Correlations between Molecular Landscape and Sonographic Image of Different Variants of Papillary Thyroid Carcinoma
Abstract
1. Introduction
2. Thyroid Tumor Classifications
2.1. New Aspects of Pathological Classifications of Thyroid Carcinoma
2.2. Sonographic Classifications of Thyroid Nodules
3. Genetic Drivers of Thyroid Carcinoma
4. Genetic Drivers and Sonographic Pattern of Different Variants of PTC
4.1. BRAF Mutations
4.2. RET/PTC Rearrangements
4.3. RAS and PAX8-PPARγ Mutations
4.4. TERT Promoter Mutations
4.5. Other Mutations in Different Variants of PTC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACR | American College of Radiology |
| ATA | American Thyroid Association |
| DSV | Diffuse sclerosing variant |
| EFV | Encapsulated follicular variant |
| ETA | European Thyroid Association |
| FAP | Familial adenomatous polyposis |
| FNAB | Fine needle aspiration biopsy |
| FV | Follicular variant |
| NIFTP | Non-invasive follicular thyroid neoplasm with papillary-like nuclear features |
| PTC | Papillary thyroid carcinoma |
| US | Ultrasound |
| WV | Warthin-like variant |
References
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek-Parulska, E.; Woliński, K.; Stangierski, A.; Gurgul, E.; Biczysko, M.; Majewski, P.; Rewaj-Łosyk, M.; Ruchała, M. Comparison of diagnostic value of conventional ultrasonography and shear wave elastography in the prediction of thyroid lesions malignancy. PLoS ONE 2013, 8, e81532. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Adamczewski, Z.; Lewiński, A. Proposed algorithm for management of patients with thyroid nodules/focal lesions, based on ultrasound (US) and fine-needle aspiration biopsy (FNAB); our own experience. Thyroid Res. 2013, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Lewiński, A.; Adamczewski, Z. Ultrasound and cytological diagnostics of thyroid—Its proper application in case of coexisting disturbing clinical signs and symptoms, suggestive of active proliferative lesion. Thyroid Res. 2015, 8 (Suppl. 1), A19. [Google Scholar] [CrossRef]
- Lewiński, A.; Adamczewski, Z. Decision making for surgery in the suspect thyroid nodule. (Proper application of ultrasound (US) and fine needle aspiration biopsy (FNAB) completed but do not replace coexisting worrying clinical signs and symptoms). Thyroid Int. 2013, 1, 3–18. [Google Scholar]
- Stasiak, M.; Tymoniuk, B.; Adamczewski, Z.; Stasiak, B.; Lewiński, A. Sonographic pattern of subacute thyroiditis is HLA-dependent. Front. Endocrinol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO classification of tumours of the thyroid gland. In WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Shin, J.H. Ultrasonographic imaging of papillary thyroid carcinoma variants. Ultrasonography 2017, 36, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P.; AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 Update. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Dobruch-Sobczak, K.S.; Krauze, A.; Migda, B.; Mlosek, K.; Słapa, R.Z.; Bakuła-Zalewska, E.; Adamczewski, Z.; Lewiński, A.; Jakubowski, W.; Dedecjus, M. Integration of sonoelastography into the TIRADS lexicon could influence the classification. Front. Endocrinol. 2019, 10, 127. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Ohori, N.P.; Hodak, S.P.; Carty, S.E.; LeBeau, S.O.; Ferris, R.L.; Yip, L.; Seethala, R.R.; Tublin, M.E.; Stang, M.T.; et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metab. 2011, 96, 3390–3397. [Google Scholar] [CrossRef]
- Menicali, E.; Moretti, S.; Voce, P.; Romagnoli, S.; Avenia, N.; Puxeddu, E. Intracellular signal transduction and modification of the tumor microenvironment induced by RET/PTCs in papillary thyroid carcinoma. Front. Endocrinol. 2012, 3, 67. [Google Scholar] [CrossRef]
- Colombo, C.; Muzza, M.; Proverbio, M.C.; Tosi, D.; Soranna, D.; Pesenti, C.; Rossi, S.; Cirello, V.; De Leo, S.; Fusco, N.; et al. Impact of mutation density and heterogeneity on papillary thyroid cancer clinical features and remission probability. Thyroid 2019, 29, 237–251. [Google Scholar] [CrossRef]
- Adeniran, A.J.; Zhu, Z.; Gandhi, M.; Steward, D.L.; Fidler, J.P.; Giordano, T.J.; Biddinger, P.W.; Nikiforov, Y.E. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am. J. Surg. Pathol. 2006, 30, 216–222. [Google Scholar] [CrossRef]
- Yip, L. Molecular markers for thyroid cancer diagnosis, prognosis, and targeted therapy. J. Surg. Oncol. 2015, 111, 43–50. [Google Scholar] [CrossRef]
- Trovisco, V.; Vieira de Castro, I.; Soares, P.; Maximo, V.; Silva, P.; Magalhaes, J.; Abrosimov, A.; Guiu, X.M.; Sobrinho-Simões, M. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J. Pathol. 2004, 202, 247–251. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Sun, S.; Li, X.; Zeng, W.; Xiong, Y.; Guo, Y.; Wang, J.; Wang, Y.; Liu, C.; et al. Ultrasound features suspicious for malignancy predict the risk of BRAF mutation in papillary thyroid carcinoma. Int. J. Clin. Exp. Med. 2017, 10, 9470–9475. [Google Scholar]
- Lee, E.J.; Song, K.H.; Kim, D.L.; Jang, Y.M.; Hwang, T.S.; Kim, S.K. The BRAF(V600E) mutation is associated with malignant ultrasonographic features in thyroid nodules. Clin. Endocrinol. 2011, 75, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Khadra, H.; Deniwar, A.; Mohsin, K.; Monlezun, D.; Kandil, E. Can suspicious ultrasound features predict BRAFV600E status in papillary thyroid cancer? Eur. Thyroid J. 2018, 7, 205–210. [Google Scholar] [CrossRef]
- Rossi, M.; Buratto, M.; Tagliati, F.; Rossi, R.; Lupo, S.; Trasforini, G.; Lanza, G.; Franceschetti, P.; Bruni, S.; Degli Uberti, E.; et al. Relevance of BRAF(V600E) mutation testing versus RAS point mutations and RET/PTC rearrangements evaluation in the diagnosis of thyroid cancer. Thyroid 2015, 25, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kabaker, A.S.; Tublin, M.E.; Nikiforov, Y.E.; Armstrong, M.J.; Hodak, S.P.; Stang, M.T.; McCoy, K.L.; Carty, S.E.; Yip, L. Suspicious ultrasound characteristics predict BRAF V600E-positive papillary thyroid carcinoma. Thyroid 2012, 22, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Kwak, J.Y.; Kim, E.K.; Kim, M.J. A taller-than-wide shape in thyroid nodules in transverse and longitudinal ultrasonographic planes and the prediction of malignancy. Thyroid 2011, 21, 1249–1253. [Google Scholar] [CrossRef]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef]
- Park, A.Y.; Son, E.J.; Kim, J.A.; Youk, J.H.; Park, Y.J.; Park, C.S.; Chang, H.S. Associations of the BRAFV600E mutation with sonographic features and clinicopathologic characteristics in a large population with conventional papillary thyroid carcinoma. PLoS ONE 2014, 9, e110868. [Google Scholar] [CrossRef]
- Fakhruddin, N.; Jabbour, M.; Novy, M.; Tamim, H.; Bahmad, H.; Farhat, F.; Zaatari, G.; Aridi, T.; Kriegshauser, G.; Oberkanins, C.; et al. BRAF and NRAS mutations in papillary thyroid carcinoma and concordance in BRAF mutations between primary and corresponding lymph node metastases. Sci. Rep. 2017, 5, 4666. [Google Scholar] [CrossRef]
- Baek, H.J.; Kim, D.W.; Shin, G.W.; Heo, Y.J.; Baek, J.W.; Lee, Y.J.; Cho, Y.J.; Park, H.K.; Ha, T.K.; Kim, D.H.; et al. Ultrasonographic features of papillary thyroid carcinomas according to their subtypes. Front. Endocrinol. 2018, 9, 223. [Google Scholar] [CrossRef]
- Chen, J.H.; Faquin, W.C.; Lloyd, R.V.; Nosé, V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod. Pathol. 2011, 24, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Watutantrige-Fernando, S.; Vianello, F.; Barollo, S.; Bertazza, L.; Galuppini, F.; Cavedon, E.; Censi, S.; Benna, C.; Ide, E.C.; Parisi, A.; et al. The hobnail variant of papillary thyroid carcinoma: Clinical/molecular characteristics of a large monocentric series and comparison with conventional histotypes. Thyroid 2018, 28, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.R.; Shin, J.H.; Hahn, S.Y.; Ko, E.Y.; Oh, Y.L. Ultrasonographic features and clinical characteristics of Warthin-like variant of papillary thyroid carcinoma. Endocr. J. 2016, 63, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Koo, J.S.; Kim, E.K.; Lee, S. Clinical and sonographic characteristics of Warthin-like variant papillary thyroid carcinomas. Med. Ultrason. 2019, 21, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, B.J.; Ren, W.W.; He, Y.P.; Li, X.L.; Zhao, C.K.; Zhang, Y.F.; Yue, W.W.; Zheng, J.Y.; Xu, H.X. Association between BRAF V600E Mutation and ultrasound features in papillary thyroid carcinoma patients with and without Hashimoto’s thyroiditis. Sci. Rep. 2017, 7, 4899. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.Y.; Kim, T.H.; Jeong, D.J.; Park, S.M.; Cho, Y.Y.; Jang, H.W.; Jung, Y.Y.; Oh, Y.L.; Yim, H.S.; Kim, Y.L.; et al. Diffuse sclerosing variant of papillary thyroid carcinoma: Major genetic alterations and prognostic implications. Histopathology 2016, 69, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yuan, J.; Wang, Y.; Zhai, Y. Association between the BRAF V600E mutation and ultrasound features of the thyroid in thyroid papillary carcinoma. Oncol. Lett. 2017, 14, 1439–1444. [Google Scholar] [CrossRef]
- Hughes, N.M.; Nae, A.; Barry, J.; Fitzgerald, B.; Feeley, L.; Sheahan, P. Sonographic differences between conventional and follicular variant papillary thyroid carcinoma. Eur. Arch. Otorhinolaryngol. 2017, 274, 2907–2913. [Google Scholar] [CrossRef]
- Rivera, M.; Ricarte-Filho, J.; Knauf, J.; Shaha, A.; Tuttle, M.; Fagin, J.A.; Ghossein, R.A. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod. Pathol. 2010, 23, 1191–1200. [Google Scholar] [CrossRef]
- Howitt, B.E.; Jia, Y.; Sholl, L.M.; Barletta, J.A. Molecular alterations in partially-encapsulated or well-circumscribed follicular variant of papillary thyroid carcinoma. Thyroid 2013, 23, 1256–1262. [Google Scholar] [CrossRef]
- Zhao, L.; Dias-Santagata, D.; Sadow, P.M.; Faquin, W.C. Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer Cytopathol. 2017, 125, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Paulson, V.A.; Shivdasani, P.; Angell, T.E.; Cibas, E.S.; Krane, J.F.; Lindeman, N.I.; Alexander, E.K.; Barletta, J.A. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features accounts for more than half of “carcinomas” harboring RAS mutations. Thyroid 2017, 27, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Geramizadeh, B.; Maleki, Z. Non-invasive follicular thyroid neoplasm with papillary-like nuclearfeatures (NIFTP): A review and update. Endocrine 2019, 64, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Baloch, Z.W.; Hodak, S.P.; Giordano, T.J.; Lloyd, R.V.; Seethala, R.R.; Wenig, B.M. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol. 2018, 4, 1125–1126. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W.; Mourão, G.F. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): A review for clinicians. Endocr. Relat. Cancer 2019, 26, R259–R266. [Google Scholar] [CrossRef]
- Chandler, J.B.; Colunga, M.; Prasad, M.L.; Callender, G.G.; Quinn, C.; Chhieng, D.; Adeniran, A.J. Identification of distinct cytomorphologic features in the diagnosis of NIFTP at the time of preoperative FNA: Implications for patient management. Cancer Cytopathol. 2017, 125, 865–875. [Google Scholar] [CrossRef]
- Afkhami, M.; Karunamurthy, A.; Chiosea, S.; Nikiforova, M.N.; Seethala, R.; Nikiforov, Y.E.; Coyne, C. Histopathologic and clinical characterization of thyroid tumors carrying the BRAF(K601E) mutation. Thyroid 2016, 26, 242–247. [Google Scholar] [CrossRef]
- Johnson, D.N.; Furtado, L.V.; Long, B.C.; Zhen, C.J.; Wurst, M.; Mujacic, I.; Kadri, S.; Segal, J.P.; Antic, T.; Cipriani, N.A. Noninvasive follicular thyroid neoplasms with papillary-like nuclear features are genetically and biologically similar to adenomatous nodules and distinct from papillary thyroid carcinomas with extensive follicular growth. Arch. Pathol. Lab. Med. 2018, 142, 838–850. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kwon, H.J.; Kim, E.K.; Moon, H.J.; Kwak, J.Y. The follicular variant of papillary thyroid carcinoma: Characteristics of preoperative ultrasonography and cytology. Ultrasonography 2016, 35, 47–54. [Google Scholar] [CrossRef]
- Hahn, S.Y.; Shin, J.H.; Lim, H.K.; Jung, S.L.; Oh, Y.L.; Choi, I.H.; Jung, C.K. Preoperative differentiation between noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) and non-NIFTP. Clin. Endocrinol. 2017, 86, 444–450. [Google Scholar] [CrossRef]
- Rosario, P.W.; da Silva, A.L.; Nunes, M.B.; Borges, M.A.R. Risk of malignancy in thyroid nodules using the American College of Radiology thyroid imaging reporting and data system in the NIFTP era. Horm. Metab. Res. 2018, 50, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W.; Silva, T.H.; de Oliveira, P.H.L. Impact of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on the risk of malignancy estimated by the ultrasonographic classification of the American Thyroid Association (ATA) in thyroid nodules >1 cm. Endocrine 2018, 60, 535–536. [Google Scholar] [CrossRef] [PubMed]
- De Napoli, L.; Bakkar, S.; Ambrosini, C.E.; Materazzi, G.; Proietti, A.; Macerola, E.; Basolo, F.; Miccoli, P. Indeterminate Single Thyroid Nodule: Synergistic impact of mutational markers and sonographic features in triaging patients to appropriate surgery. Thyroid 2016, 26, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W. Ultrasonography and cytology as predictors of noninvasive follicular thyroid (NIFTP) neoplasm with papillary-like nuclear features: Importance of the differential diagnosis with the invasive encapsulated follicular variant of papillary thyroid cancer. Clin. Endocrinol. 2017, 87, 635–636. [Google Scholar] [CrossRef]
- Fukahori, M.; Yoshida, A.; Hayashi, H.; Yoshihara, M.; Matsukuma, S.; Sakuma, Y.; Koizume, S.; Okamoto, N.; Kondo, T.; Masuda, M.; et al. The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: New insights from a single center and a large patient cohort. Thyroid 2012, 22, 683–689. [Google Scholar] [CrossRef]
- Ibrahimpasic, T.; Ghossein, R.; Shah, J.P.; Ganly, I. Poorly differentiated carcinoma of the thyroid gland: Current status and future prospects. Thyroid 2019, 29, 311–321. [Google Scholar] [CrossRef]
- Bonhomme, B.; Godbert, Y.; Perot, G.; Al Ghuzlan, A.; Bardet, S.; Belleannée, G.; Crinière, L.; Do Cao, C.; Fouilloux, G.; Guyetant, S.; et al. Molecular pathology of anaplastic thyroid carcinomas: A retrospective study of 144 cases. Thyroid 2017, 27, 682–692. [Google Scholar] [CrossRef]
- Oh, E.J.; Lee, S.; Bae, J.S.; Kim, Y.; Jeon, S.; Jung, C.K. TERT promoter mutation in an aggressive cribriform morular variant of papillary thyroid carcinoma. Endocr. Pathol. 2017, 28, 49–53. [Google Scholar] [CrossRef]
- Kim, T.H.; Ki, C.S.; Hahn, S.Y.; Oh, Y.L.; Jang, H.W.; Kim, S.W.; Chung, J.H.; Shin, J.H. Ultrasonographic prediction of highly aggressive telomerase reverse transcriptase (TERT) promoter-mutated papillary thyroid cancer. Endocrine 2017, 57, 234–240. [Google Scholar] [CrossRef]
- Ravella, L.; Lopez, J.; Descotes, F.; Lifante, J.C.; David, C.; Decaussin-Petrucci, M. DICER1 mutated, solid/trabecular thyroid papillary carcinoma in an 11-year-old child. Ann. Pathol. 2018, 38, 316–320. [Google Scholar] [CrossRef]
- Trovisco, V.; Soares, P.; Soares, R.; Magalhães, J.; Sá-Couto, P.; Sobrinho-Simões, M. A new BRAF gene mutation detected in a case of a solid variant of papillary thyroid carcinoma. Hum. Pathol. 2005, 36, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, S.W.; Shin, S.J.; Kim, Y.M.; Rhee, Y.; Jeon, B.I.; Moon, W.C.; Oh, M.R.; Lim, S.K. Papillary thyroid carcinoma associated with familial adenomatous polyposis: Molecular analysis of pathogenesis in a family and review of the literature. Endocr. J. 2004, 1, 317–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cetta, F.; Pelizzo, M.R.; Curia, M.C.; Barbarisi, A. Genetics and clinicopathological findings in thyroid carcinomas associated with familial adenomatous polyposis. Am. J. Pathol. 1999, 155, 7–9. [Google Scholar] [CrossRef]
- Jung, C.K.; Choi, Y.J.; Lee, K.Y.; Bae, J.S.; Kim, H.J.; Yoon, S.K.; Son, Y.I.; Chung, J.H.; Oh, Y.L. The cytological, clinical, and pathological features of the cribriform-morular variant of papillary thyroid carcinoma and mutation analysis of CTNNB1 and BRAF genes. Thyroid 2009, 19, 905–913. [Google Scholar] [CrossRef]
- Uchino, S.; Noguchi, S.; Yamashita, H.; Yamashita, H.; Watanabe, S.; Ogawa, T.; Tsuno, A.; Murakami, A.; Miyauchi, A. Mutational analysis of the APC gene in cribriform-morular variant of papillary thyroid carcinoma. World J. Surg. 2006, 30, 775–779. [Google Scholar] [CrossRef]
- Xu, B.; Yoshimoto, K.; Miyauchi, A.; Kuma, S.; Mizusawa, N.; Hirokawa, M.; Sano, T. Cribriform-morular variant of papillary thyroid carcinoma: A pathological and molecular genetic study with evidence of frequent somatic mutations in exon 3 of the beta-catenin gene. J. Pathol. 2003, 199, 58–67. [Google Scholar] [CrossRef]
- Chong, Y.; Shin, J.H.; Oh, Y.L.; Han, B.; Ko, E.Y. Cribriform-morular variant of papillary thyroid carcinoma: Ultrasonographic and clinical characteristics. Thyroid 2013, 23, 45–49. [Google Scholar] [CrossRef]
- Pradhan, D.; Sharma, A.; Mohanty, S.K. Cribriform-morular variant of papillary thyroid carcinoma. Pathol. Res. Pract. 2015, 211, 712–716. [Google Scholar] [CrossRef]
- Giannelli, S.M.; McPhaul, L.; Nakamoto, J.; Gianoukakis, A.G. Familial adenomatous polyposis-associated, cribriform morular variant of papillary thyroid carcinoma harboring a K-RAS mutation: Case presentation and review of molecular mechanisms. Thyroid 2014, 24, 1184–1189. [Google Scholar] [CrossRef]
- Cameselle-Teijeiro, J.; Menasce, L.P.; Yap, B.K.; Colaco, R.J.; Castro, P.; Celestino, R.; Ruiz-Ponte, C.; Soares, P.; Sobrinho-Simoes, M. Cribriform-morular variant of papillary thyroid carcinoma: Molecular characterization of a case with neuroendocrine differentiation and aggressive behavior. Am. J. Clin. Pathol. 2009, 131, 134–142. [Google Scholar] [CrossRef]
- Kwon, M.J.; Rho, Y.S.; Jeong, J.C.; Shin, H.S.; Lee, J.S.; Cho, S.J.; Nam, E.S. Cribriform-morular variant of papillary thyroid carcinoma: A study of 3 cases featuring the PIK3CA mutation. Hum. Pathol. 2015, 46, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
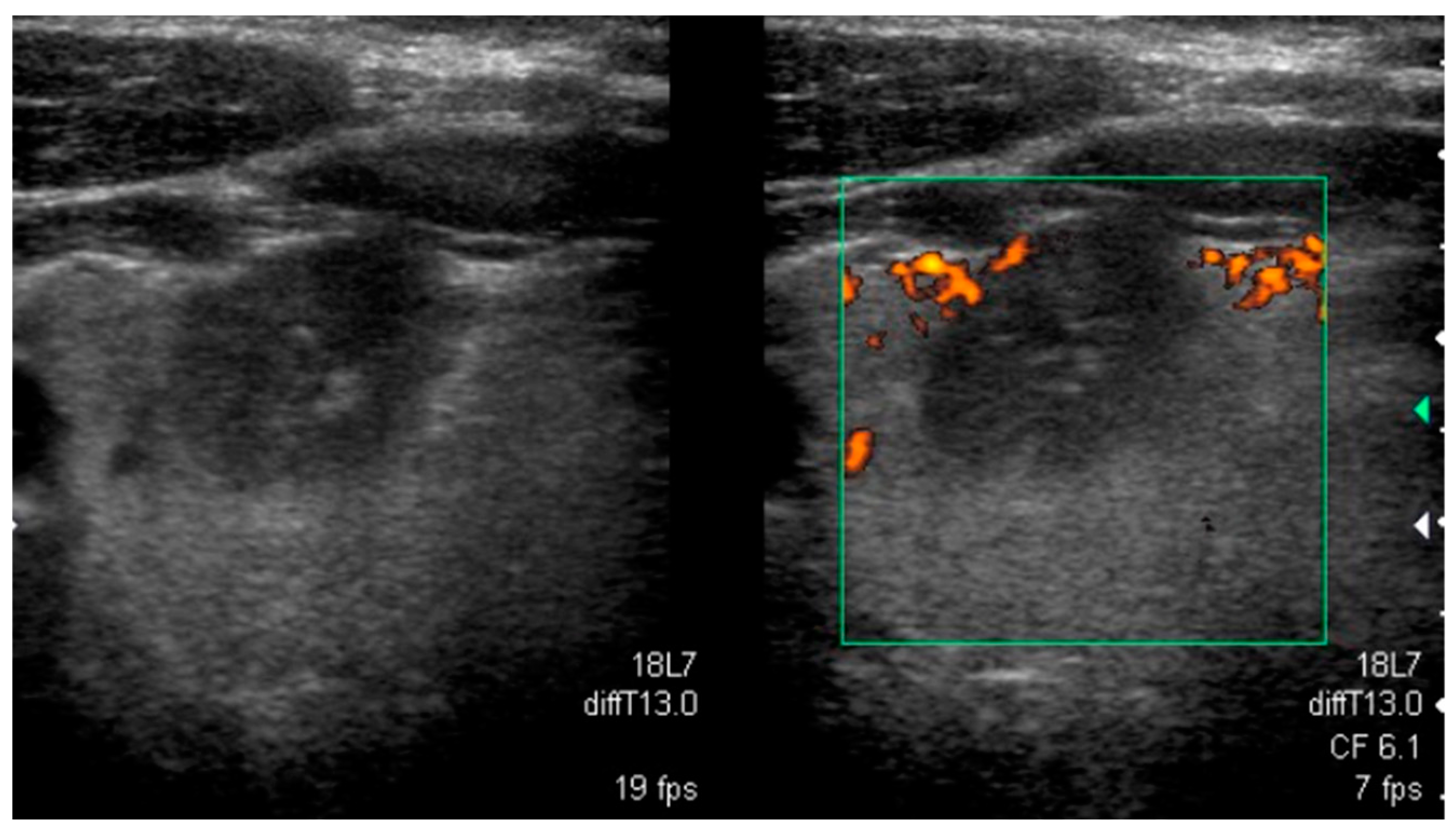
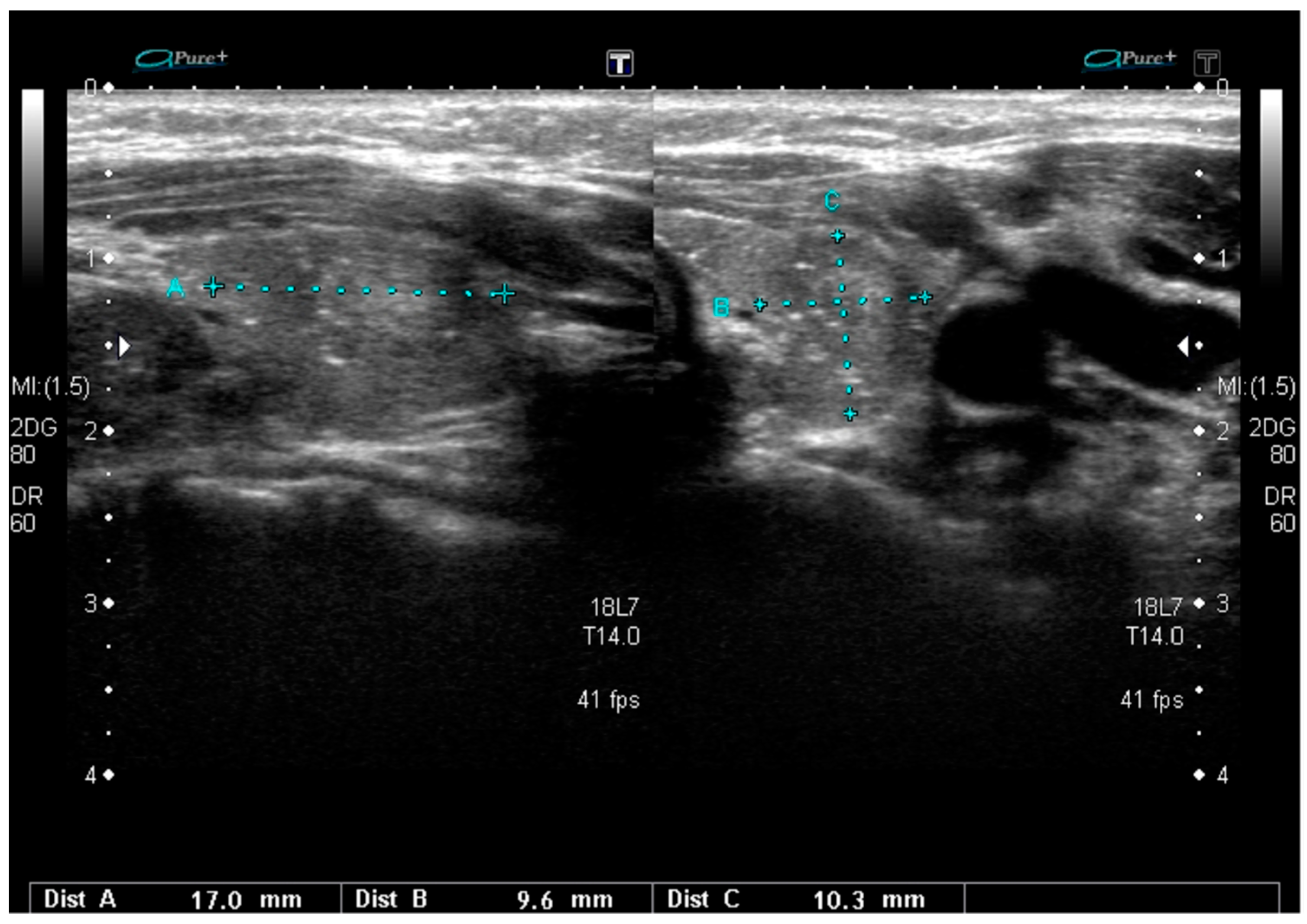
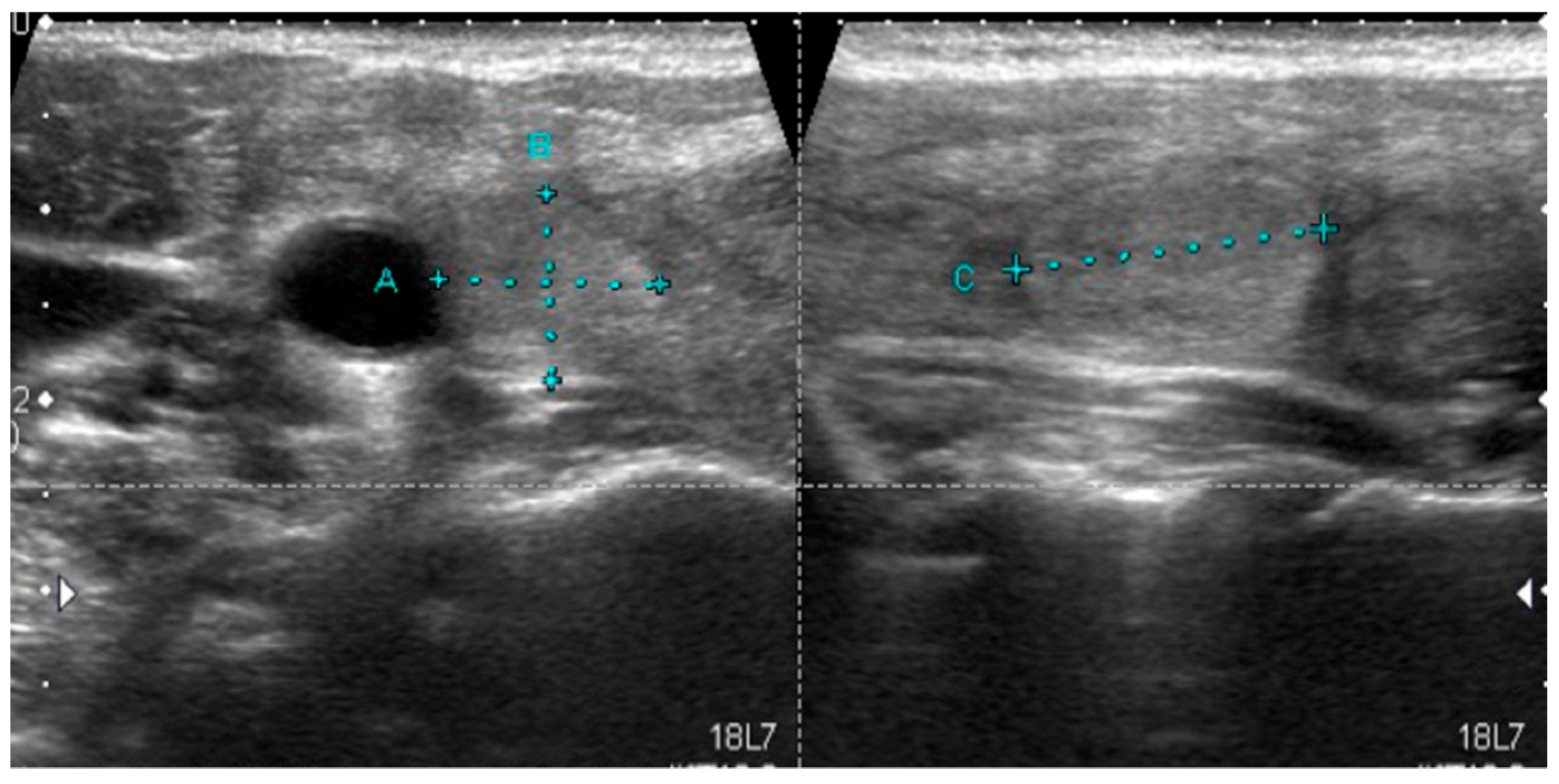
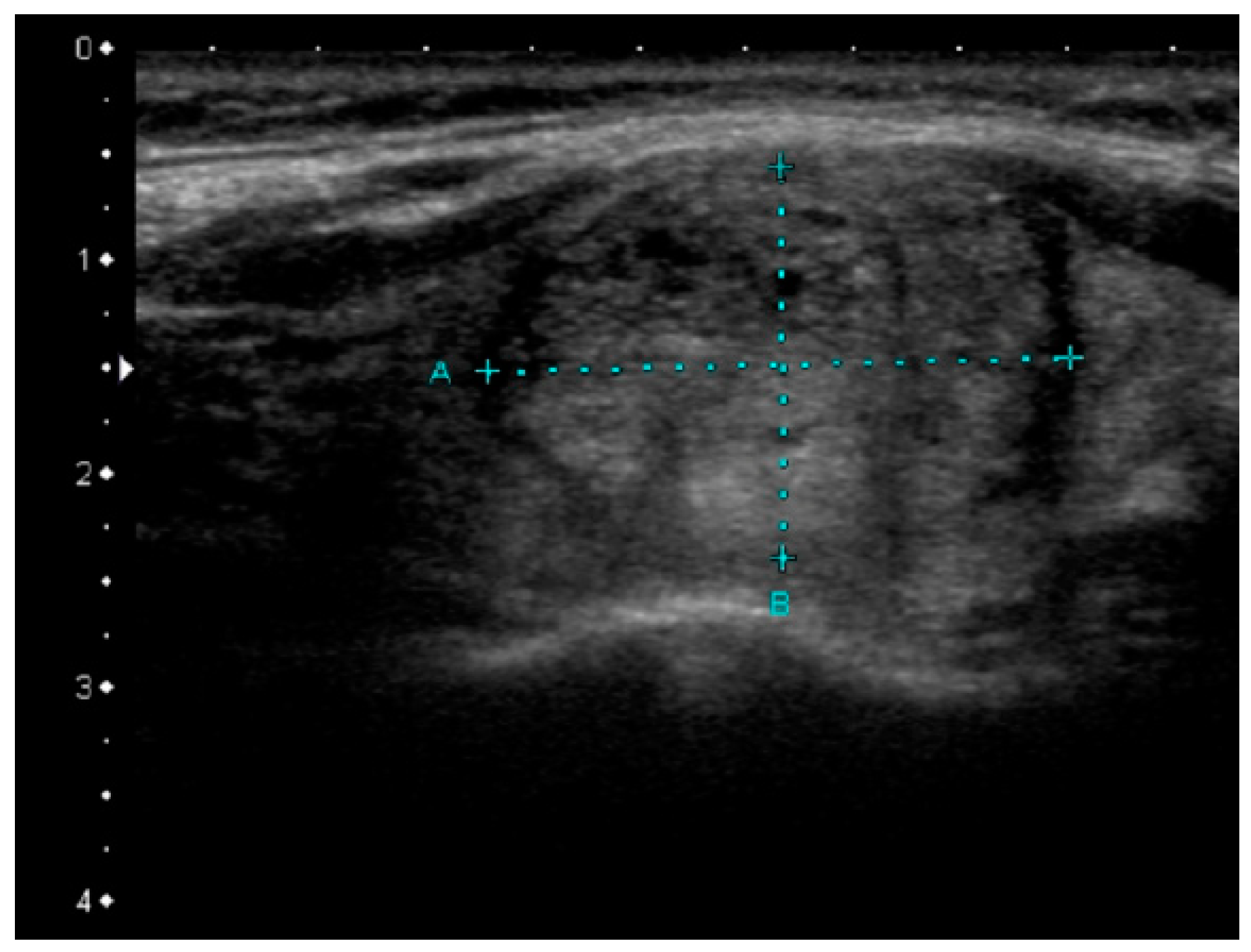
| Mutation | Tumor US Features and References | PTC Variant |
|---|---|---|
| BRAFV600E | solid structure [12,23,31,34,35] hypoechogenicity [12,23,24,25,26,31,34,35] microcalcifications [12,22,23,24,25,26,31,33] macrocalcifications [31] taller-then-wide shape [23,29,34,35]/non-parallel orientations [31] spiculated/microlobulated/irregular margin [12,23,24,31] absent halo [26] ill-defined margin [22,26] lymph node metastases [12,24,33] extrathyroidal extension [12,24] mixed type vascularity [31] | classic, tall cell, columnar cell hobnail Warthin-like solid microcarcinoma |
| iso- or hypoechogenicity [31] smooth or spiculated/microlobulated margins [31] no microcalcifications [31] possible macrocalcifications [31] non-parallel orientation [31] mixed vascularity [31] | Hürthle cell | |
| BRAFK601E | hypoechogenicity [31,39] spiculated/microlobulated margins [31,39] microcalcifications (20%) [31,39] | infiltrative FV PTC |
| RAS | hypoechogenicity [31] smooth margins [31] no microcalcifications [31] ovoid-to-round shape [29] mixed vascularization [29] non-parallel orientation [29,39] | invasive EFV PTC |
| iso-/hyperechogenicity [46,50,51] well-defined margins [46,50,51] no calcifications [46,50,51] | NIFTP | |
| RET/PTC rearrangement | diffuse involvement of one or both thyroid lobes [12,37] scattered microcalcifications [12,37] heterogeneous, mostly hypoechoic [12] ill-defined margins [12,37] nodule may not be defined (a “snowstorm” pattern) [12] hypo-/isoechogenicity [12,37] lymph node involvement with nodal microcalcifications [12,37] | diffuse sclerosing |
| small nodule size [19] hypoechogenicity [19] microcalcifications [19] ill-defined margins [19] | classic | |
| PAX8-PPARγ | hypoechogenicity [31] smooth margins [31] no calcifications [31] ovoid-to-round shape [31] mixed vascularization [31] | invasive EFV PTC |
| CTNNB1 APC | multiple tumors [12,65,66] solid structure [12,65,66] hypoechogenicity [12,65] oval shape [12,65] well circumscribed [12,65] absence of hypoechoic halo [12] absence of calcifications [12,65] | cribriform-morular |
| TERT promoter | hypoechogenicity [12,33] microcalcifications [12,33] non-parallel orientation [60] microlobulated margins [60] | cribriform-morular hobnail classic |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewiński, A.; Adamczewski, Z.; Zygmunt, A.; Markuszewski, L.; Karbownik-Lewińska, M.; Stasiak, M. Correlations between Molecular Landscape and Sonographic Image of Different Variants of Papillary Thyroid Carcinoma. J. Clin. Med. 2019, 8, 1916. https://doi.org/10.3390/jcm8111916
Lewiński A, Adamczewski Z, Zygmunt A, Markuszewski L, Karbownik-Lewińska M, Stasiak M. Correlations between Molecular Landscape and Sonographic Image of Different Variants of Papillary Thyroid Carcinoma. Journal of Clinical Medicine. 2019; 8(11):1916. https://doi.org/10.3390/jcm8111916
Chicago/Turabian StyleLewiński, Andrzej, Zbigniew Adamczewski, Arkadiusz Zygmunt, Leszek Markuszewski, Małgorzata Karbownik-Lewińska, and Magdalena Stasiak. 2019. "Correlations between Molecular Landscape and Sonographic Image of Different Variants of Papillary Thyroid Carcinoma" Journal of Clinical Medicine 8, no. 11: 1916. https://doi.org/10.3390/jcm8111916
APA StyleLewiński, A., Adamczewski, Z., Zygmunt, A., Markuszewski, L., Karbownik-Lewińska, M., & Stasiak, M. (2019). Correlations between Molecular Landscape and Sonographic Image of Different Variants of Papillary Thyroid Carcinoma. Journal of Clinical Medicine, 8(11), 1916. https://doi.org/10.3390/jcm8111916







