. J. Shoulder Elb. Surg. 2018, 27, 1596–1601. [Google Scholar] [CrossRef]
- Erickson, B.J.; Bohl, D.D.; Cole, B.J.; Verma, N.N.; Nicholson, G.; Romeo, A.A.; Harris, J.D. Reverse total shoulder arthroplasty: Indications and techniques across the world. Am. J. Orthop. 2018, 47. [Google Scholar] [CrossRef]
- Drake, G.N.; O’Connor, D.P.; Edwards, T.B. Indications for reverse total shoulder arthroplasty in rotator cuff disease. Clin. Orthop. Relat. Res. 2010, 468, 1526–1533. [Google Scholar] [CrossRef]
- Nolan, B.M.; Ankerson, E.; Wiater, J.M. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin. Orthop. Relat. Res. 2011, 469, 2476–2482. [Google Scholar] [CrossRef]
- Jobin, C.M.; Galdi, B.; Anakwenze, O.A.; Ahmad, C.S.; Levine, W.N. Reverse shoulder arthroplasty for the management of proximal humerus fractures. J. Am. Acad. Orthop. Surg. 2015, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- National Joint Registry for England, Wales; Northern Ireland and the Isle of Man (NJR). UK National Jt. Registry 16th Annual Report; NJR: London, UK, 2018. [Google Scholar]
- Angst, F.; Schwyzer, H.-K.; Aeschlimann, A.; Simmen, B.R.; Goldhahn, J. Measures of adult shoulder function: Disabilities of the arm, shoulder, and hand questionnaire (DASH) and its short version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder. Arthritis Care Res. 2011, 63, S174–S188. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.R. An evaluation of the constant-murley shoulder assessment. J. Bone Jt. Surg. Br. 1997, 79, 695–696. [Google Scholar] [CrossRef]
- Bjornholdt, K.T.; Brandsborg, B.; Soballe, K.; Nikolajsen, L. Persistent pain is common 1–2 years after shoulder replacement. Acta Orthop. 2015, 86, 71–77. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).