Serum Zinc Level Classification System: Usefulness in Patients with Liver Cirrhosis
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. ALBI Score and ALBI Grade
2.3. Serum Zn Classification and Our Study
2.4. Statistical Analyses
3. Results
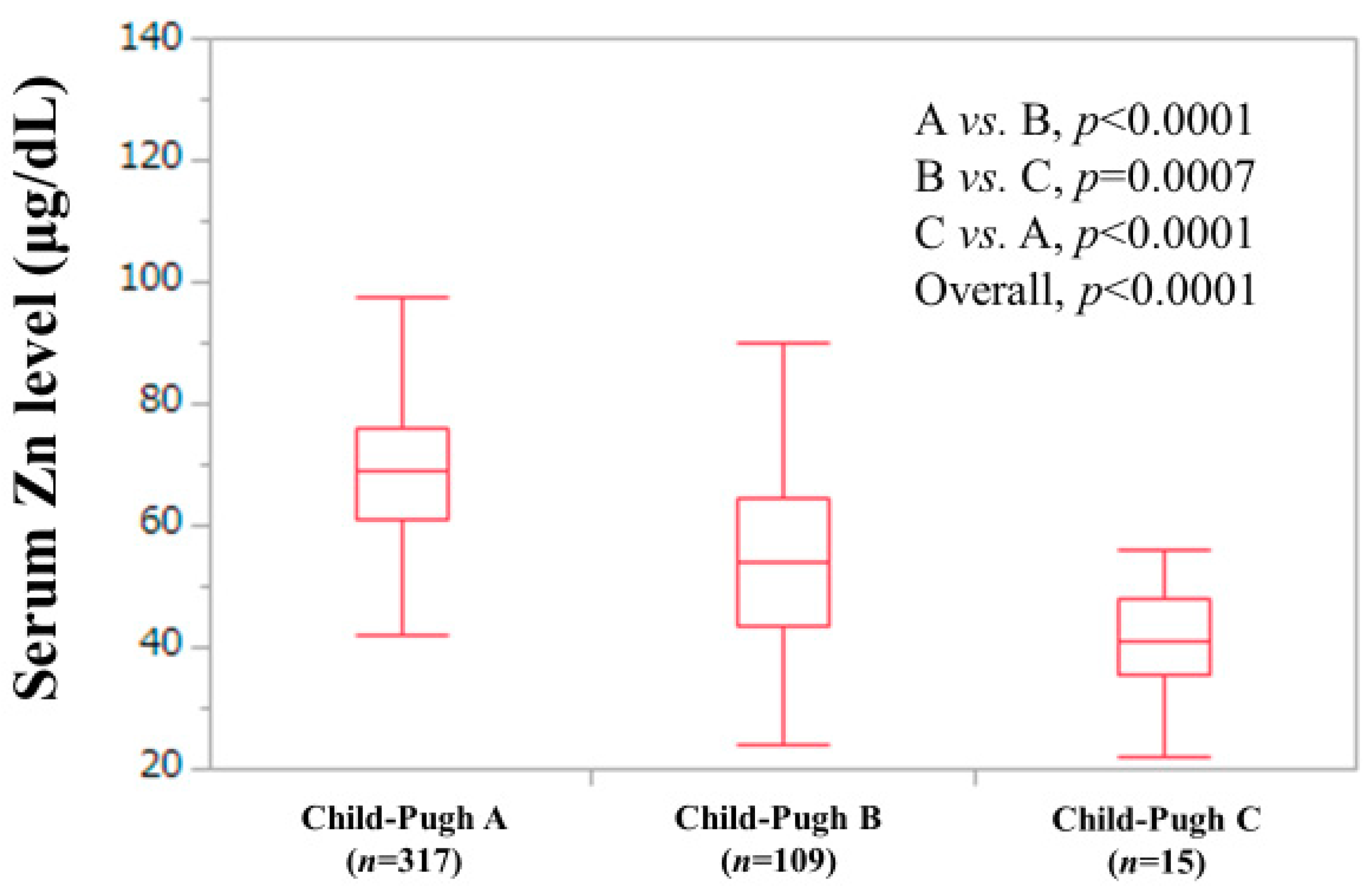
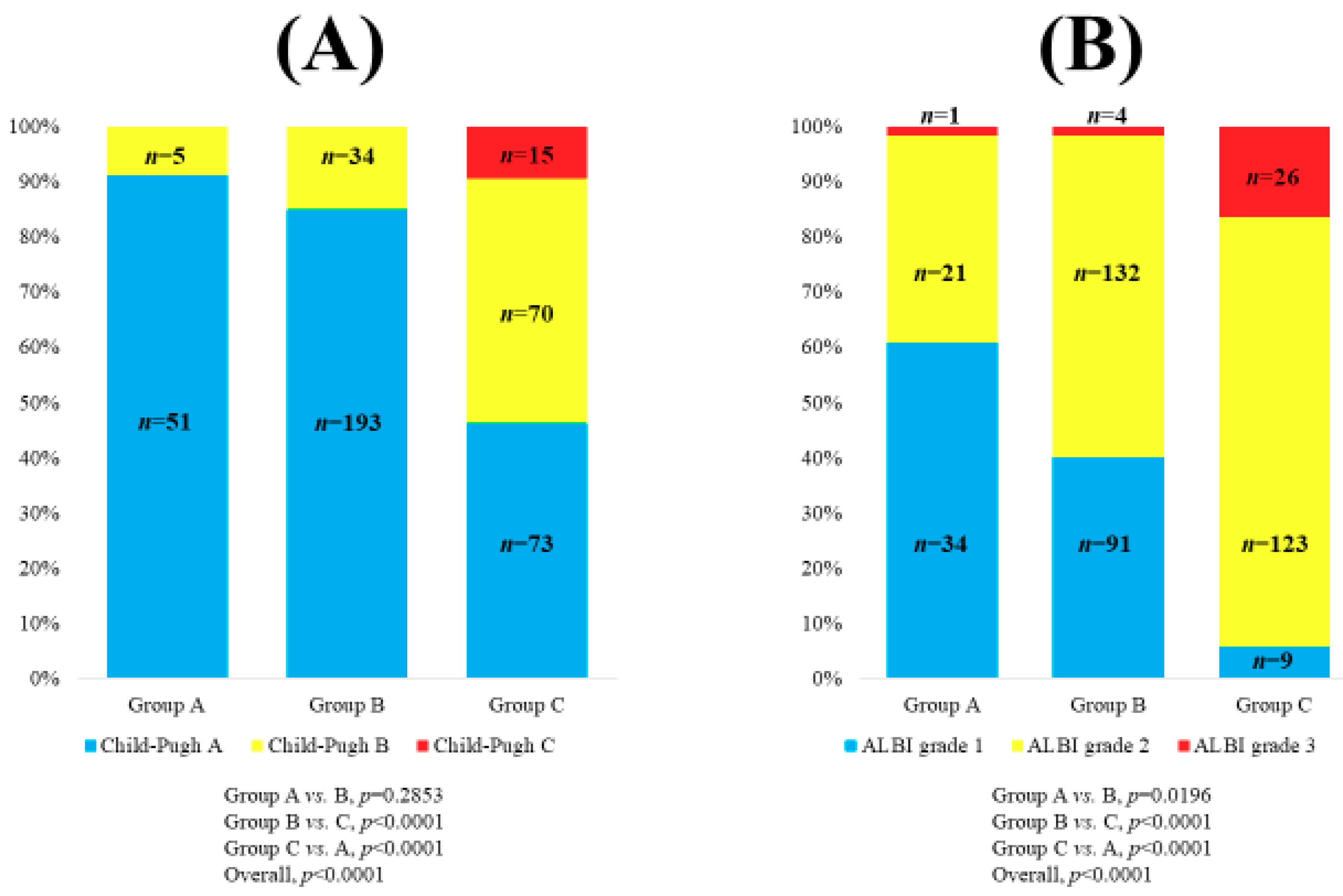
3.1. Baseline Characteristics
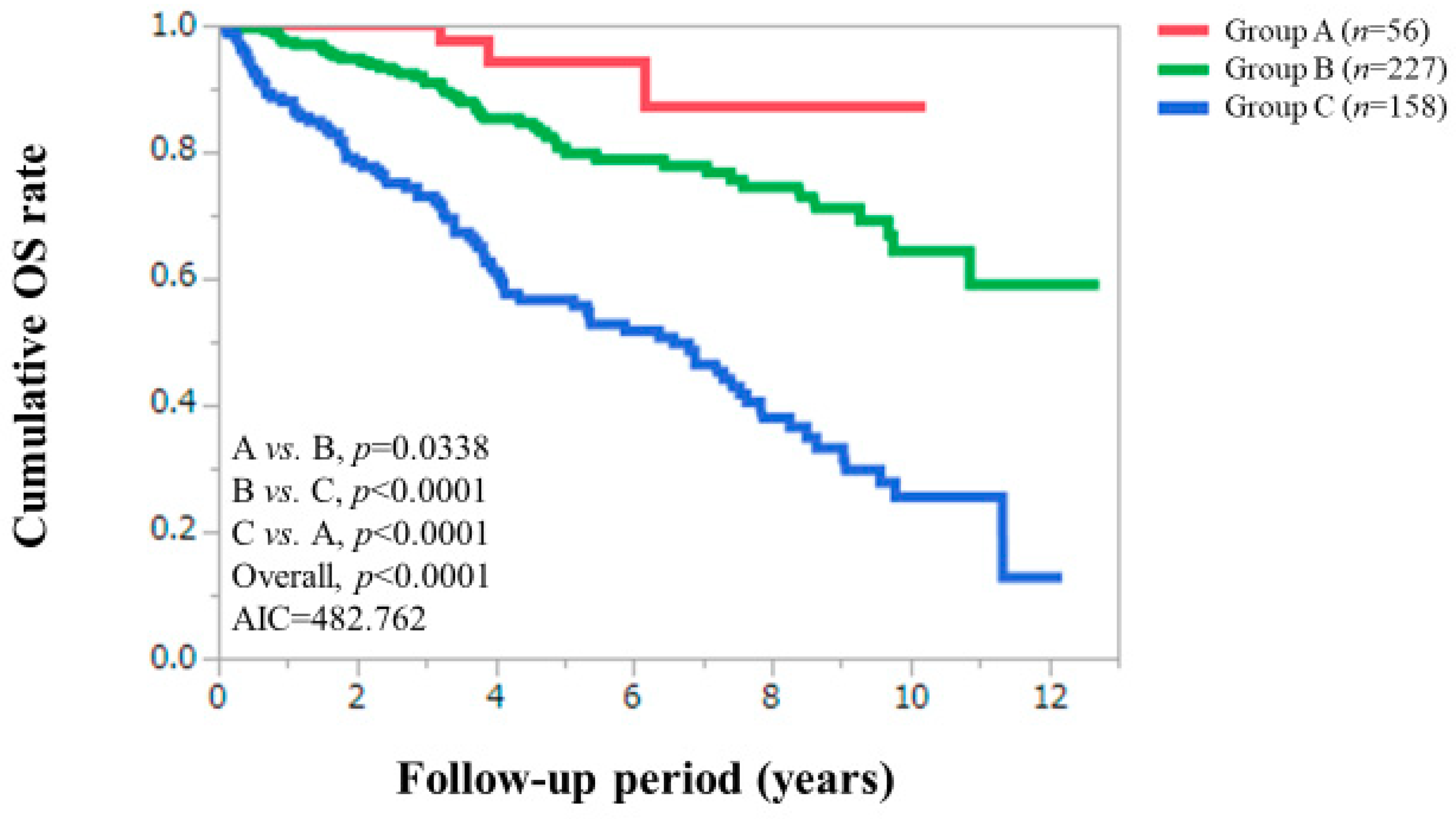
3.2. Cumulative OS Rates According to Serum Zn Level Classification
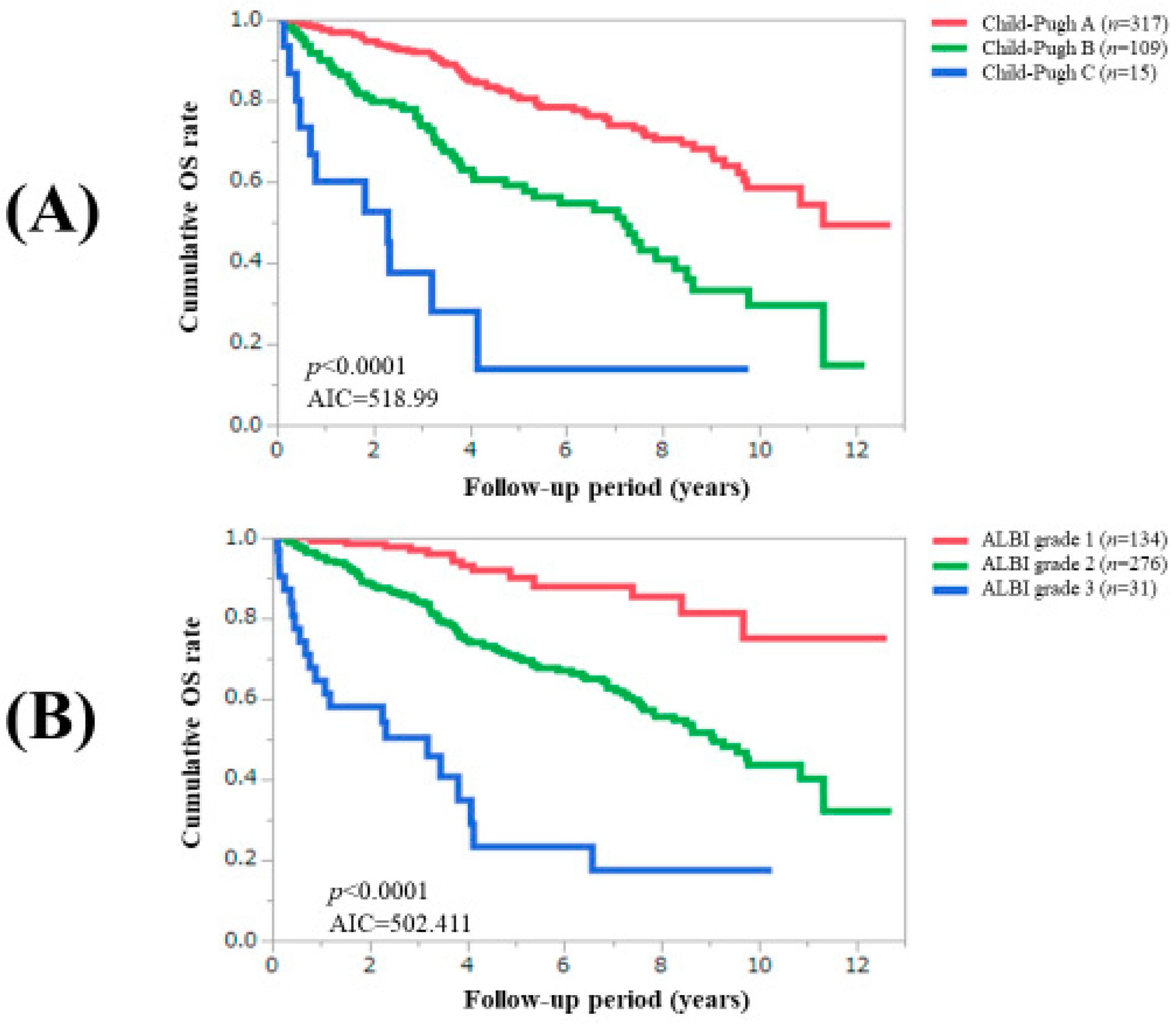
3.3. Comparison of Prognostic Impact among C–P Classification, ALBI Grade and Zn Classification System
3.4. Causes of Death According to Serum Zn Level Classification
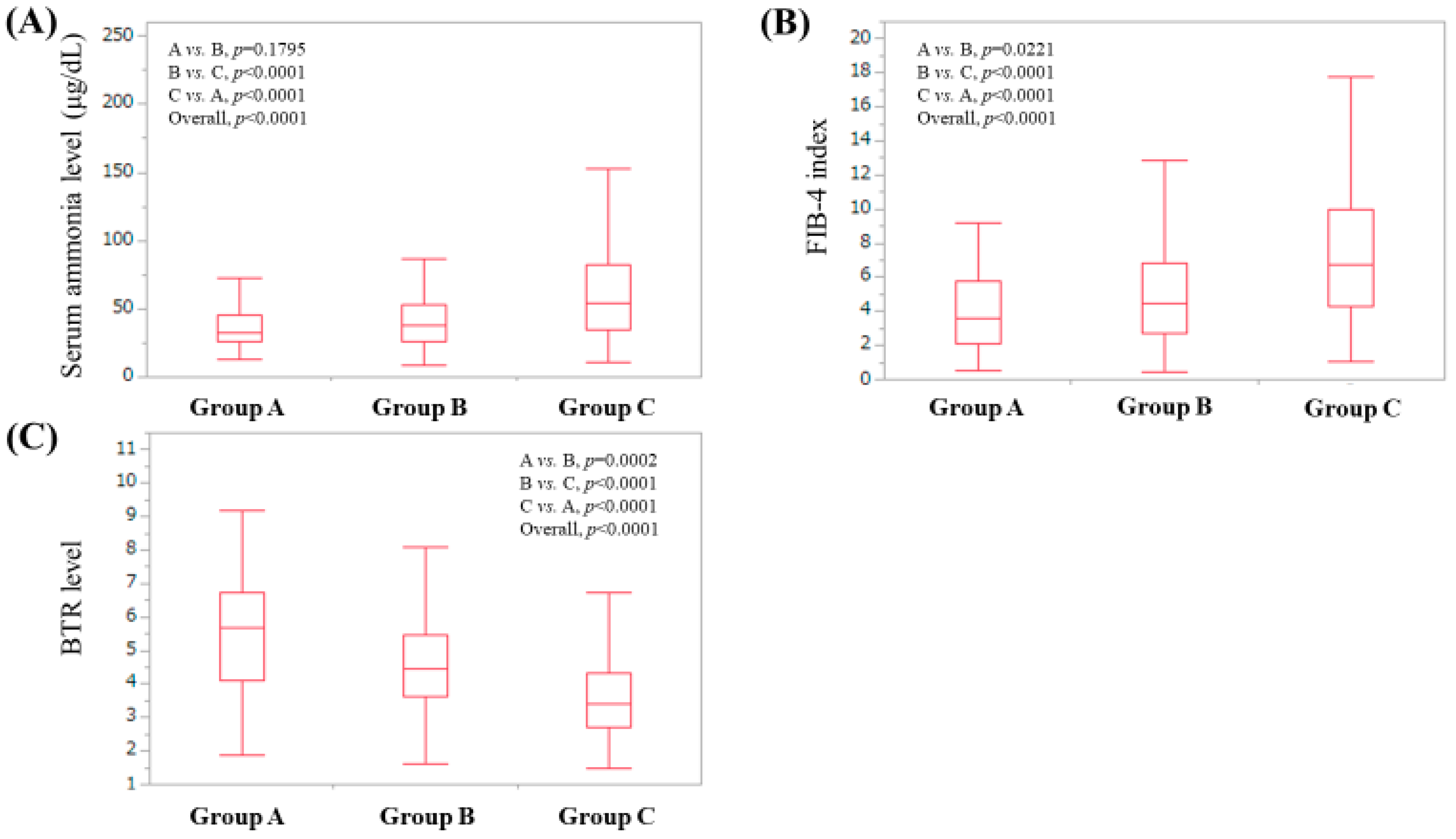
3.5. Serum Ammonia Level According to Serum Zn Level Classification
3.6. FIB-4 Index According to Serum Zn Level Classification
3.7. Branched-Chain Amino Acid to Tyrosine Ratio (BTR) Value According to Serum Zn Level Classification
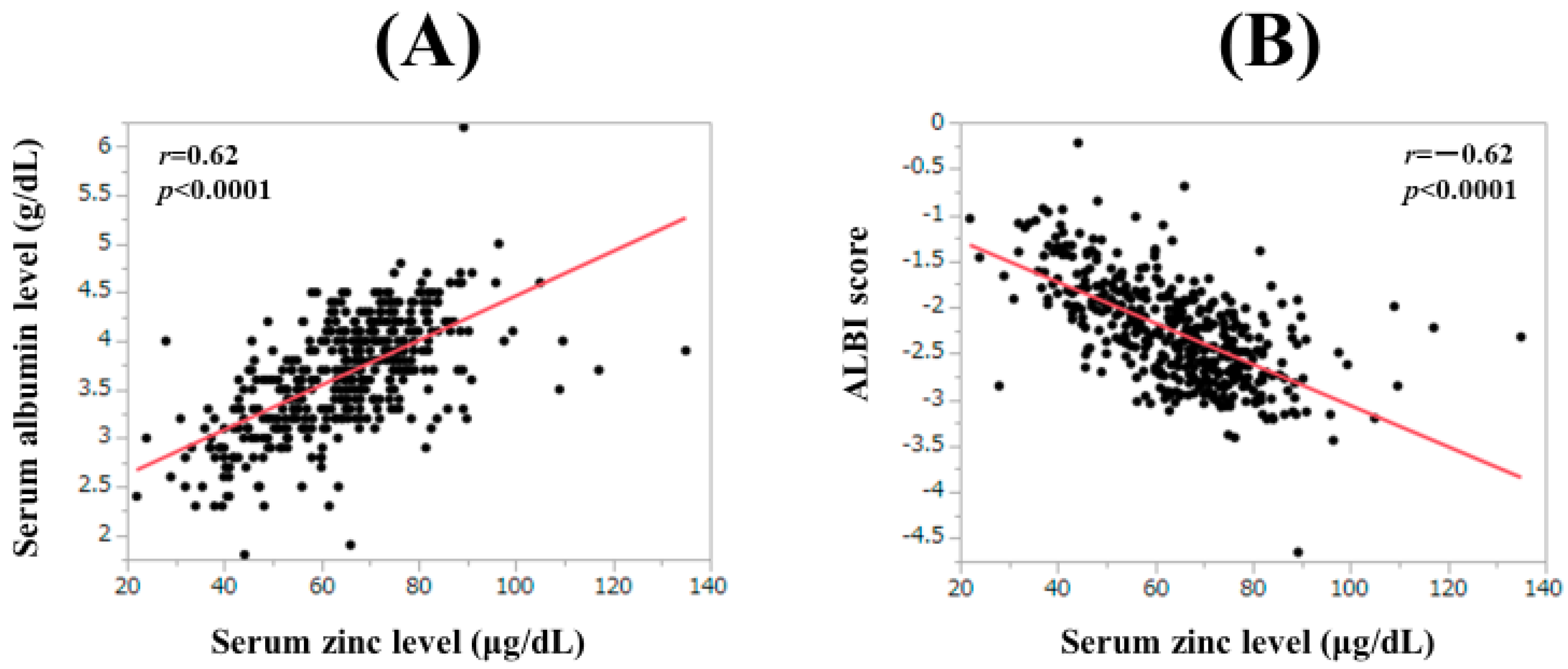
3.8. Correlation between Serum Zn Level and Baseline Data
3.9. Uni- and Multivariate Analyses of Factors Linked to OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Thandassery, R.B.; Montano-Loza, A.J. Role of Nutrition and Muscle in Cirrhosis. Curr. Treat. Options Gastroenterol. 2016, 14, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Gunsar, F.; Raimondo, M.L.; Jones, S.; Terreni, N.; Wong, C.; Patch, D.; Sabin, C.; Burroughs, A.K. Nutritional status and prognosis in cirrhotic patients. Aliment. Pharmacol. Ther. 2006, 24, 563–572. [Google Scholar] [CrossRef]
- Osaki, Y.; Nishikawa, H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol. Res. 2015, 45, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhong, J.H.; Su, Z.Y.; Huang, J.F.; Lu, S.D.; Xiang, B.D.; Ma, L.; Qi, L.N.; Ou, B.N.; Li, L.Q. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br. J. Surg. 2016, 103, 725–734. [Google Scholar] [CrossRef]
- Toyoda, H.; Lai, P.B.; O’Beirne, J.; Chong, C.C.; Berhane, S.; Reeves, H.; Manas, D.; Fox, R.P.; Yeo, W.; Mo, F.; et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: Application of the ALBI grade. Br. J. Cancer 2016, 114, 744–750. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Michitaka, K.; Toyoda, H.; Tada, T.; Ueki, H.; Kaneto, M.; Aibiki, T.; Okudaira, T.; Kawakami, T.; et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2016, 31, 1031–1036. [Google Scholar] [CrossRef]
- Edeline, J.; Blanc, J.F.; Johnson, P.; Campillo-Gimenez, B.; Ross, P.; Ma, Y.T.; King, J.; Hubner, R.A.; Sumpter, K.; Darby, S.; et al. A multicentre comparison between Child Pugh and Albumin-Bilirubin scores in patients treated with sorafenib for Hepatocellular Carcinoma. Liver Int. 2016, 36, 1821–1828. [Google Scholar] [CrossRef]
- Fujita, K.; Oura, K.; Yoneyama, H.; Ting, T.S.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Nomura, T.; Morishita, A.; Tsutsui, K.; et al. Albumin-bilirubin score indicates liver fibrosis staging and prognosis in chronic hepatitis C patients. Hepatol. Res. 2019, 49, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Yan, X.; Li, M.; Xia, J.; Liu, Y.; Chen, Y.; Jia, B.; Zhu, L.; Zhu, C.; et al. Albumin-Bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig. Liver Dis. 2019, 51, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Lee, K.C.; Wang, Y.W.; Yang, Y.Y.; Hou, M.C.; Huo, T.I.; Lin, H.C. Correlation and prognostic accuracy between noninvasive liver fibrosismarkers and portal pressure in cirrhosis: Role of ALBI score. PLoS ONE 2018, 13, e0208903. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Association between Sarcopenia and Depression in Patients with Chronic Liver Diseases. J. Clin. Med. 2019, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Tsuji, K.; Takaguchi, K.; Itobayashi, E.; Kariyama, K.; Ochi, H.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Validation of Modified ALBI Grade for More Detailed Assessment of Hepatic Function in Hepatocellular Carcinoma Patients: A Multicenter Analysis. Liver Cancer 2019, 8, 121–129. [Google Scholar] [CrossRef]
- Grüngreiff, K. Zinc in liver disease. J. Trace Elem. Exp. Med. 2002, 15, 67–78. [Google Scholar] [CrossRef]
- Alker, W.; Haase, H. Zinc and Sepsis. Nutrients 2018, 10, 976. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Serum Zinc Concentration and Sarcopenia: A Close Linkage in Chronic Liver Diseases. J. Clin. Med. 2019, 8, 336. [Google Scholar] [CrossRef]
- Stamoulis, I.; Kouraklis, G.; Theocharis, S. Zinc and the liver: An active interaction. Dig. Dis. Sci. 2007, 52, 1595–1612. [Google Scholar] [CrossRef]
- Wastney, M.E.; Aamodt, R.L.; Rumble, W.F.; Henkin, R.I. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am. J. Physiol. 1986, 251, R398–R408. [Google Scholar] [CrossRef]
- Livingstone, C. Zinc: Physiology, deficiency, and parenteral nutrition. Nutr. Clin. Pract. 2015, 30, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M. Zinc Deficiency is Associated with Poor Glycemic Control. J. Coll. Physicians Surg. Pak. 2019, 29, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Shigefuku, R.; Iwasa, M.; Katayama, K.; Eguchi, A.; Kawaguchi, T.; Shiraishi, K.; Ito, T.; Suzuki, K.; Koreeda, C.; Ohtake, T.; et al. Hypozincemia is associated with human hepatocarcinogenesis in hepatitis C virus-related liver cirrhosis. Hepatol. Res. 2019, 49, 1127–1135. [Google Scholar] [CrossRef]
- Chavez-Tapia, N.C.; Cesar-Arce, A.; Barrientos-Gutiérrez, T.; Villegas-López, F.A.; Méndez-Sanchez, N.; Uribe, M. A systematic review and meta-analysis of the use of oral zinc in the treatment of hepatic encephalopathy. Nutr. J. 2013, 12, 74. [Google Scholar] [CrossRef]
- Kudo, M.; Zheng, R.Q.; Kim, S.R.; Okabe, Y.; Osaki, Y.; Iijima, H.; Itani, T.; Kasugai, H.; Kanematsu, M.; Ito, K.; et al. Diagnostic accuracy of imaging for liver cirrhosis compared to histologically proven liver cirrhosis. A multicenter collaborative study. Intervirology 2008, 51, 17–26. [Google Scholar] [CrossRef]
- Zarski, J.P.; Sturm, N.; Guechot, J.; Paris, A.; Zafrani, E.S.; Asselah, T.; Boisson, R.C.; Bosson, J.L.; Guyader, D.; Renversez, J.C.; et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: The ANRS HCEP-23 study. J. Hepatol. 2012, 56, 55–62. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Gurusamy, K.S.; Ntaoula, S.; Cholongitas, E.; Davidson, B.R.; Burroughs, A.K. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Hepatol. 2011, 54, 650–659. [Google Scholar] [CrossRef]
- Kumada, H.; Okanoue, T.; Onji, M.; Moriwaki, H.; Izumi, N.; Tanaka, E.; Chayama, K.; Sakisaka, S.; Takehara, T.; Oketani, M.; et al. Study Group for the Standardization of Treatment of Viral Hepatitis Including Cirrhosis, Ministry of Health, Labour and Welfare of Japan. Guidelines for the treatment of chronic hepatitis and cirrhosis due to hepatitis C virus infection for the fiscal year 2008 in Japan. Hepatol. Res. 2010, 40, 8–13. [Google Scholar]
- Kappus, M.R.; Mendoza, M.S.; Nguyen, D.; Medici, V.; McClave, S.A. Sarcopenia in Patients with Chronic Liver Disease: Can It Be Altered by Diet and Exercise? Curr. Gastroenterol. Rep. 2016, 18, 43. [Google Scholar] [CrossRef]
- Kokudo, N.; Hasegawa, K.; Akahane, M.; Igaki, H.; Izumi, N.; Ichida, T.; Uemoto, S.; Kaneko, S.; Kawasaki, S.; Ku, Y.; et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015, 45, 12464. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Kawaguchi, T.; Shiraishi, K.; Ito, T.; Suzuki, K.; Koreeda, C.; Ohtake, T.; Iwasa, M.; Tokumoto, Y.; Endo, R.; et al. The Prevalence and Implication of Zinc Deficiency in Patients with Chronic Liver Disease. J. Clin. Med. Res. 2018, 10, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Grungreiff, K.; Reinhold, D.; Wedemeyer, H. The role of zinc in liver cirrhosis. Ann. Hepatol. 2016, 15, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Tsuji, K.; Hiraoka, A.; Michitaka, K.; Deguchi, A.; Ishikawa, T.; Imai, M.; Ochi, H.; et al. Impact of albumin-bilirubin grade on survival in patients with hepatocellular carcinoma who received sorafenib: An analysis using time-dependent receiver operating characteristic. J. Gastroenterol. Hepatol. 2019, 34, 1066–1073. [Google Scholar] [CrossRef]
- Ueshima, K.; Nishida, N.; Hagiwara, S.; Aoki, T.; Minami, T.; Chishina, H.; Takita, M.; Minami, Y.; Ida, H.; Takenaka, M.; et al. Impact of Baseline ALBI Grade on the Outcomes of Hepatocellular Carcinoma Patients Treated with Lenvatinib: A Multicenter Study. Cancers 2019, 11, 952. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J. Cachexia Sarcopenia Muscle 2017, 8, 915–925. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Feld, J.J. What Are the Benefits of a Sustained Virologic Response to Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? Gastroenterology 2019, 156, 446–460.e2. [Google Scholar] [CrossRef]
- Pradat, P.; Virlogeux, V.; Trépo, E. Epidemiology and Elimination of HCV-Related Liver Disease. Viruses 2018, 10, 545. [Google Scholar] [CrossRef]
- Tong, M.J.; Pan, C.Q.; Han, S.B.; Lu, D.S.; Raman, S.; Hu, K.Q.; Lim, J.K.; Hann, H.W.; Min, A.D. An expert consensus for the management of chronic hepatitis B in Asian Americans. Aliment. Pharmacol. Ther. 2018, 47, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Stempniak, M.; Hostomska, Z.; Nodes, B.R.; Hostomsky, Z. The NS3 proteinase domain of hepatitis C virus is a zinccontaining enzyme. J. Virol. 1997, 71, 2881–2886. [Google Scholar] [PubMed]
- Tellinghuisen, T.L.; Marcotrigiano, J.; Gorbalenya, A.E.; Rice, C.M. The NS5A protein of hepatitis C virus is a zinc metalloprotein. J. Biol. Chem. 2004, 279, 48576–48587. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Okawa, O.; Shirahashi, R.; Tokutomi, N.; Tamano, M. Changes in serum zinc levels in hepatitis C patients before and after treatment with direct-acting antiviral agents. Hepatol. Res. 2019, 13409. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Cases (n = 441) |
|---|---|
| Age (years) | 66 (59.5, 72.5) |
| Gender, male/female | 239 (54.2)/202 (45.8) |
| Cause of liver disease, HBV/HCV/others | 46 (10.4)/253 (57.4)/142 (32.2) |
| Child–Pugh classification, A/B/C | 317 (71.9)/109 (24.7)/15 (3.4) |
| MELD score | 4.87 (2.20, 7.36) |
| Ascites, yes/no | 59 (13.4)/382 (86.6) |
| Hepatic encephalopathy, yes/no | 6 (1.4)/435 (98.6) |
| Body mass index (kg/m2) | 23.1 (20.5, 25.7) |
| Total bilirubin (mg/dL) | 1.0 (0.7, 1.5) |
| Serum albumin (g/dL) | 3.7 (3.2, 4.05) |
| ALBI score | −2.31 (−2.67, −1.92) |
| ALBI grade, 1/2/3 | 134 (30.4)/276 (62.6)/31 (7.0) |
| Prothrombin time (%) | 76.9 (66.0, 86.3) |
| Platelet count (×104/mm3) | 9.7 (6.9, 13.6) |
| AST (IU/l) | 39 (27, 60) |
| ALT (IU/l) | 30.5 (20, 49) |
| Total cholesterol (mg/dL) | 151.5 (129.25, 177) |
| Fasting blood glucose (mg/dL) | 102 (92.25, 118) |
| Serum creatinine (mg/dL) | 0.67 (0.57, 0.79) |
| Serum sodium (mmol/L) | 140 (138, 141) |
| Serum Zn level (μg/dL) | 65 (54, 74.4) |
| Serum Zn level classification, <130, ≥80 μg/dL/<80, ≥60 μg/dL/<60 μg/dL | 56 (12.7)/227 (51.5)/158 (35.8) |
| Branched-chain amino acid to tyrosine ratio | 4.13 (3.235, 5.375) |
| Serum ammonia (µg/dL) | 40 (29, 62) |
| FIB-4 index | 5.09 (3.18, 7.77) |
| Variables | r | p Value |
|---|---|---|
| Age | −0.10 | 0.0420 |
| Body mass index | −0.04 | 0.4015 |
| Total bilirubin | −0.27 | <0.0001 |
| Serum albumin | 0.62 | <0.0001 |
| ALBI score | −0.62 | <0.0001 |
| Prothrombin time | 0.35 | <0.0001 |
| Platelet count | 0.14 | 0.0031 |
| AST | −0.27 | <0.0001 |
| ALT | −0.13 | 0.0050 |
| Total cholesterol | 0.19 | <0.0001 |
| Fasting blood glucose | −0.06 | 0.1909 |
| Serum creatinine | −0.02 | 0.6695 |
| Serum sodium | 0.27 | <0.0001 |
| BTR | 0.42 | <0.0001 |
| Serum ammonia | −0.34 | <0.0001 |
| MELD score | −0.29 | <0.0001 |
| FIB-4 index | −0.32 | <0.0001 |
| Variables | n | p Value |
|---|---|---|
| Age ≥ 66 years, yes/no | 234/207 | 0.0010 |
| Gender, male/female | 239/202 | 0.1605 |
| HBV/HCV/others | 46/253/142 | 0.0140 |
| Child–Pugh classification, A/B/C | 317/109/15 | <0.0001 |
| Body mass index ≥ 23.1 kg/m2, yes/no | 221/220 | 0.0366 |
| Total bilirubin ≥ 1.0 mg/dL, yes/no | 253/188 | 0.0177 |
| Serum albumin ≥ 3.7 g/dL, yes/no | 232/209 | <0.0001 |
| ALBI grade, 1/2/3 | 134/276/31 | <0.0001 |
| MELD score ≥ 4.87, yes/no | 220/221 | 0.0003 |
| Prothrombin time ≥ 76.9%, yes/no | 221/220 | 0.0020 |
| Platelet count ≥ 9.7 × 104/mm3, yes/no | 223/216 | 0.0065 |
| AST ≥ 39 IU/l, yes/no | 224/216 | 0.4417 |
| ALT ≥ 30.5 IU/l, yes/no | 220/220 | 0.3604 |
| Total cholesterol ≥ 151.5 mg/dL, yes/no | 216/216 | 0.0969 |
| Fasting blood glucose ≥ 102 mg/dL, yes/no | 226/206 | 0.5393 |
| Serum creatinine ≥ 0.67 mg/dL, yes/no | 223/218 | 0.0010 |
| Serum sodium ≥ 140 mmol/L, yes/no | 266/175 | 0.0070 |
| Zn, <130, ≥ 80 μg/dL/<80, ≥ 60 μg/dL/<60 μg/dL | 56/227/158 | <0.0001 |
| BTR ≥ 4.13, yes/no | 220/217 | 0.0149 |
| Serum ammonia ≥ 40 μg/dL, yes/no | 227/199 | <0.0001 |
| FIB-4 index ≥ 5.09, yes/no | 220/219 | <0.0001 |
| Variables | Multivariate Analysis | ||
|---|---|---|---|
| HR | 95% CI | p Value | |
| Serum ammonia level ≥ 40 µg/dL | 1.995 | 1.540–2.583 | <0.0001 |
| FIB-4 index ≥ 5.09 | 1.210 | 0.926–1.582 | 0.1622 |
| MELD score ≥ 4.87 | 1.139 | 0.880–1.473 | 0.3234 |
| Serum sodium ≥ 140 mmol/L | 0.870 | 0.672–1.126 | 0.2894 |
| ALBI grade | |||
| Grade 1 | 1.000 | Reference | |
| Grade 2 | 1.127 | 0.524–2.429 | 0.7600 |
| Grade 3 | 1.540 | 1.163–2.041 | 0.0026 |
| Child–Pugh classification | |||
| Child–Pugh A | 1.000 | Reference | |
| Child–Pugh B | 1.042 | 0.715–1.519 | 0.8309 |
| Child–Pugh C | 2.409 | 0.791–7.339 | 0.1219 |
| Serum Zn classification by JSCN | |||
| Group A | 1.000 | Reference | |
| Group B | 2.003 | 1.438–2.790 | <0.0001 |
| Group C | 2.533 | 1.640–3.911 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Serum Zinc Level Classification System: Usefulness in Patients with Liver Cirrhosis. J. Clin. Med. 2019, 8, 2057. https://doi.org/10.3390/jcm8122057
Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, Ikeda N, Takashima T, Aizawa N, Takata R, et al. Serum Zinc Level Classification System: Usefulness in Patients with Liver Cirrhosis. Journal of Clinical Medicine. 2019; 8(12):2057. https://doi.org/10.3390/jcm8122057
Chicago/Turabian StyleNishikawa, Hiroki, Hirayuki Enomoto, Kazunori Yoh, Yoshinori Iwata, Yoshiyuki Sakai, Kyohei Kishino, Naoto Ikeda, Tomoyuki Takashima, Nobuhiro Aizawa, Ryo Takata, and et al. 2019. "Serum Zinc Level Classification System: Usefulness in Patients with Liver Cirrhosis" Journal of Clinical Medicine 8, no. 12: 2057. https://doi.org/10.3390/jcm8122057
APA StyleNishikawa, H., Enomoto, H., Yoh, K., Iwata, Y., Sakai, Y., Kishino, K., Ikeda, N., Takashima, T., Aizawa, N., Takata, R., Hasegawa, K., Ishii, N., Yuri, Y., Nishimura, T., Iijima, H., & Nishiguchi, S. (2019). Serum Zinc Level Classification System: Usefulness in Patients with Liver Cirrhosis. Journal of Clinical Medicine, 8(12), 2057. https://doi.org/10.3390/jcm8122057




