Association between Albumin–Globulin Ratio and Mortality in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Collection of Demographic, Medical, and Laboratory Data and Study Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics
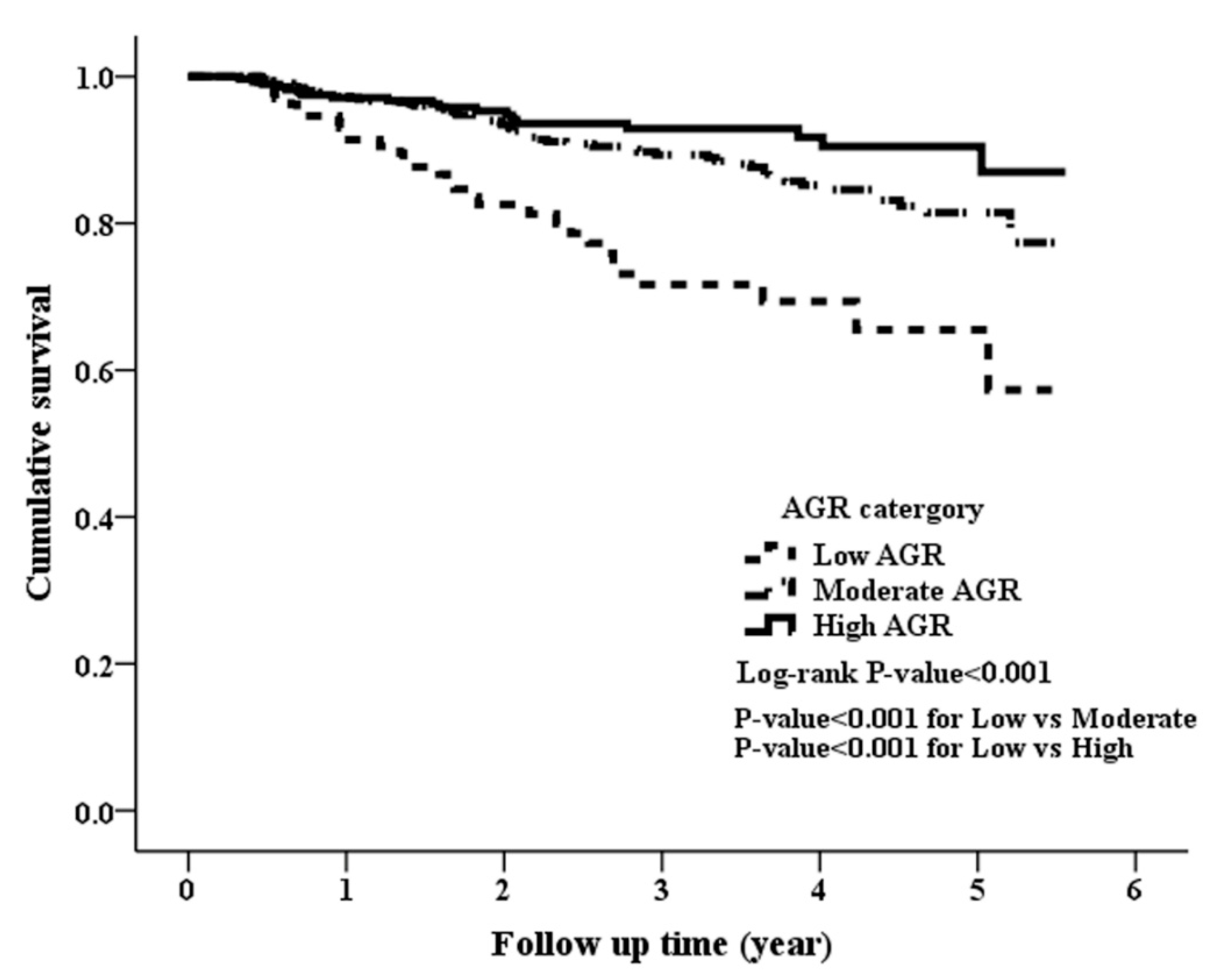
3.2. Clinical Outcomes among the Study Patients
3.3. Adjusted Associations of AGR Groups with Clinical Outcomes
3.4. Sensitivity Analysis
3.5. Stratified Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stevens, P.E.; O’Donoghue, D.J.; de Lusignan, S.; Van Vlymen, J.; Klebe, B.; Middleton, R.; Hague, N.; New, J.; Farmer, C.K. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007, 72, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Rothenbacher, D. Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health 2008, 8, 117. [Google Scholar] [CrossRef]
- Saran, R.; Li, Y.; Robinson, B.; Ayanian, J.; Balkrishnan, R.; Bragg-Gresham, J.; Chen, J.T.; Cope, E.; Gipson, D.; He, K.; et al. US Renal Data System 2014 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2015, 65, S1–S305. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Murray, A.M.; Li, S.; Herzog, C.A.; McBean, A.M.; Eggers, P.W.; Collins, A.J. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J. Am. Soc. Nephrol. 2005, 16, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Azab, B.; Kedia, S.; Shah, N.; Vonfrolio, S.; Lu, W.; Naboush, A.; Mohammed, F.; Bloom, S.W. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. Int. J. Colorectal Dis. 2013, 28, 1629–1636. [Google Scholar] [CrossRef]
- Azab, B.N.; Bhatt, V.R.; Vonfrolio, S.; Bachir, R.; Rubinshteyn, V.; Alkaied, H.; Habeshy, A.; Patel, J.; Picon, A.I.; Bloom, S.W. Value of the pretreatment albumin to globulin ratio in predicting long-term mortality in breast cancer patients. Am. J. Surg. 2013, 206, 764–770. [Google Scholar] [CrossRef]
- Du, X.J.; Tang, L.L.; Mao, Y.P.; Sun, Y.; Zeng, M.S.; Kang, T.B.; Jia, W.H.; Lin, A.H.; Ma, J. The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PLoS ONE 2014, 9, e94473. [Google Scholar] [CrossRef]
- Duran, A.O.; Inanc, M.; Karaca, H.; Dogan, I.; Berk, V.; Bozkurt, O.; Jia, W.H.; Lin, A.H.; Ma, J. Albumin-globulin ratio for prediction of long-term mortality in lung adenocarcinoma patients. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 6449–6453. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Kor, C.T.; Su, W.W.; Hsu, Y.C. Risk factors and prediction score for chronic pancreatitis: A nationwide population-based cohort study. World J. Gastroenterol. 2018, 24, 5034–5045. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Gatta, A.; Verardo, A.; Bolognesi, M. Hypoalbuminemia. Intern. Emerg. Med. 2012, 3, 193–199. [Google Scholar] [CrossRef]
- Caraceni, P.; Domenicali, M.; Tovoli, A.; Napoli, L.; Ricci, C.S.; Tufoni, M.; Bernardi, M. Clinical indications for the albumin use: Still a controversial issue. Eur. J. Intern. Med. 2013, 24, 721–728. [Google Scholar] [CrossRef]
- Seaton, K. Albumin concentration controls cancer. J. Natl. Med. Assoc. 2001, 93, 490–493. [Google Scholar]
- Kaysen, G.A. Serum albumin concentration in dialysis patients: Why does it remain resistant to therapy? Kidney Int. Suppl. 2003, 87, 92–98. [Google Scholar] [CrossRef]
- Das, U.N. Albumin to globulin ratio and/or plasma albumin in predicting long-term mortality. Am. J. Surg. 2014, 208, 157–158. [Google Scholar] [CrossRef]
- Busch, M.; Franke, S.; Müller, A.; Wolf, M.; Gerth, J.; Ott, U.; Niwa, T.; Stein, G. Potential cardiovascular risk factors in chronic kidney disease: AGEs, total homocysteine and metabolites, and the C-reactive protein. Kidney Int. 2004, 66, 338–347. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Panichi, V.; Paoletti, S.; Consani, C. Inflammatory pattern in hemodiafiltration. Contrib. Nephrol. 2008, 161, 185–190. [Google Scholar] [PubMed]
- Carrero, J.J.; Yilmaz, M.I.; Lindholm, B.; Stenvinkel, P. Cytokine dysregulation in chronic kidney disease: How can we treat it? Blood Purif. 2008, 26, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y. Consequences of chronic inflammation in peritoneal dialysis. Semin. Nephrol. 2011, 31, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, J.; Heimburger, O.; Lindholm, B.; Quereshi, A.R. Elevated serum C-reactive protein is strong predictor of increased mortality and low serum albumin in hemodialysis (HD) (abstract). J. Am. Soc. Nephrol. 1995, 6, 573. [Google Scholar]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, S.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef]
- Menon, V.; Greene, T.; Wang, X.; Pereira, A.A.; Marcovina, S.M.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Levey, A.S.; Sarnak, M.J. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005, 68, 766–772. [Google Scholar] [CrossRef]
- Jovanovic, D.B.; Stosović, M.D.; Gojakovic, B.M.; Jovanovic, N.Z.; Stanojevic-Stosovic, M.L.J.; Simic-Ogrizovic, S.P.; Naumovic, R.T. Inflammatory markers as mortality predictors in continuous ambulatory peritoneal dialysis patients. Ren. Fail. 2015, 37, 230–236. [Google Scholar] [CrossRef]
- Du, X.J.; Tang, L.L.; Mao, Y.P.; Guo, R.; Sun, Y.; Lin, A.H.; Ma, J. Circulating EBV DNA, Globulin and Nodal Size Predict Distant Metastasis after Intensity-Modulated Radiotherapy in Stage II Nasopharyngeal Carcinoma. J. Cancer. 2016, 20, 664–670. [Google Scholar] [CrossRef]
- Suh, B.; Park, S.; Shin, D.W.; Yun, J.M.; Keam, B.; Yang, H.K.; Ahn, E.; Lee, H.; Park, J.H.; Cho, B. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann. Oncol. 2014, 25, 2260–2266. [Google Scholar] [CrossRef]
- Cuppari, L.; Meireles, M.S.; Ramos, C.I.; Kamimura, M.A. Subjective global assessment for the diagnosis of protein-energy wasting in nondialysis-dependent chronic kidney disease patients. J. Ren. Nutr. 2014, 24, 385–389. [Google Scholar] [CrossRef]
- Sanches, F.M.; Avesani, C.M.; Kamimura, M.A.; Lemos, M.M.; Axelsson, J.; Vasselai, P.; Draibe, S.A.; Cuppari, L. Waist circumference and visceral fat in CKD: A cross-sectional study. Am. J. Kidney Dis. 2008, 52, 66–73. [Google Scholar] [CrossRef]
- Campbell, K.L.; Ash, S.; Davies, P.S.; Bauer, J.D. Randomized controlled trial of nutritional counseling on body composition and dietary intake in severe CKD. Am. J. Kidney Dis. 2008, 51, 748–758. [Google Scholar] [CrossRef]
- Amparo, F.C.; Kamimura, M.A.; Molnar, M.Z.; Cuppari, L.; Lindholm, B.; Amodeo, C.; Carrero, J.J.; Cordeiro, A.C. Diagnostic validation and prognostic significance of the Malnutrition-Inflammation Score in nondialyzed chronic kidney disease patients. Nephrol Dial. Transplant. 2015, 30, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]

| Low AGR | Moderate AGR | High AGR | p-Value | |
|---|---|---|---|---|
| (AGR < 1.0) | (1.0 ≤ AGR < 1.3) | (AGR ≥ 1.3) | ||
| Number of Patients | 138 | 535 | 283 | -- |
| Sex, men | 65 (47.1%) | 276 (51.59%) | 188 (66.43%) | <0.004 |
| Age (years) | 69.7 ± 12.8 | 68.6 ± 12.3 | 65.4 ± 13.6 | <0.001 |
| Body mass index (kg/m2) | 26.34 ± 5.23 | 25.34 ± 4.24 | 25.00 ± 4.1 | 0.011 |
| Smoking | ||||
| Never | 89 (64.49%) | 371 (69.35%) | 176 (62.19%) | 0.103 |
| Current | 15 (10.87%) | 60 (11.21%) | 37 (13.07%) | 0.694 |
| Ever | 34 (24.64%) | 104 (19.44%) | 70 (24.73%) | 0.147 |
| Alcohol | ||||
| Never | 108 (78.26%) | 419 (78.32%) | 194 (68.55%) | 0.006 |
| Current | 6 (4.35%) | 39 (7.29%) | 40 (14.13%) | 0.001 |
| Ever | 24 (17.39%) | 77 (14.39%) | 49 (17.31%) | 0.461 |
| CKD stages | ||||
| Stage 3 | 32 (23.19%) | 238 (44.49%) | 164 (57.95%) | <0.001 |
| Stage 4 | 62 (44.93%) | 183 (34.21%) | 79 (27.92%) | <0.001 |
| Stage 5 | 44 (31.88%) | 114 (21.31%) | 40 (14.13%) | <0.001 |
| Comorbidity Disease | ||||
| Gout | 32 (23.19%) | 141 (26.36%) | 81 (28.62%) | 0.489 |
| Hypertension | 107 (77.54%) | 395 (73.83%) | 201 (71.02%) | 0.354 |
| Diabetes mellitus | 86 (62.32%) | 270 (50.47%) | 105 (37.1%) | <0.001 |
| Cardiovascular disease | 70 (50.72%) | 208 (38.88%) | 82 (28.98%) | <0.001 |
| Chronic Lung disease | 27 (19.57%) | 72 (13.46%) | 31 (10.95%) | 0.053 |
| Medication use | ||||
| NSAID | 4 (2.9%) | 17 (3.18%) | 13 (4.59%) | 0.526 |
| ACEI/ARB | 83 (60.14%) | 310 (57.94%) | 152 (53.71%) | 0.368 |
| Statin | 66 (47.83%) | 244 (45.61%) | 120 (42.4%) | 0.523 |
| Erythropoiesis-stimulating agents | 23 (16.67%) | 68 (12.71%) | 20 (7.07%) | 0.008 |
| Vitamin D | 6 (4.35%) | 17 (3.18%) | 11 (3.89%) | 0.754 |
| Calcium supplement | 17 (12.32%) | 40 (7.48%) | 17 (6.01%) | 0.071 |
| Calcium channel blockers | 79 (57.25%) | 308 (57.57%) | 134 (47.35%) | 0.016 |
| Pentoxifylline | 72 (52.17%) | 308 (57.57%) | 183 (64.66%) | 0.032 |
| Laboratory data | ||||
| AGR | 0.8 (0.7–0.9) | 1.2 (1.1–1.2) | 1.5 (1.4–1.6) | <0.001 |
| Albumin (g/dL) | 3.2 (2.8–3.4) | 3.7 (3.5–3.9) | 4 (3.8–4.2) | <0.001 |
| Blood urea nitrogen (mg/dL) | 40 (29–53) | 32 (25–50) | 28 (23–39) | <0.001 |
| Calcium (mg/dL) | 8.9 (8.5–9.2) | 9.2 (8.9–9.4) | 9.3 (9–9.5) | <0.001 |
| Cholesterol (mg/dL) | 170.5 (142–207) | 174 (148–200) | 177 (149–204) | 0.625 |
| Creatinine (mg/dL) | 2.76 (2.01–4.38) | 2.18 (1.66–3.34) | 1.95 (1.67–2.75) | <0.001 |
| eGFR (mL/min/1.73 m2) | 20.82 (11.67–28.44) | 27.75 (16.38–36.13) | 33.39 (21.29–39.39) | <0.001 |
| Hemoglobin (g/dL) | 9.95 (9.1–11.2) | 11.1 (9.7–12.5) | 12.1 (10.5–13.5) | <0.001 |
| HbA1c (%) | 6.2 (5.7–7.3) | 6 (5.6–6.9) | 5.8 (5.5–6.3) | <0.001 |
| Potassium (mEq/L) | 4.3 (3.8–4.7) | 4.4 (4.1–4.8) | 4.4 (4.1–4.7) | 0.024 |
| Sodium (mEq/L) | 138 (136–139) | 139 (137–140) | 139 (138–140) | <0.001 |
| Phosphate (mg/dL) | 4.3 (3.7–4.7) | 4 (3.6–4.6) | 3.8 (3.5–4.3) | <0.001 |
| Triglyceride (mg/dL) | 112.5 (81–174) | 119 (87–171) | 131 (95–188) | 0.042 |
| UPCR (mg/g) | 1822.05 (584.5–5290.9) | 943.3 (269.4–2215.5) | 430 (137.2–1403.5) | <0.001 |
| Uric Acid (mg/dL) | 7.8 (6.4–8.7) | 7.5 (6.5–8.5) | 7.2 (6.4–8.1) | 0.014 |
| WBC (× 103/μL) | 7.3 (5.9–9) | 6.7 (5.6–8) | 6.4 (5.4–7.8) | 0.002 |
| AGR Category †: Low AGR Group: AGR ≤ 1.0 (as Reference Group); Moderate AGR Group: 1.1 ≤ AGR < 1.3; High AGR Group: AGR ≥ 1.3 | All-Cause Mortality | CVD Mortality | |||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | ||
| (A)Crude model | Low | 1 | 1 | ||
| Moderate | 0.56 (0.43,0.74) | <0.001 | 0.61 (0.41,0.91) | 0.014 | |
| High | 0.46 (0.34,0.63) | <0.001 | 0.27 (0.16,0.46) | <0.001 | |
| Model 1 | Low | 1 | 1 | ||
| Moderate | 0.57 (0.36,0.9) | 0.016 | 0.52 (0.28,0.98) | 0.043 | |
| High | 0.49 (0.27,0.9) | 0.021 | 0.27 (0.1,0.74) | 0.010 | |
| Model 2 | Low | 1 | |||
| Moderate | 0.72 (0.54,0.97) | 0.028 | 0.78 (0.52,1.18) | 0.237 | |
| High | 0.72 (0.52,0.99) | 0.046 | 0.46 (0.27,0.8) | 0.006 | |
| (B) sensitivity tests | |||||
| (i) AGR as a continuous variable in model 1 | -- | 0.27 (0.13,0.61) | 0.001 | 0.21 (0.07,0.67) | 0.009 |
| (ii) AGR as a continuous variable in model 2 | -- | 0.49 (0.32,0.78) | 0.002 | 0.37 (0.19,0.72) | 0.003 |
| Maximum Standardization Difference Between Groups | ||
|---|---|---|
| Before IPW a (%) | After IPW a (%) | |
| Gender, male | 0.259 | 0.180 * |
| Age (years) | 0.224 | 0.035 |
| Body mass index | 0.205 | 0.080 |
| Smoking | 0.101 | 0.016 |
| Alcohol | 0.113 | 0.071 |
| Comorbidity disease | ||
| Gout | 0.082 | 0.032 |
| Hypertension | 0.099 | 0.036 |
| Diabetes mellitus | 0.336 | 0.076 |
| Cardiovascular disease | 0.299 | 0.109 * |
| Chronic Lung disease | 0.167 | 0.094 |
| Medication use | ||
| NSAID | 0.061 | 0.012 |
| ACEI/ARB | 0.086 | 0.053 |
| Statin | 0.073 | 0.059 |
| Erythropoiesis-stimulating agents | 0.199 | 0.047 |
| Vitamin D | 0.042 | 0.040 |
| Calcium supplement | 0.157 | 0.021 |
| Calcium channel blockers | 0.137 | 0.084 |
| Pentoxifylline | 0.169 | 0.119* |
| Subgroup | All-Cause Mortality | CVD Mortality | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Interaction | Hazard Ratio (95% CI) | P Interaction | |
| Male | 0.24(0.13,0.44) | 0.918 | 0.13(0.03,0.64) | 0.929 |
| Female | 0.33(0.09,1.15) | 0.17(0.03,1.01) | ||
| Age < 65 (years) | 0.08(0.01,0.55) | 0.793 | 0.35(0.01,10.78) | 0.834 |
| Age ≧ 65 (years) | 0.50(0.20,1.22) | 0.25(0.07,0.93) | ||
| DM | 0.36(0.12,1.09) | 0.157 | 0.314(0.06,1.61) | 0.742 |
| No DM | 0.21(0.07,0.66) | 0.112(0.02,0.62) | ||
| Hypertension | 0.23(0.08,0.64) | 0.560 | 0.113(0.03,0.51) | 0.252 |
| No hypertension | 0.30(0.08,1.15) | 0.339(0.06,1.84) | ||
| Gout | 0.56(0.11,2.97) | 0.459 | 0.363(0.03,4.39) | 0.792 |
| No gout | 0.16(0.07,0.39) | 0.153(0.04,0.56) | ||
| Chronic lung disease | 0.35(0.09,1.42) | 0.405 | 1.448(0.14,15.26) | 0.130 |
| No chronic lung disease | 0.24(0.10,0.62) | 0.116(0.03,0.42) | ||
| CVD | 0.47(0.17,1.30) | 0.108 | 0.37(0.08,1.7) | 0.895 |
| No CVD | 0.17(0.05,0.58) | 0.125(0.02,0.87) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-P.; Hsieh, Y.-P.; Kor, C.-T.; Chiu, P.-F. Association between Albumin–Globulin Ratio and Mortality in Patients with Chronic Kidney Disease. J. Clin. Med. 2019, 8, 1991. https://doi.org/10.3390/jcm8111991
Wu P-P, Hsieh Y-P, Kor C-T, Chiu P-F. Association between Albumin–Globulin Ratio and Mortality in Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2019; 8(11):1991. https://doi.org/10.3390/jcm8111991
Chicago/Turabian StyleWu, Pin-Pin, Yao-Peng Hsieh, Chew-Teng Kor, and Ping-Fang Chiu. 2019. "Association between Albumin–Globulin Ratio and Mortality in Patients with Chronic Kidney Disease" Journal of Clinical Medicine 8, no. 11: 1991. https://doi.org/10.3390/jcm8111991
APA StyleWu, P.-P., Hsieh, Y.-P., Kor, C.-T., & Chiu, P.-F. (2019). Association between Albumin–Globulin Ratio and Mortality in Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 8(11), 1991. https://doi.org/10.3390/jcm8111991




