Early Post-Operative Intervention of Whole-Body Vibration in Patients After Total Knee Arthroplasty: A Pilot Study
Abstract
:1. Introduction
2. Experimental Section
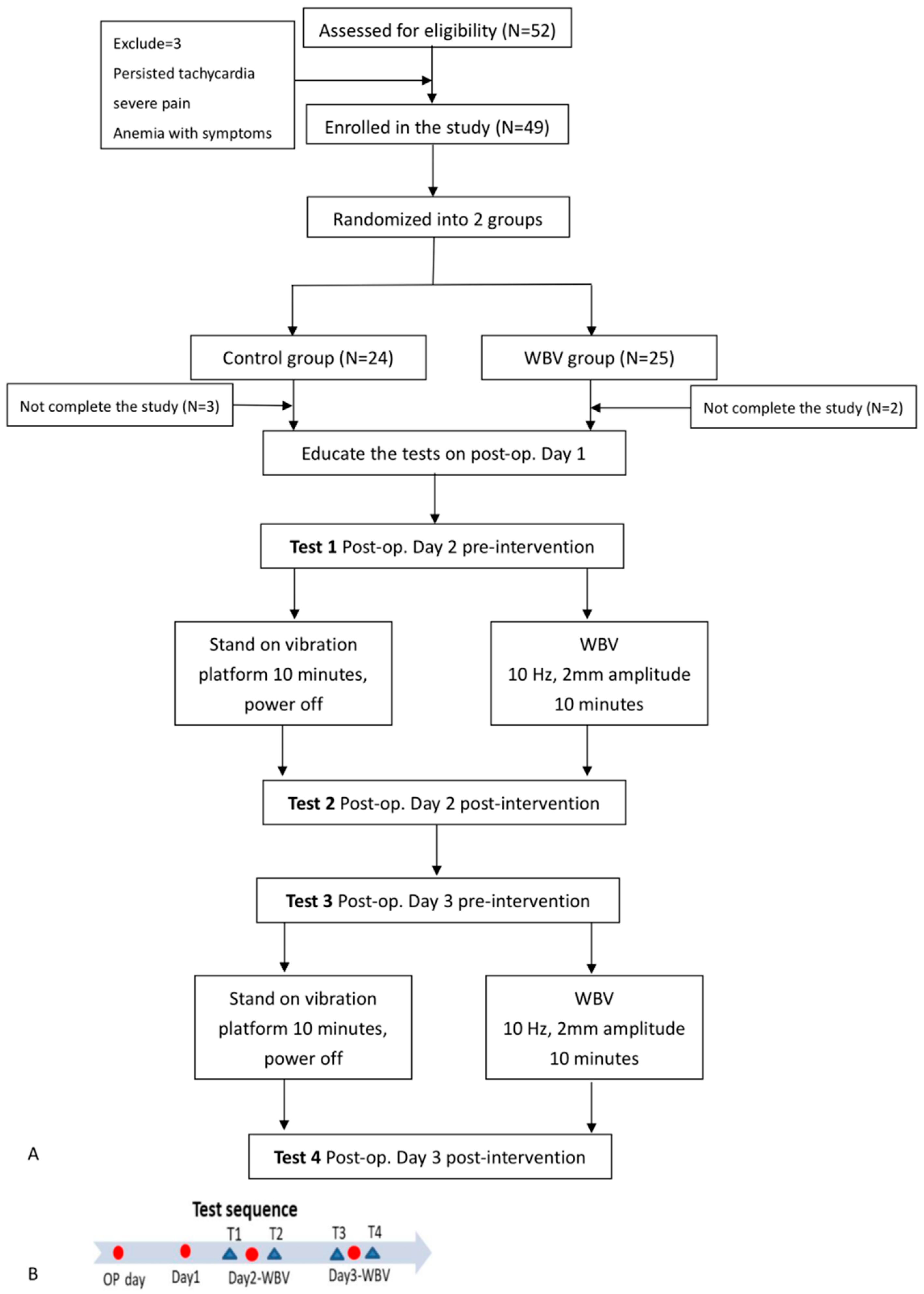
2.1. Study Population
2.2. Design of Post-Operative Exercise Interventions
2.3. Outcome Measures
2.3.1. Pain Scale (0–10)
2.3.2. Knee Joint Range of Motion
2.3.3. Leg Circumference
2.3.4. Knee Extensor Muscle Strength
2.3.5. Functional Performance Test with Modified Five Times Sit to Stand Test and Modified Timed Up and Go (TUG) Test
2.4. Statistics
3. Results
3.1. Characteristics of the Participants
3.2. Effect of Whole Body Vibration (WBV) on All Outcome Measures During Serial Testing
3.3. Effect of Whole Body Vibration (WBV) on Pain Reduction and Lower Leg Circumference Change
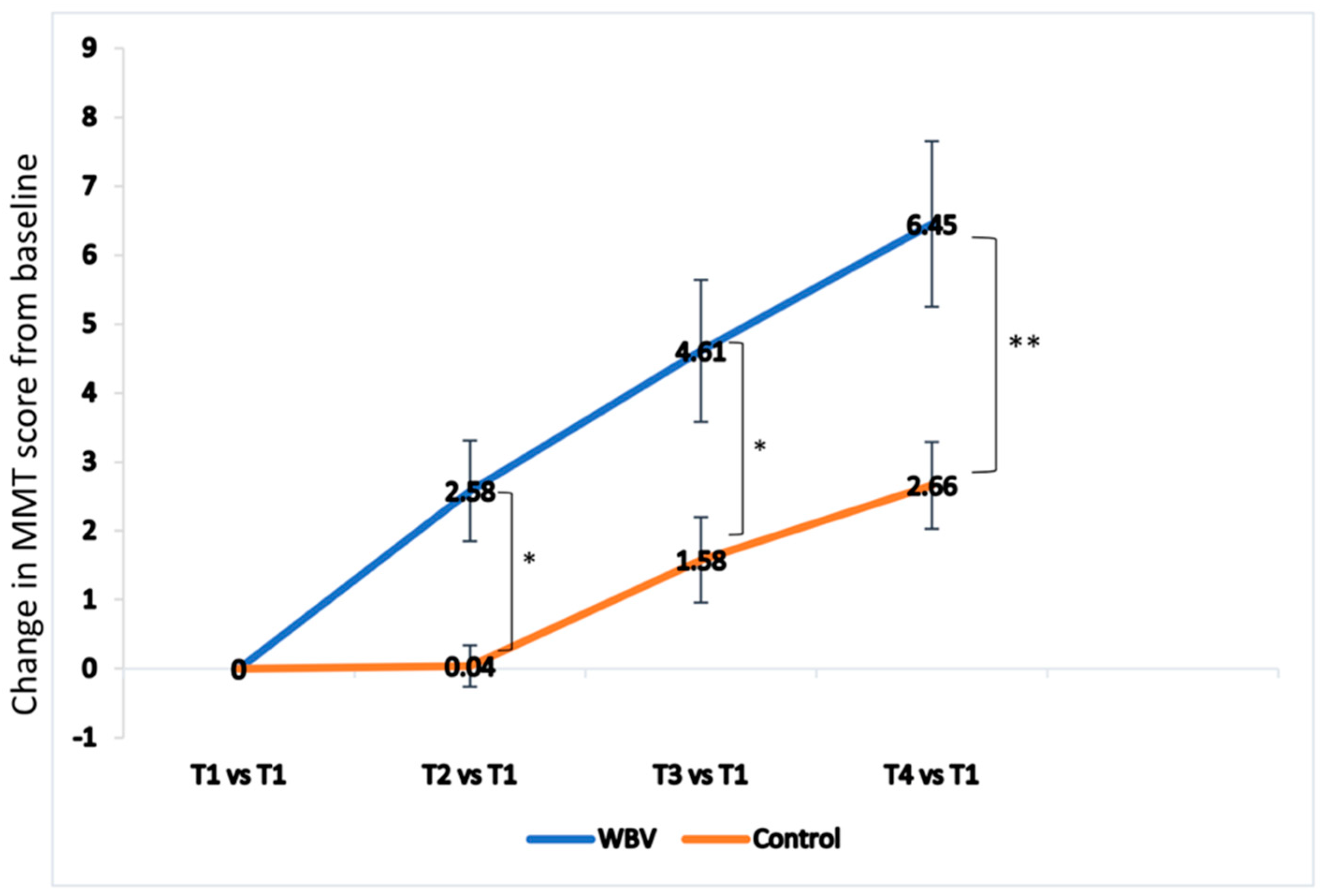
3.4. Effect of Whole Body Vibration (WBV) on Knee Extensor Strength and Knee Joint Range of Motion (ROM)
3.5. Effect of Whole Body Vibration (WBV) on Functional Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leech, R.D.; Eyles, J.; Batt, M.E.; Hunter, D.J. Lower extremity osteoarthritis: Optimising musculoskeletal health is a growing global concern: A narrative review. Br. J. Sports Med. 2019, 53, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Bridgett, L.; March, L.; Hoy, D.; Penserga, E.; Brooks, P. The epidemiology of osteoarthritis in Asia. Int. J. Rheum. Dis. 2011, 14, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Gourdine, J. Review of Nonsurgical Treatment Guidelines for Lower Extremity Osteoarthritis. Orthop. Nurs. 2019, 38, 303–308. [Google Scholar] [CrossRef]
- Van Manen, M.D.; Nace, J.; Mont, M.A. Management of primary knee osteoarthritis and indications for total knee arthroplasty for general practitioners. J. Am. Osteopath. Assoc. 2012, 112, 709–715. [Google Scholar]
- Ferket, B.S.; Feldman, Z.; Zhou, J.; Oei, E.H.; Bierma-Zeinstra, S.M.; Mazumdar, M. Impact of total knee replacement practice: Cost effectiveness analysis of data from the Osteoarthritis Initiative. BMJ 2017, 356, j1131. [Google Scholar] [CrossRef]
- Noble, P.C.; Gordon, M.J.; Weiss, J.M.; Reddix, R.N.; Conditt, M.A.; Mathis, K.B. Does total knee replacement restore normal knee function? Clin. Orthop. Relat. Res. 2005, 157–165. [Google Scholar] [CrossRef]
- Bade, M.J.; Kohrt, W.M.; Stevens-Lapsley, J.E. Outcomes before and after total knee arthroplasty compared to healthy adults. J. Orthop. Sports Phys. Ther. 2010, 40, 559–567. [Google Scholar] [CrossRef]
- Mizner, R.L.; Petterson, S.C.; Stevens, J.E.; Vandenborne, K.; Snyder-Mackler, L. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J. Bone Jt. Surg. Am. 2005, 87, 1047–1053. [Google Scholar] [CrossRef]
- Holm, B.; Kristensen, M.T.; Bencke, J.; Husted, H.; Kehlet, H.; Bandholm, T. Loss of knee-extension strength is related to knee swelling after total knee arthroplasty. Arch. Phys. Med. Rehabil. 2010, 91, 1770–1776. [Google Scholar] [CrossRef]
- Stevens, J.E.; Mizner, R.L.; Snyder-Mackler, L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J. Orthop. Res. 2003, 21, 775–779. [Google Scholar] [CrossRef]
- Mizner, R.L.; Petterson, S.C.; Snyder-Mackler, L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J. Orthop. Sports Phys. Ther. 2005, 35, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, M.; Elmallah, R.D.; Mistry, J.B.; Bhave, A.; Cherian, J.J.; McGinn, T.L.; Harwin, S.F.; Mont, M.A. Nonpharmacologic Pain Management and Muscle Strengthening following Total Knee Arthroplasty. J. Knee Surg. 2016, 29, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.B.; Elmallah, R.D.; Bhave, A.; Chughtai, M.; Cherian, J.J.; McGinn, T.; Harwin, S.F.; Mont, M.A. Rehabilitative Guidelines after Total Knee Arthroplasty: A Review. J. Knee Surg. 2016, 29, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Abercromby, A.F.; Amonette, W.E.; Layne, C.S.; McFarlin, B.K.; Hinman, M.R.; Paloski, W.H. Vibration exposure and biodynamic responses during whole-body vibration training. Med. Sci. Sports Exerc. 2007, 39, 1794–1800. [Google Scholar] [CrossRef]
- Sitja-Rabert, M.; Rigau, D.; Fort Vanmeerghaeghe, A.; Romero-Rodriguez, D.; Bonastre Subirana, M.; Bonfill, X. Efficacy of whole body vibration exercise in older people: A systematic review. Disabil. Rehabil. 2012, 34, 883–893. [Google Scholar] [CrossRef]
- Bemben, D.; Stark, C.; Taiar, R.; Bernardo-Filho, M. Relevance of Whole-Body Vibration Exercises on Muscle Strength/Power and Bone of Elderly Individuals. Dose-Response 2018, 16, 1559325818813066. [Google Scholar] [CrossRef]
- Jepsen, D.B.; Thomsen, K.; Hansen, S.; Jorgensen, N.R.; Masud, T.; Ryg, J. Effect of whole-body vibration exercise in preventing falls and fractures: A systematic review and meta-analysis. BMJ Open 2017, 7, e018342. [Google Scholar] [CrossRef]
- Tseng, S.Y.; Lai, C.L.; Chang, K.L.; Hsu, P.S.; Lee, M.C.; Wang, C.H. Influence of Whole-Body Vibration Training Without Visual Feedback on Balance and Lower-Extremity Muscle Strength of the Elderly: A Randomized Controlled Trial. Medicine 2016, 95, e2709. [Google Scholar] [CrossRef]
- Anwer, S.; Alghadir, A.; Zafar, H.; Al-Eisa, E. Effect of whole body vibration training on quadriceps muscle strength in individuals with knee osteoarthritis: A systematic review and meta-analysis. Physiotherapy 2016, 102, 145–151. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.Q.; Chen, B.L.; Huang, L.Y.; Liu, Y. Whole-Body Vibration Exercise for Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2015, 2015, 758147. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Lee, S.; Hu, X.; Wang, L. Effect of adding whole-body vibration training to squat training on physical function and muscle strength in individuals with knee osteoarthritis. J. Musculoskelet. Neuronal Interact. 2019, 19, 333–341. [Google Scholar] [PubMed]
- Wang, P.; Yang, L.; Liu, C.; Wei, X.; Yang, X.; Zhou, Y.; Jiang, H.; Lei, Z.; Reinhardt, J.D.; He, C. Effects of Whole Body Vibration Exercise associated with Quadriceps Resistance Exercise on functioning and quality of life in patients with knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2016, 30, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Bokaeian, H.R.; Bakhtiary, A.H.; Mirmohammadkhani, M.; Moghimi, J. The effect of adding whole body vibration training to strengthening training in the treatment of knee osteoarthritis: A randomized clinical trial. J. Bodyw. Mov. Ther. 2016, 20, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.W.; Myrer, J.W.; Hunter, I.; Feland, J.B.; Hopkins, J.T.; Draper, D.O.; Eggett, D. Whole-body vibration strengthening compared to traditional strengthening during physical therapy in individuals with total knee arthroplasty. Physiother. Theory Pract. 2010, 26, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.A.; Derhon, V.; Brandalize, M.; Brandalize, D.; Rossi, L.P. Evaluation of knee range of motion: Correlation between measurements using a universal goniometer and a smartphone goniometric application. J. Bodyw. Mov. Ther. 2017, 21, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, C.; Armijo-Olivo, S.; De la Fuente, C.; Fuentes, J.; Javier Chirosa, L. Absolute Reliability and Concurrent Validity of Hand Held Dynamometry and Isokinetic Dynamometry in the Hip, Knee and Ankle Joint: Systematic Review and Meta-analysis. Open Med. 2017, 12, 359–375. [Google Scholar] [CrossRef]
- Kim, W.K.; Kim, D.K.; Seo, K.M.; Kang, S.H. Reliability and validity of isometric knee extensor strength test with hand-held dynamometer depending on its fixation: A pilot study. Ann. Rehabil. Med. 2014, 38, 84–93. [Google Scholar] [CrossRef]
- Pincivero, D.M.; Salfetnikov, Y.; Campy, R.M.; Coelho, A.J. Angle- and gender-specific quadriceps femoris muscle recruitment and knee extensor torque. J. Biomech. 2004, 37, 1689–1697. [Google Scholar] [CrossRef]
- Applebaum, E.V.; Breton, D.; Feng, Z.W.; Ta, A.T.; Walsh, K.; Chasse, K.; Robbins, S.M. Modified 30-second Sit to Stand test predicts falls in a cohort of institutionalized older veterans. PLoS ONE 2017, 12, e0176946. [Google Scholar] [CrossRef]
- Kang, L.; Han, P.; Wang, J.; Ma, Y.; Jia, L.; Fu, L.; Yu, H.; Chen, X.; Niu, K.; Guo, Q. Timed Up and Go Test can predict recurrent falls: A longitudinal study of the community-dwelling elderly in China. Clin. Interv. Aging 2017, 12, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Baczkowicz, D.; Skiba, G.; Czerner, M.; Majorczyk, E. Gait and functional status analysis before and after total knee arthroplasty. Knee 2018, 25, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Lapsley, J.E.; Balter, J.E.; Kohrt, W.M.; Eckhoff, D.G. Quadriceps and hamstrings muscle dysfunction after total knee arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Kwon, B.S.; Park, J.W.; Cha, D.Y.; Nam, K.Y.; Sim, K.B.; Chang, J.; Lee, H.J. Therapeutic effect of whole body vibration on chronic knee osteoarthritis. Ann. Rehabil. Med. 2013, 37, 505–515. [Google Scholar] [CrossRef]
- Avelar, N.C.; Simao, A.P.; Tossige-Gomes, R.; Neves, C.D.; Rocha-Vieira, E.; Coimbra, C.C.; Lacerda, A.C. The effect of adding whole-body vibration to squat training on the functional performance and self-report of disease status in elderly patients with knee osteoarthritis: A randomized, controlled clinical study. J. Altern. Complement. Med. 2011, 17, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.M.; Liao, L.R.; Kwok, T.C.; Pang, M.Y. The effect of vertical whole-body vibration on lower limb muscle activation in elderly adults: Influence of vibration frequency, amplitude and exercise. Maturitas 2016, 88, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Corum, M.; Basoglu, C.; Yakal, S.; Sahinkaya, T.; Aksoy, C. Effects of whole body vibration training on isokinetic muscular performance, pain, function, and quality of life in female patients with patellofemoral pain: A randomized controlled trial. J. Musculoskelet. Neuronal Interact. 2018, 18, 473–484. [Google Scholar]
- Kiiski, J.; Heinonen, A.; Jarvinen, T.L.; Kannus, P.; Sievanen, H. Transmission of vertical whole body vibration to the human body. J. Bone Miner. Res. 2008, 23, 1318–1325. [Google Scholar] [CrossRef]
- Rhea, M.R.; Bunker, D.; Marin, P.J.; Lunt, K. Effect of iTonic whole-body vibration on delayed-onset muscle soreness among untrained individuals. J. Strength Cond. Res. 2009, 23, 1677–1682. [Google Scholar] [CrossRef]
- Bosco, C.; Iacovelli, M.; Tsarpela, O.; Cardinale, M.; Bonifazi, M.; Tihanyi, J.; Viru, M.; De Lorenzo, A.; Viru, A. Hormonal responses to whole-body vibration in men. Eur. J. Appl. Physiol. 2000, 81, 449–454. [Google Scholar] [CrossRef]
- Ritzmann, R.; Kramer, A.; Gruber, M.; Gollhofer, A.; Taube, W. EMG activity during whole body vibration: Motion artifacts or stretch reflexes? Eur. J. Appl. Physiol. 2010, 110, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Kahrizi, S.; Razi, M.; Faghihzadeh, S. The effect of whole-body vibration training on the lower extremity muscles’ electromyographic activities in patients with knee osteoarthritis. Med. J. Islamic Repub. Iran 2017, 31, 107. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.A.; McNair, P.J. Quadriceps arthrogenic muscle inhibition: Neural mechanisms and treatment perspectives. Semin. Arthritis Rheum. 2010, 40, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctot, K.L. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: A meta-analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Simao, A.P.; Mendonca, V.A.; Avelar, N.C.P.; da Fonseca, S.F.; Santos, J.M.; de Oliveira, A.C.C.; Tossige-Gomes, R.; Ribeiro, V.G.C.; Neves, C.D.C.; Balthazar, C.H.; et al. Whole Body Vibration Training on Muscle Strength and Brain-Derived Neurotrophic Factor Levels in Elderly Woman with Knee Osteoarthritis: A Randomized Clinical Trial Study. Front. Physiol. 2019, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Lythgo, N.; Eser, P.; de Groot, P.; Galea, M. Whole-body vibration dosage alters leg blood flow. Clin. Physiol. Funct. Imaging 2009, 29, 53–59. [Google Scholar] [CrossRef]
- Mahbub, M.H.; Hiroshige, K.; Yamaguchi, N.; Hase, R.; Harada, N.; Tanabe, T. A systematic review of studies investigating the effects of controlled whole-body vibration intervention on peripheral circulation. Clin. Physiol. Funct. Imaging 2019, 39, 363–377. [Google Scholar] [CrossRef]
- Games, K.E.; Sefton, J.M.; Wilson, A.E. Whole-body vibration and blood flow and muscle oxygenation: A meta-analysis. J. Athl. Train. 2015, 50, 542–549. [Google Scholar] [CrossRef]
- Ritzmann, R.; Krause, A.; Freyler, K.; Gollhofer, A. Acute whole-body vibration increases reciprocal inhibition. Hum. Mov. Sci. 2018, 60, 191–201. [Google Scholar] [CrossRef]
- Ko, M.C.; Wu, L.S.; Lee, S.; Wang, C.C.; Lee, P.F.; Tseng, C.Y.; Ho, C.C. Whole-body vibration training improves balance control and sit-to-stand performance among middle-aged and older adults: A pilot randomized controlled trial. Eur. Rev. Aging Phys. Act. 2017, 14, 11. [Google Scholar] [CrossRef]
| WBV | Control | p-Value | |
|---|---|---|---|
| n | 25 | 24 | |
| Age, mean (SD), years | 71.1 (8.0) | 71.8 (5.8) | 0.738 |
| Sex, n (%) | |||
| Male | 4 (16.0) | 5 (20.8) | |
| Female | 21 (84.0) | 19 (79.2) | 0.662 |
| Height, mean (SD), cm | 154.2 (6.5) | 154.0 (8.2) | 0.925 |
| Body weight, mean (SD), kg | 64.2 (12.9) | 67.1 (13.1) | 0.445 |
| BMI, mean (SD), kg/m2 | 27.1 (5.7) | 28.2 (4.8) | 0.473 |
| T1 | T2 | T3 | T4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBV | Control | WBV | Control | WBV | Control | WBV | Control | |||||
| Lsmean (SE) | Lsmean (SE) | p-Value | Lsmean (SE) | Lsmean (SE) | p-Value | Lsmean (SE) | Lsmean (SE) | p-Value | Lsmean (SE) | Lsmean (SE) | p-Value | |
| NRS | 4.12 (0.41) | 3.96 (0.48) | 0.793 | 3.68 (0.33) | 3.88 (0.48) | 0.733 | 3.40 (0.37) | 2.67 (0.34) | 0.147 | 2.88 (0.28) | 2.42 (0.28) | 0.239 |
| Thigh circumference | 43.56 (1.09) | 44.31 (1.91) | 0.725 | 44.55 (1.72) | 45.16 (1.19) | 0.767 | 43.72 (1.08) | 44.9 (1.29) | 0.476 | 43.36 (1.08) | 44.75 (1.29) | 0.400 |
| Calf circumference | 34.20 (0.75) | 34.95 (1.23) | 0.596 | 34.39 (0.69) | 36.26 (0.89) | 0.101 | 32.73 (1.16) | 36.44 (0.87) | 0.014 | 33.67 (0.71) | 36.25 (0.84) | 0.023 |
| Knee extensor strength | 14.21 (1.08) | 13.61 (1.00) | 0.726 | 16.79 (1.40) | 13.26 (1.09) | 0.051 | 18.83 (1.38) | 14.81 (0.83) | 0.017 | 20.67 (1.53) | 15.88 (0.88) | 0.010 |
| AROM | 76.20 (3.41) | 70.42 (3.97) | 0.265 | 82.4 (3.08) | 72.92 (3.43) | 0.044 | 83.60 (2.07) | 78.75 (2.36) | 0.124 | 89.80 (1.84) | 81.67 (2.06) | 0.024 |
| PROM | 88.80 (3.22) | 81.46 (3.37) | 0.117 | 92.4 (3.15) | 83.54 (3.19) | 0.053 | 92.20 (2.08) | 88.33 (2.14) | 0.194 | 97.60 (1.66) | 92.50 (1.75) | 0.039 |
| Five-time sit to stand | 39.46 (5.03) | 39.13 (4.21) | 0.958 | 36.73 (5.36) | 33.77 (3.95) | 0.652 | 33.32 (4.42) | 34.19 (2.88) | 0.868 | 28.19 (3.29) | 31.98 (2.93) | 0.383 |
| TUG test | 73.58 (5.75) | 82.56 (10.78) | 0.455 | 66.29 (6.43) | 73.41 (10.73) | 0.562 | 65.75 (5.72) | 66.82 (5.23) | 0.888 | 59.51 (5.12) | 68.42 (8.17) | 0.349 |
| WBV Lsmean (SE) | Control Lsmean (SE) | Difference of Least Square Means (SE; 95% CI) | p-Value | |
|---|---|---|---|---|
| NRS and lower leg circumference | ||||
| NRS baseline | 4.12 (0.40) | 3.96 (0.47) | ||
| Change from baseline to 4 times | −0.40 (0.07) | −0.58 (0.16) | 0.18 (0.17; −0.16 to 0.53) | 0.294 |
| Thigh circumference baseline | 43.56 (1.06) | 44.31 (1.87) | ||
| Change from baseline to 4 times | −0.14 (0.17) | 0.11 (0.38) | −0.25 (0.42; −1.06 to 0.57) | 0.550 |
| Calf circumference baseline | 34.2 (0.73) | 34.95 (1.21) | ||
| Change from baseline to 4 times | −0.33 (0.12) | 0.41 (0.34) | −0.73 (0.37; −1.45 to −0.01) | 0.045 * |
| Knee extensor strength and ROM | ||||
| Knee extensor strength baseline | 14.21 (1.05) | 13.22 (0.98) | ||
| Change from baseline to 4 times | 2.14 (0.44) | 0.95 (0.25) | 1.19 (0.5; 0.20 to 2.18) | 0.018 * |
| AROM baseline | 76.20 (3.34) | 70.42 (3.89) | ||
| Change from baseline to 4 times | 4.20 (0.89) | 3.96 (1.11) | 0.24 (1.43; −2.55 to 3.04) | 0.865 |
| PROM baseline | 88.80 (3.15) | 81.46 (3.30) | ||
| Change from baseline to 4 times | 2.62 (0.95) | 3.79 (1.00) | −1.17 (1.38; −3.88 to 1.54) | 0.397 |
| Functional activities | ||||
| Five time sit to stand baseline | 39.46 (4.93) | 39.13 (4.12) | ||
| Change from baseline to 4 times | −3.72 (1.08) | −2.10 (1.08) | −1.62 (1.53; −4.62 to 1.38) | 0.290 |
| TUG test baseline | 73.58 (5.64) | 82.56 (10.55) | ||
| Change from baseline to 4 times | −4.27 (1.46) | −4.90 (2.05) | 0.63 (2.51; −4.29 to 5.55) | 0.803 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, Y.-H.; Chien, S.-H.; Tu, H.-P.; Fu, J.C.-M.; Tsai, S.-T.; Chen, Y.-S.; Chen, Y.-J.; Chen, C.-H. Early Post-Operative Intervention of Whole-Body Vibration in Patients After Total Knee Arthroplasty: A Pilot Study. J. Clin. Med. 2019, 8, 1902. https://doi.org/10.3390/jcm8111902
Hsiao Y-H, Chien S-H, Tu H-P, Fu JC-M, Tsai S-T, Chen Y-S, Chen Y-J, Chen C-H. Early Post-Operative Intervention of Whole-Body Vibration in Patients After Total Knee Arthroplasty: A Pilot Study. Journal of Clinical Medicine. 2019; 8(11):1902. https://doi.org/10.3390/jcm8111902
Chicago/Turabian StyleHsiao, Yu-Hsuan, Song-Hsiung Chien, Hung-Pin Tu, Jimmy Chun-Ming Fu, Shih-Ting Tsai, Ying-Shan Chen, Yi-Jen Chen, and Chia-Hsin Chen. 2019. "Early Post-Operative Intervention of Whole-Body Vibration in Patients After Total Knee Arthroplasty: A Pilot Study" Journal of Clinical Medicine 8, no. 11: 1902. https://doi.org/10.3390/jcm8111902
APA StyleHsiao, Y.-H., Chien, S.-H., Tu, H.-P., Fu, J. C.-M., Tsai, S.-T., Chen, Y.-S., Chen, Y.-J., & Chen, C.-H. (2019). Early Post-Operative Intervention of Whole-Body Vibration in Patients After Total Knee Arthroplasty: A Pilot Study. Journal of Clinical Medicine, 8(11), 1902. https://doi.org/10.3390/jcm8111902





