Real-World Experience of Olaparib Maintenance in High-Grade Serous Recurrent Ovarian Cancer Patients with BRCA1/2 Mutation: A Korean Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Treatment and Study Assessments
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Exposure
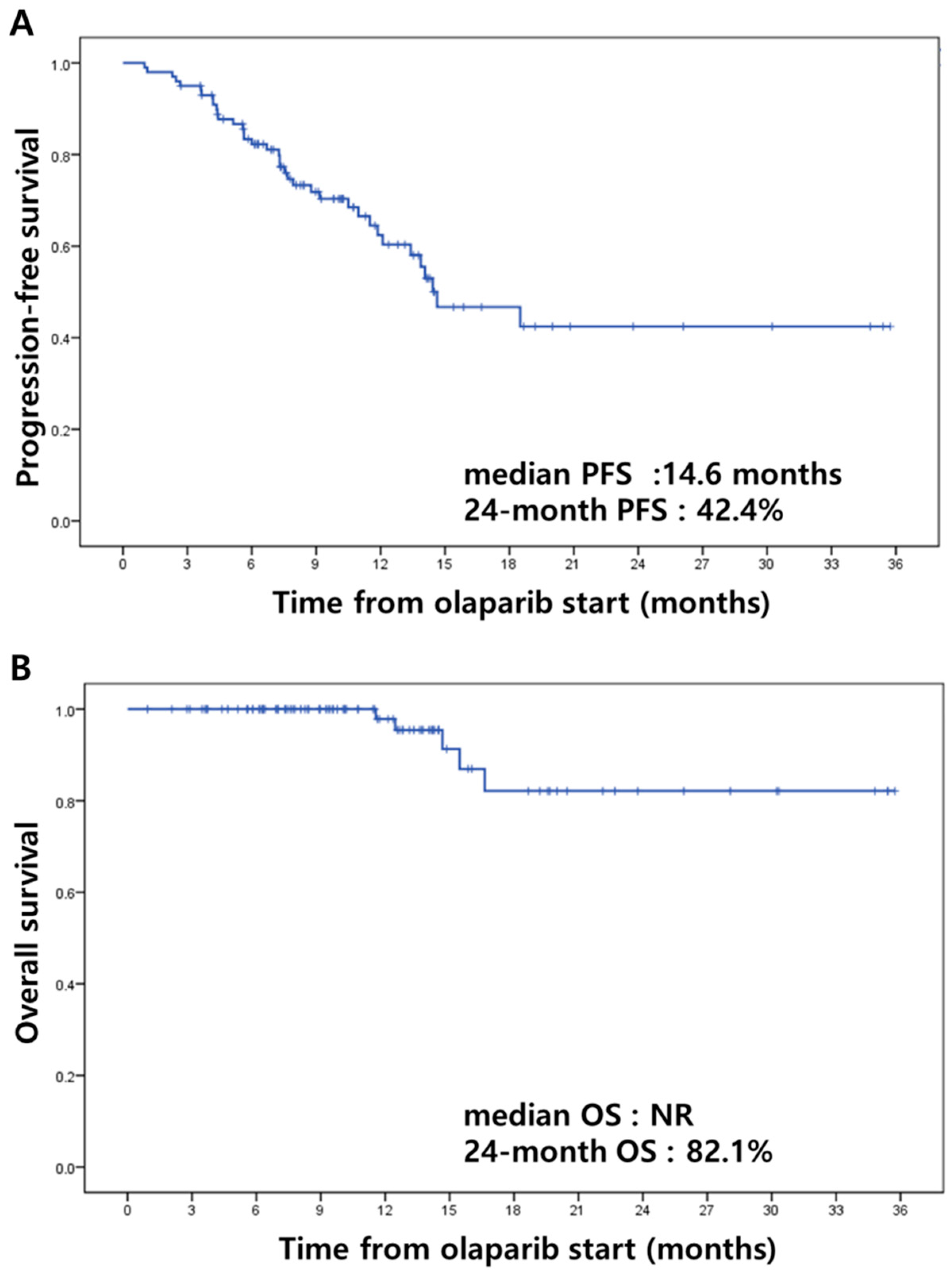
3.3. Efficacy
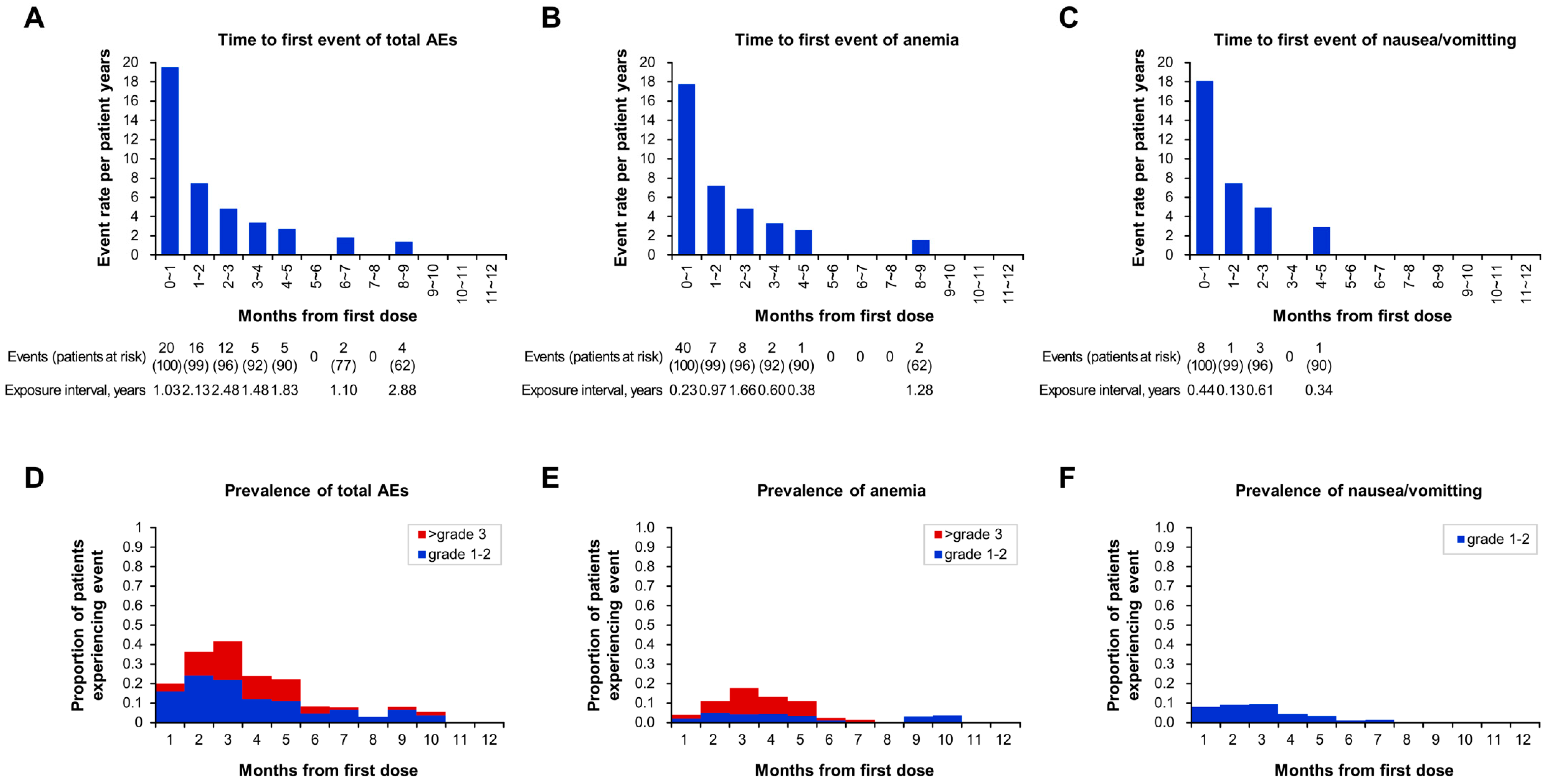
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Hanker, L.C.; Loibl, S.; Burchardi, N.; Pfisterer, J.; Meier, W.; Pujade-Lauraine, E.; Ray-Coquard, I.; Sehouli, J.; Harter, P.; du Bois, A. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2012, 23, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. New Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Evers, B.; Drost, R.; Schut, E.; de Bruin, M.; van der Burg, E.; Derksen, P.W.; Holstege, H.; Liu, X.; van Drunen, E.; Beverloo, H.B.; et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 3916–3925. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.; Zander, S.A.; Derksen, P.W.; de Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.; Gebski, V.; Gibbs, E.; Davies, L.; Bloomfield, R.; Hilpert, F.; Wenzel, L.B.; Eek, D.; Rodrigues, M.; Clamp, A.; et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): A placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018, 19, 1126–1134. [Google Scholar] [CrossRef]
- Friedlander, M.; Matulonis, U.; Gourley, C.; du Bois, A.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br. J. Cancer 2018, 119, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Friedlander, M.; Shannon, C.; Goh, J.; Scott, C.; Mileshkin, L. Practical considerations for clinicians for transitioning patients on maintenance therapy with olaparib capsules to the tablet formulation of olaparib. Asia Pac. J. Clin. Oncol. 2018, 14, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, S.; Kim, J.H. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J. Korean Med. Sci. 2018, 33, e213. [Google Scholar] [CrossRef] [PubMed]
- Garrison, L.P., Jr.; Neumann, P.J.; Erickson, P.; Marshall, D.; Mullins, C.D. Using real-world data for coverage and payment decisions: The ISPOR Real-World Data Task Force report. Value Health J. Int. Soc. Pharm. Outcomes Res. 2007, 10, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, N.; Jahnz-Różyk, K. The evolving landscape for real world evidence in Poland: physicians’ perspective. J. Health Policy Outcomes Res. 2015, 15–33. [Google Scholar] [CrossRef]
| Characteristics | N = 100 |
|---|---|
| Age, years | |
| Median (Range) | 54 (29~79) |
| Initial FIGO stage, n (%) | |
| IA | 1 (1.0) |
| IB | 1 (1.0) |
| IC | 6 (6.0) |
| IIA | 1 (1.0) |
| IIB | 3 (3.0) |
| IIIA | 2 (2.0) |
| IIIB | 6 (6.0) |
| IIIC | 47 (47.0) |
| IV | 33 (33.0) |
| Initial residual status, n (%) | |
| No residual | 41 (41.0) |
| 0.1~1 cm | 45 (45.0) |
| >1 cm | 13 (13.0) |
| Initial CA-125 level | 720.5 (14.2~11,552.2) |
| Concurrent breast cancer, n (%) | 18 (18.0) |
| Family history of breast and ovarian cancer, n (%) | 23 (23.0) |
| Platinum-free interval Duration, n (%) | |
| 6–12 months | 32 (32.0) |
| >12 months | 68 (68.0) |
| Median (range), months | 14.6 (6.0~86.3) |
| Objective response to most recent chemotherapy, n (%) | |
| Complete | 46 (46.0) |
| Partial | 53 (53.0) |
| Unknown | 1 (1.0) |
| Number of previous chemotherapy regimen, n (%) | |
| 2 | 63 (63.0) |
| 3 | 26 (26.0) |
| 4 | 7 (7.0) |
| ≥5 | 4 (4.0) |
| Median (range) | 2 (2~13) |
| Previous bevacizumab exposure, n (%) | 16 (16.0) |
| BRCA mutation status, n (%) | |
| Germline | |
| BRCA1 | 69 (69.0) |
| BRCA2 | 24 (2.0) |
| BRCA1/2 | 1 (1.0) |
| Somatic | |
| BRCA1 | 3 (3.0) |
| BRCA2 | 1 (1.0) |
| BRCA1/2 | 2 (2.0) |
| Outcomes | |
|---|---|
| Observation period after olaparib | |
| Median (range), months | 10.2 (1.0~35.7) |
| Olaparib maintenance period | |
| Olaparib users/total patients at time period (%) | |
| ≥6 months | 53/85 (62.4) |
| ≥12 months | 23/43 (53.5) |
| ≥24 months | 4/8 (50.0) |
| Best overall response with olaparib in patients with previous PR from most recent chemotherapy, n (%) | N = 53 |
| CR | 12 (22.6) |
| PR | 4 (7.5) |
| SD | 33 (62.3) |
| PD | 2 (3.8) |
| NE | 2 (3.8) |
| Type of dose modification due to AE, number of patients (%) | N = 100 |
| Dose reduction | 36 (36.0) |
| Drug discontinuation | 4 (4.0) |
| Treatment applied other than dose reduction | 11 (11.0) |
| Recurrence event, n (%) | 37 (37.0) |
| Death event, n (%) | 5 (5.0) |
| Total episodes, n (%) | Grade | Characterization of AEs | Management | |||||
|---|---|---|---|---|---|---|---|---|
| Grade 1–2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Median time to onset of first event, months, median (range) | Dose reduction, n (%) | Drug discontinuation, n (%) | Treatment applied with continuation (without dose reduction), n (%) | ||
| Any adverse event | 64 (64.0) | 39 (39.0) | 19 (19.0) | 6 (6.0) | 1.9 (0.1~8.8) | 41 (41.0) | 8 (6.0) | 15 (15.0) |
| Hematological | 37 (37.0) | 14 (14.0) | 17 (17.0) | 6 (6.0) | 2.1 (0.1~8.8) | 22 (22.0) | 6 (6.0) | 9 (9.0) |
| Anemia | 24 (24.0) | 10 (10.0) | 8 (8.0) | 6 (6.0) | 2.1 (0.7~2.8) | 15 (14.0) | 3 (3.0) | 6 (6.0) |
| Neutropenia | 7 (7.0) | 7 (7.0) | 1.8 (0.1~8.8) | 4 (4.0) | 2 (2.0) | 1 (1.0) | ||
| Thrombocytopenia | 4 (4.0) | 2 (2.0) | 2 (2.0) | 2.5 (0.1~3.5) | 3 (3.0) | 1 (1.0) | ||
| Serum ALT/AST elevated | 2 (2.0) | 2 (2.0) | 4.6 | 2 (2.0) | ||||
| Non hematological | 27 (27.0) | 25 (25.0) | 2 (2.0) | 1.6 (0.4~6.7) | 19 (19.0) | 2 (2.0) | 6 (6.0) | |
| Nausea/vomiting | 13 (13.0) | 13 (13.0) | 2.5 (0.4~4.1) | 9 (9.0) | 1 (1.0) | 3 (3.0) | ||
| Fatigue | 6 (6.0) | 6 (6.0) | 1.9 (0.5~6.7) | 5 (5.0) | 1 (1.0) | |||
| Oral mucositis | 2 (2.0) | 1 (1.0) | 1(1.0) | 4.2 | 2 (2.0) | |||
| Peripheral neuropathy | 2 (2.0) | 2 (2.0) | 1.4 | 1 (1.0) | 1 (1.0) | |||
| Urticaria | 1 (1.0) | 1 (1.0) | 0.8 | 1 (1.0) | ||||
| Dizziness | 1 (1.0) | 1 (1.0) | 4.7 | 1 (1.0) | ||||
| Headache | 1 (1.0) | 1 (1.0) | 2.5 | 1 (1.0) | ||||
| Soft tissue infection | 1 (1.0) | 1(1.0) | 1.7 | 1 (1.0) |
| Number of Episodes, n (%) | Reduction Dose, n (%) | After Dose Reduction Initiation, n (%) | Median Time to Onset of First Event, Months, Median (Range) | DR Duration, Months, Median (Range) | ||||
|---|---|---|---|---|---|---|---|---|
| 25% DR | 50% DR | Dose normalized after AE resolved | Maintain DR | Discontinuation after DR | ||||
| Any adverse event | 41 (100.0) | 20 (48.8) | 21 (51.2) | 8 (19.5) | 28 (68.3) | 5 (12.2) | 1.9 (0.1~6.7) | 6.5 (1.0~32.4) |
| Grade 1–2 | 24 (100.0) | 15 (62.5) | 9 (37.5) | 3 (12.5) | 19 (79.2) | 2 (8.3) | 1.9 (0.1~6.7) | 5.6 (1.0~32.4) |
| Grade 3 | 12 (100.0) | 3 (25.0) | 9 (75.0) | 4 (33.3) | 5 (41.7) | 3 (25.0) | 1.6 (0.1~4.6) | 5.1 (1.0~30.8) |
| Grade 4 | 5 (100.0) | 2 (40.0) | 3 (60.0) | 1 (20.0) | 4 (80.0) | 0 | 2.1 (1.7~2.8) | 8.1 (1.2~11.1) |
| Hematological | 22 (100.0) | 8 (36.4) | 14 (63.6) | 6 (27.3) | 12 (54.5) | 4 (18.2) | 1.8 (0.1~4.6) | 5.4 (1.0~30.8) |
| Grade 1–2 | 5 (100.0) | 3 (60.0) | 2 (40.0) | 1 (20.0) | 3 (60.0) | 1 (20.0) | 1.8 (0.1~3.7) | 4.0 (1.0~8.3) |
| Grade 3 | 12 (100.0) | 3 (25.0) | 9 (75.0) | 4 (33.3) | 5 (41.7) | 3 (25.0) | 1.6 (0.1~4.6) | 5.1 (1.0~30.8) |
| Grade 4 | 5 (100.0) | 2 (40.0) | 3 (60.0) | 1 (20.0) | 4 (80.0) | 0 | 2.1 (1.7~2.8) | 8.1 (1.2~11.1) |
| Non hematological | 19 (100.0) | 12 (63.2) | 7 (36.8) | 2 (10.5) | 16 (84.2) | 1 (5.3) | 1.9 (0.4~6.7) | 7.2 (1.0~32.4) |
| Grade 1–2 | 19 (100.0) | 12 (63.2) | 7 (36.8) | 2 (10.5) | 16 (84.2) | 1 (5.3) | 1.9 (0.4~6.7) | 7.2 (1.0~32.4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paik, E.S.; Lee, Y.J.; Lee, J.-Y.; Shin, W.; Park, S.-Y.; Kim, S.I.; Kim, J.-W.; Choi, C.H.; Kim, B.-G. Real-World Experience of Olaparib Maintenance in High-Grade Serous Recurrent Ovarian Cancer Patients with BRCA1/2 Mutation: A Korean Multicenter Study. J. Clin. Med. 2019, 8, 1920. https://doi.org/10.3390/jcm8111920
Paik ES, Lee YJ, Lee J-Y, Shin W, Park S-Y, Kim SI, Kim J-W, Choi CH, Kim B-G. Real-World Experience of Olaparib Maintenance in High-Grade Serous Recurrent Ovarian Cancer Patients with BRCA1/2 Mutation: A Korean Multicenter Study. Journal of Clinical Medicine. 2019; 8(11):1920. https://doi.org/10.3390/jcm8111920
Chicago/Turabian StylePaik, E Sun, Yong Jae Lee, Jung-Yun Lee, Wonkyo Shin, Sang-Yoon Park, Se Ik Kim, Jae-Weon Kim, Chel Hun Choi, and Byoung-Gie Kim. 2019. "Real-World Experience of Olaparib Maintenance in High-Grade Serous Recurrent Ovarian Cancer Patients with BRCA1/2 Mutation: A Korean Multicenter Study" Journal of Clinical Medicine 8, no. 11: 1920. https://doi.org/10.3390/jcm8111920
APA StylePaik, E. S., Lee, Y. J., Lee, J.-Y., Shin, W., Park, S.-Y., Kim, S. I., Kim, J.-W., Choi, C. H., & Kim, B.-G. (2019). Real-World Experience of Olaparib Maintenance in High-Grade Serous Recurrent Ovarian Cancer Patients with BRCA1/2 Mutation: A Korean Multicenter Study. Journal of Clinical Medicine, 8(11), 1920. https://doi.org/10.3390/jcm8111920






