Modular Strategies to Build Cell-Free and Cell-Laden Scaffolds towards Bioengineered Tissues and Organs
Abstract
1. Introduction to Tissue Engineering Scaffolds and Bottom-Up Fabrication
2. Microparticles (µPs) as Building Blocks for Modular Tissue Engineering Scaffolds
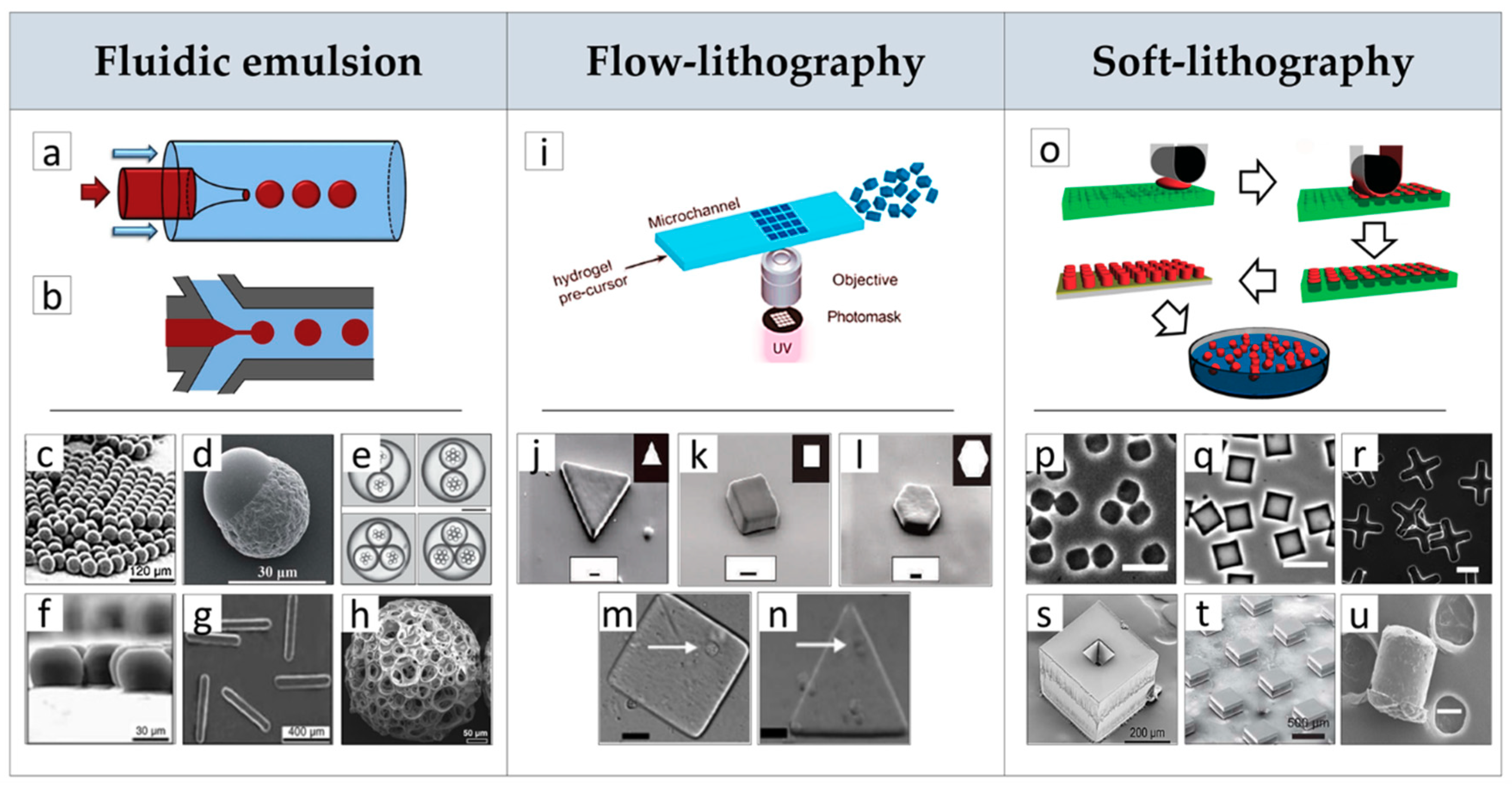
2.1. µPs Fabrication by Advanced Processes
2.2. µPs as Building Blocks for In Vitro and In Vivo Modular Tissue Engineering (TE) Scaffolds
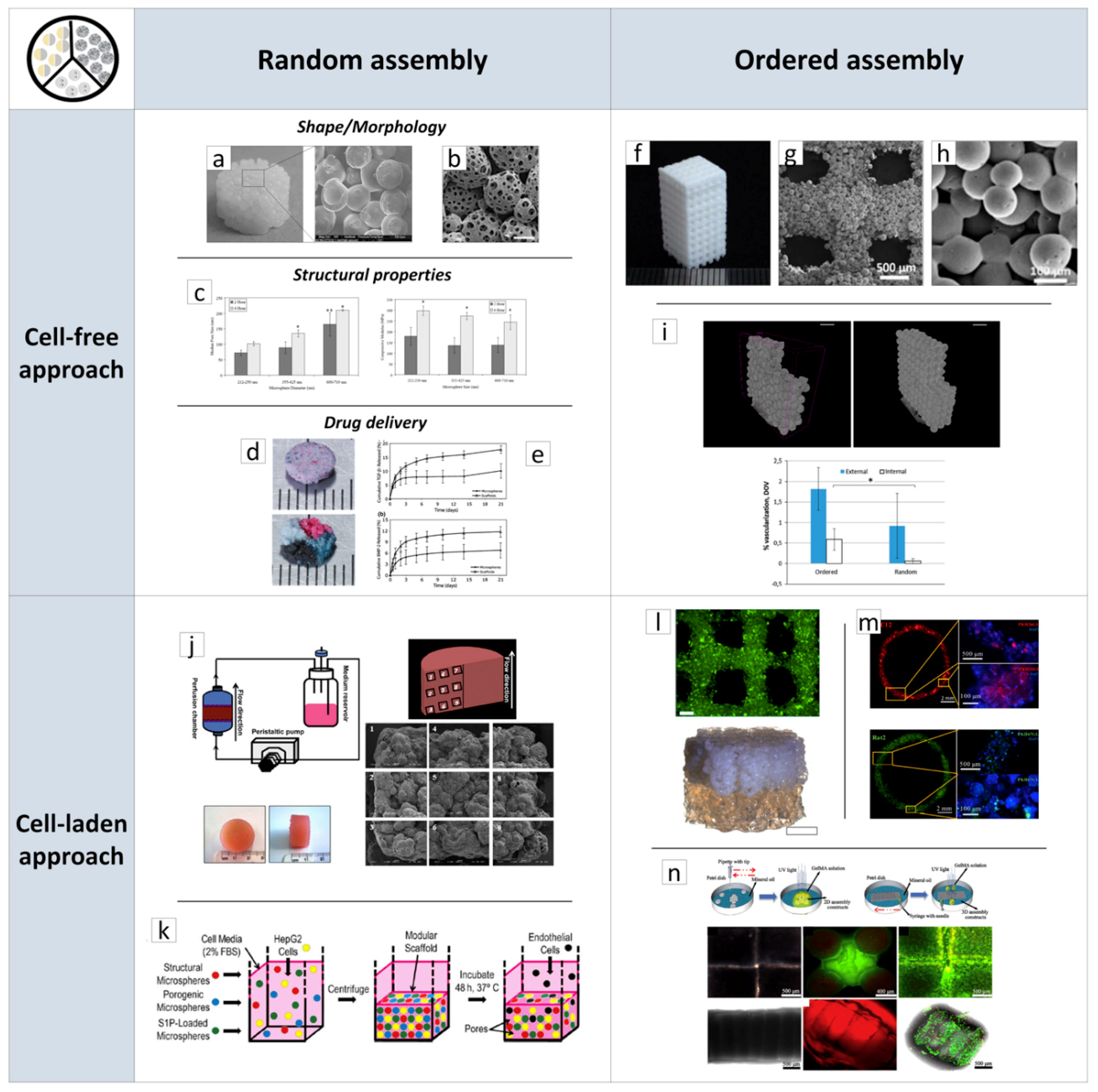
2.2.1. Porous Scaffolds Prepared by the Random/Ordered Assembly of µPs
2.2.2. Porous µPs as µ-Scaffolds for In Vitro Tissue Building
3. Layer-by-Layer Approaches for Scaffolds’ Fabrication
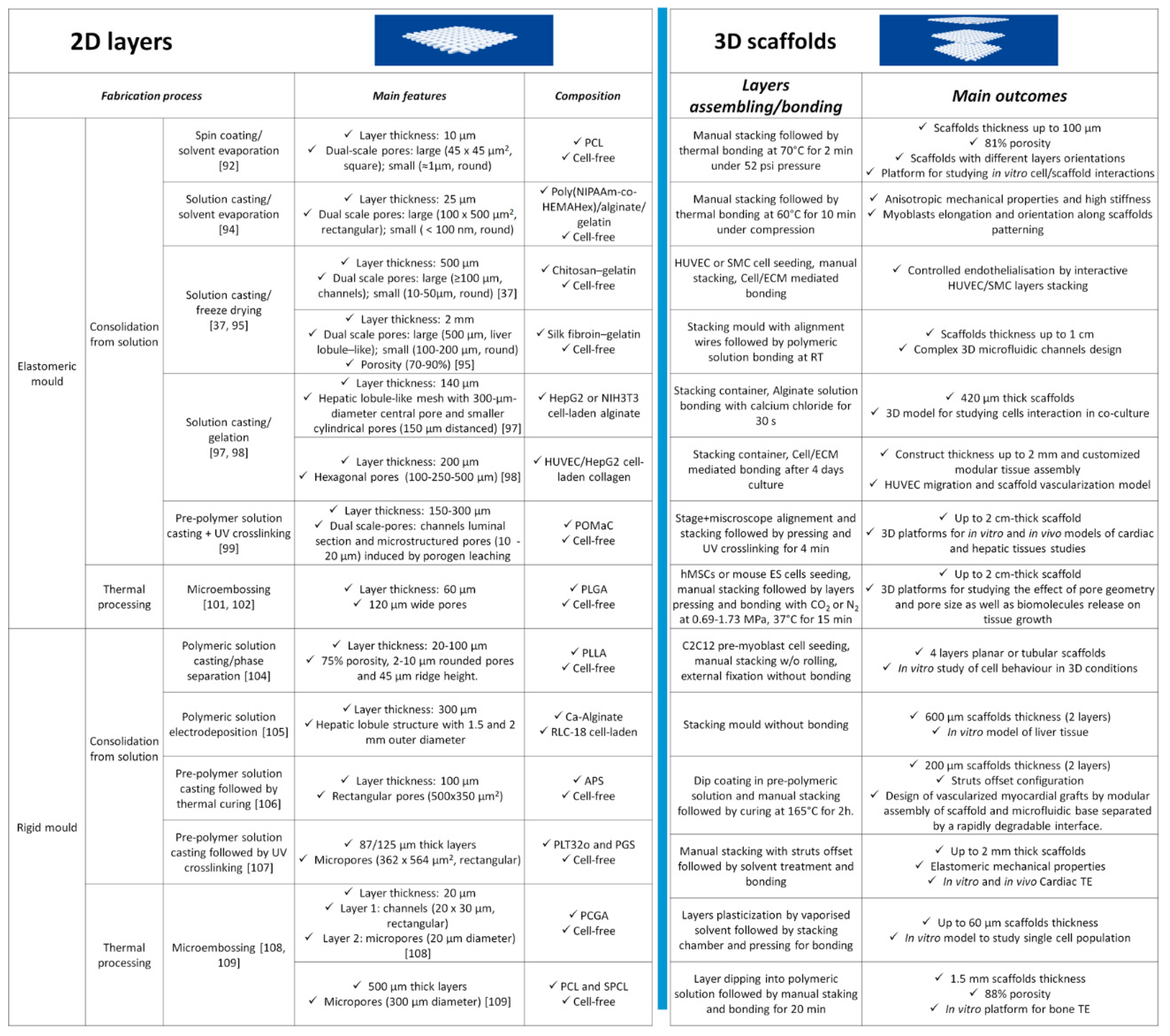
3.1. Discontinuous Processes
3.2. Continuous Processes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AL | Alendronate |
| ALP | Alkaline phosphatase activity |
| AM | Additive manufacturing |
| APS | Poly (ester-amide),1:2 poly (1,3-diamino-2-hydroxypropane-co -polyol sebacate) |
| 3D | Three-dimensional |
| BMP | Bone morphogenic protein |
| BSA | Bovine serum albumin |
| CAD | Computer-aided design |
| CPC | Cardiac progenitor cell |
| Dex | Dexamethasone |
| ECM | Extracellular matrix |
| ES cells | Embryonic stem cells |
| FDM | Fused deposition modeling |
| HA | Hydroxyapatite |
| HDF | Human dermal fibroblast |
| HIF | Hypoxia-inducible factor |
| hMSC | Human mesenchymal stem cell |
| HUVEC | Human umbilical vein endothelial cell |
| ITOP | Integrated tissue–organ printer |
| μCPP | Microscale continuous projection printing method |
| µP | Microparticle |
| µTP | Micro-tissue precursor |
| MSC | Mesenchymal stem cell |
| NIH3T3 | Mouse embryo fibroblast cell line |
| NPC | Neural progenitor cell |
| OPC | Oligodendrocyte progenitor cell |
| Pa | Pascal |
| PBS | Phosphate-buffered saline |
| PCGA | Poly (ε-caprolcatone–co-glycolic acid) |
| PCL | Polycaprolactone |
| PDMS | Polydimethylsiloxane |
| PEG | Poly-ethylene glycol |
| PEGDM | Poly (ethylene glycol) dimethyl ether |
| PEGDA | Polyethylene glycol diacrylate |
| PEGDMA | Polyethylen glycol dimethacrylate |
| PGS | Poly (glycerol sebacate) |
| PLA | Poly-lactic acid |
| PLGA | Polylactic-co-glycolic acid |
| PLLA | Poly(L-lactic acid) |
| PLT32o | Poly (limonene thioether) |
| Poly(NIPAAm-co-HEMAHex) | Poly (N-isopropylacrylamide–co-2-hydroxyethylmethacrylate-6-hydroxyhexanoate) |
| POMaC | Poly (octamethylene maleate (anhydride) citrate |
| PSC | Pluripotent stem cell |
| PU | Polyurethane |
| PVA | Poly (vinyl alcohol) |
| REM | Replica molding |
| RLC | Rat liver cells |
| RNA | RiboNucleic Acid |
| S1P | Sphingosine 1-phosphate |
| SEAL | StampEd Assembly of polymer Layers |
| SLS | Selective laser sintering |
| SMC | Smooth muscle cell |
| SPCL | Starch-polycaprolactone |
| TCP | Tricalcium phosphate |
| TE | Tissue engineering |
| TGF | Transforming growth factor |
| UV | Ultraviolet |
| VEGF | Vascular endothelial growth factor |
References
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Stuart, M.A.C.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Coccoli, V.; Orsi, S.; Ambrosio, L.; Netti, P.A. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials 2008, 29, 4800–4807. [Google Scholar] [CrossRef] [PubMed]
- Netti, P.A. Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing series in biomaterials; Woodhead Publishing: Cambridge, UK, 2014; ISBN 978-0-85709-703-3. [Google Scholar]
- Moioli, E.K.; Clark, P.A.; Xin, X.; Lal, S.; Mao, J.J. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 308–324. [Google Scholar] [CrossRef]
- Schantz, J.-T.; Chim, H.; Whiteman, M. Cell Guidance in Tissue Engineering: SDF-1 Mediates Site-Directed Homing of Mesenchymal Stem Cells within Three-Dimensional Polycaprolactone Scaffolds. Tissue Eng. 2007, 13, 2615–2624. [Google Scholar] [CrossRef]
- Iannone, M.; Ventre, M.; Pagano, G.; Giannoni, P.; Quarto, R.; Netti, P.A. Defining an optimal stromal derived factor-1 presentation for effective recruitment of mesenchymal stem cells in 3D: Optimal SDF-1 Presentation for MSCs Recruitment. Biotechnol. Bioeng. 2014, 111, 2303–2316. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Saunders, R.L.; Hammer, D.A.; Burdick, J.A. Fiber alignment directs cell motility over chemotactic gradients. Biotechnol. Bioeng. 2013, 110, 1249–1254. [Google Scholar] [CrossRef]
- Ma, J.; Both, S.K.; Yang, F.; Cui, F.-Z.; Pan, J.; Meijer, G.J.; Jansen, J.A.; van den Beucken, J.J.J.P. Concise Review: Cell-Based Strategies in Bone Tissue Engineering and Regenerative Medicine. STEM CELLS Transl. Med. 2014, 3, 98–107. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Reinwald, Y.; Johal, R.; Ghaemmaghami, A.; Rose, F.; Howdle, S.; Shakesheff, K.; Howdle, S.; Shakesheff, K. Interconnectivity and permeability of supercritical fluid-foamed scaffolds and the effect of their structural properties on cell distribution. Polymers 2014, 55, 435–444. [Google Scholar] [CrossRef]
- Harley, B.A.C.; Kim, H.-D.; Zaman, M.H.; Yannas, I.V.; Lauffenburger, D.A.; Gibson, L.J. Microarchitecture of Three-Dimensional Scaffolds Influences Cell Migration Behavior via Junction Interactions. Biophys. J. 2008, 95, 4013–4024. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Wang, Z.; Lu, J.; Liu, J.; Chen, G.; Lv, R.; Wang, J.; Lin, K.; Zhang, J.; Huang, X. The Correlation between the Internal Structure and Vascularization of Controllable Porous Bioceramic Materials In Vivo: A Quantitative Study. Tissue Eng. Part A 2010, 16, 3791–3803. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Zhang, W. Control of Scaffold Degradation in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef]
- Huang, J.; Gräter, S.V.; Corbellini, F.; Rinck, S.; Bock, E.; Kemkemer, R.; Kessler, H.; Ding, J.; Spatz, J.P.; Rinck-Jahnke, S. Impact of Order and Disorder in RGD Nanopatterns on Cell Adhesion. Nano Lett. 2009, 9, 1111–1116. [Google Scholar] [CrossRef]
- Sun, A.X.; Lin, H.; Fritch, M.R.; Shen, H.; Alexander, P.G.; Dehart, M.; Tuan, R.S. Chondrogenesis of human bone marrow mesenchymal stem cells in 3-dimensional, photocrosslinked hydrogel constructs: Effect of cell seeding density and material stiffness. Acta Biomater. 2017, 58, 302–311. [Google Scholar] [CrossRef]
- Swinehart, I.T.; Badylak, S.F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis: ECM Bioscaffolds in Development and Healing. Dev. Dyn. 2016, 245, 351–360. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Guldberg, R.E. Materials Science and Design Principles of Growth Factor Delivery Systems in Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2019, 8, 1801000. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Panzetta, V.; Embrione, V.; Netti, P.A. Crosstalk between focal adhesions and material mechanical properties governs cell mechanics and functions. Acta Biomater. 2015, 23, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Kim, D.; Mitchel, J.A.; Bellamkonda, R.V. Topography, cell response, and nerve regeneration. Annu. Rev. Biomed. Eng. 2010, 12, 203–231. [Google Scholar] [CrossRef]
- Carmeliet, P.; Conway, E.M. Growing better blood vessels. Nat. Biotechnol. 2001, 19, 1019–1020. [Google Scholar] [CrossRef]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef]
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef]
- Elbert, D.L. Bottom-up tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Kesireddy, V.; Kasper, F.K. Approaches for building bioactive elements into synthetic scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 6773–6786. [Google Scholar] [CrossRef] [PubMed]
- Leferink, A.; Schipper, D.; Arts, E.; Vrij, E.; Rivron, N.; Karperien, M.; Mittmann, K.; Van Blitterswijk, C.; Moroni, L.; Truckenmuller, R. Engineered Micro-Objects as Scaffolding Elements in Cellular Building Blocks for Bottom-Up Tissue Engineering Approaches. Adv. Mater. 2014, 26, 2592–2599. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Wei, H.-J.; Yeh, Y.-C.; Wang, J.-J.; Lin, W.-W.; Lee, T.-Y.; Hwang, S.-M.; Choi, S.-W.; Xia, Y.; Chang, Y.; et al. Injectable PLGA porous beads cellularized by hAFSCs for cellular cardiomyoplasty. Biomaterials 2012, 33, 4069–4077. [Google Scholar] [CrossRef] [PubMed]
- Maya, I.C.; Guarino, V. Introduction to electrofluidodynamic techniques. Part I. In Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 1–17. [Google Scholar]
- Wang, L.; Chen, Y.; Qian, J.; Tan, Y.; Huangfu, S.; Ding, Y.; Ding, S.; Jiang, B. A bottom-up method to build 3d scaffolds with predefined vascular network. J. Mech. Med. Boil. 2013, 13, 1340008. [Google Scholar] [CrossRef]
- Giannitelli, S.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Cheng, D.; Hou, J.; Hao, L.; Cao, X.; Gao, H.; Fu, X.; Wang, Y. Bottom-up topography assembly into 3D porous scaffold to mediate cell activities. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1056–1063. [Google Scholar] [CrossRef]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.-S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef]
- Cho, D.-I.D.; Yoo, H.J. Microfabrication Methods for Biodegradable Polymeric Carriers for Drug Delivery System Applications: A Review. J. Microelectromech. Syst. 2015, 24, 10–18. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Levato, R.; Mateos-Timoneda, M.A.; Engel, E.; Netti, P.A.; Planell, J.A. Modular polylactic acid microparticle-based scaffolds prepared via microfluidic emulsion/solvent displacement process: Fabrication, characterization, and in vitro mesenchymal stem cells interaction study. J. Biomed. Mater. Res. A 2013, 101A, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, W.; Ma, T.; Dong, H. One-step fabrication of polymeric hybrid particles with core–shell, patchy, patchy Janus and Janus architectures via a microfluidic-assisted phase separation process. RSC Adv. 2015, 5, 79969–79975. [Google Scholar] [CrossRef]
- De Alteriis, R.; Vecchione, R.; Attanasio, C.; De Gregorio, M.; Porzio, M.; Battista, E.; Netti, P.A. A method to tune the shape of protein-encapsulated polymeric microspheres. Sci. Rep. 2015, 5, 12634. [Google Scholar] [CrossRef]
- Dendukuri, D.; Pregibon, D.C.; Collins, J.; Hatton, T.A.; Doyle, P.S. Continuous-flow lithography for high-throughput microparticle synthesis. Nat. Mater. 2006, 5, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Salinas, A.J. Ceramics as bone repair materials. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–178. ISBN 978-0-08-102451-5. [Google Scholar]
- Xia, Y.; Pack, D.W. Uniform biodegradable microparticle systems for controlled release. Chem. Eng. Sci. 2015, 125, 129–143. [Google Scholar] [CrossRef]
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing polymeric microparticles for biomedical and industrial applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Ma, S.; Mukherjee, N. Microfluidics Fabrication of Soft Microtissues and Bottom-Up Assembly. Adv. Biosyst. 2018, 2, 1800119. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.-H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef]
- Choi, S.-W.; Yeh, Y.-C.; Zhang, Y.S.; Sung, H.-W.; Xia, Y. Uniform beads with controllable pore sizes for biomedical applications. Small 2010, 6, 1492–1498. [Google Scholar] [CrossRef]
- Baah, D.; Floyd-Smith, T. Microfluidics for particle synthesis from photocrosslinkable materials. Microfluid. Nanofluid. 2014, 17, 431–455. [Google Scholar] [CrossRef]
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of Monodisperse Particles by Using Microfluidics: Control over Size, Shape, and Composition. Angew. Chem. 2005, 117, 3865. [Google Scholar] [CrossRef]
- Panda, P.; Ali, S.; Lo, E.; Chung, B.G.; Hatton, T.A.; Khademhosseini, A.; Doyle, P.S. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip 2008, 8, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Canelas, D.A.; Herlihy, K.P.; DeSimone, J.M. Top-down particle fabrication: Control of size and shape for diagnostic imaging and drug delivery: Top-down particle fabrication. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Higuita, N.; Dai, Z.; Kaletunç, G.; Hansford, D.J. Fabrication of pH-sensitive microparticles for drug delivery applications using soft lithography techniques. In Proceedings of the Mater. Res. Soc. Symp. Proc. 2008, 1095, 7–12. [Google Scholar]
- Guan, J.; Ferrell, N.; Lee, L.J.; Hansford, D.J. Fabrication of polymeric microparticles for drug delivery by soft lithography. Biomaterials 2006, 27, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Nguyen, T.D.; Linehan, A.R.; Yang, D.; Behrens, A.M.; Rose, S.; Tochka, Z.L.; Tzeng, S.Y.; Norman, J.J.; Anselmo, A.C.; et al. Fabrication of fillable microparticles and other complex 3D microstructures. Science 2017, 359, 1138–1142. [Google Scholar] [CrossRef]
- Wang, H.; Leeuwenburgh, S.C.G.; Li, Y.; Jansen, J.A. The Use of Micro- and Nanospheres as Functional Components for Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2012, 18, 24–39. [Google Scholar] [CrossRef]
- Qutachi, O.; Vetsch, J.R.; Gill, D.; Cox, H.; Scurr, D.J.; Hofmann, S.; Müller, R.; Quirk, R.A.; Shakesheff, K.M.; Rahman, C.V. Injectable and porous PLGA microspheres that form highly porous scaffolds at body temperature. Acta Biomater. 2014, 10, 5090–5098. [Google Scholar] [CrossRef]
- Jeon, J.H.; Bhamidipati, M.; Sridharan, B.; Scurto, A.M.; Berkland, C.J.; Detamore, M.S. Tailoring of processing parameters for sintering microsphere-based scaffolds with dense-phase carbon dioxide. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101B, 330–337. [Google Scholar] [CrossRef]
- Bhamidipati, M.; Sridharan, B.; Scurto, A.M.; Detamore, M.S. Subcritical CO2 sintering of microspheres of different polymeric materials to fabricate scaffolds for tissue engineering. Mater. Sci. Eng. C 2013, 33, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Ghanbar, H.; Luo, C.; Bakhshi, P.; Day, R.; Edirisinghe, M. Preparation of porous microsphere-scaffolds by electrohydrodynamic forming and thermally induced phase separation. Mater. Sci. Eng. C 2013, 33, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Mikael, P.E.; Amini, A.R.; Basu, J.; Arellano-Jimenez, M.J.; Laurencin, C.T.; Sanders, M.M.; Carter, C.B.; Nukavarapu, S.P. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: Fabrication, in vitro and in vivo evaluation. Biomed. Mater. 2014, 9, 35001. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Nair, L.; Laurencin, C.T. Fabrication, characterization, and in vitro evaluation of poly(lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors. J. Biomed. Mater. Res. A 2009, 91A, 679–691. [Google Scholar] [CrossRef]
- Borden, M. Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials 2003, 24, 597–609. [Google Scholar] [CrossRef]
- Brown, J.L.; Nair, L.S.; Laurencin, C.T. Solvent/non-solvent sintering: A novel route to create porous microsphere scaffolds for tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 396–406. [Google Scholar] [CrossRef]
- Shi, X.; Ren, L.; Tian, M.; Yu, J.; Huang, W.; Du, C.; Wang, D.-A.; Wang, Y. In vivo and in vitro osteogenesis of stem cells induced by controlled release of drugs from microspherical scaffolds. J. Mater. Chem. 2010, 20, 9140. [Google Scholar] [CrossRef]
- Jaklenec, A.; Wan, E.; Murray, M.E.; Mathiowitz, E. Novel scaffolds fabricated from protein-loaded microspheres for tissue engineering. Biomaterials 2008, 29, 185–192. [Google Scholar] [CrossRef]
- Singh, M.; Morris, C.P.; Ellis, R.J.; Detamore, M.S.; Berkland, C. Microsphere-Based Seamless Scaffolds Containing Macroscopic Gradients of Encapsulated Factors for Tissue Engineering. Tissue Eng. Part C Methods 2008, 14, 299–309. [Google Scholar] [CrossRef]
- Dormer, N.H.; Singh, M.; Wang, L.; Berkland, C.J.; Detamore, M.S. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann. Biomed. Eng. 2010, 38, 2167–2182. [Google Scholar] [CrossRef]
- Jiang, T.; Nukavarapu, S.P.; Deng, M.; Jabbarzadeh, E.; Kofron, M.D.; Doty, S.B.; Abdel-Fattah, W.I.; Laurencin, C.T. Chitosan–poly (lactide-co-glycolide) microsphere-based scaffolds for bone tissue engineering: In vitro degradation and in vivo bone regeneration studies. Acta Biomater. 2010, 6, 3457–3470. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Lyne, D.V.; Laflin, A.D.; Zabel, T.A.; Barragan, M.; Bunch, J.T.; Pacicca, D.M.; Detamore, M.S. Microsphere-Based Osteochondral Scaffolds Carrying Opposing Gradients of Decellularized Cartilage And Demineralized Bone Matrix. ACS Biomater. Sci. Eng. 2017, 3, 1955–1963. [Google Scholar] [CrossRef]
- Tedesco, M.T.; Di Lisa, D.; Massobrio, P.; Colistra, N.; Pesce, M.; Catelani, T.; Dellacasa, E.; Raiteri, R.; Martinoia, S.; Pastorino, L. Soft chitosan microbeads scaffold for 3D functional neuronal networks. Biomaterials 2018, 156, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, H.; Shuang, J.; Wang, J.; Ma, J.; Zhang, S. Microsphere-based selective laser sintering for building macroporous bone scaffolds with controlled microstructure and excellent biocompatibility. Colloids Surf. B Biointerfaces 2015, 135, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Rossi, L.; Attanasio, C.; Vilardi, E.; De Gregorio, M.; Netti, P.A. Vasculogenic potential evaluation of bottom-up, PCL scaffolds guiding early angiogenesis in tissue regeneration. J. Mater. Sci. Mater. Electron. 2016, 27, 107. [Google Scholar] [CrossRef]
- Urciuolo, F.; Imparato, G.; Totaro, A.; Netti, P.A. Building a Tissue in Vitro from the Bottom Up: Implications in Regenerative Medicine. Methodist DeBakey Cardiovasc. J. 2013, 9, 213–217. [Google Scholar] [CrossRef]
- Casale, C.; Imparato, G.; Urciuolo, F.; Netti, P.A. Endogenous human skin equivalent promotes in vitro morphogenesis of follicle-like structures. Biomaterials 2016, 101, 86–95. [Google Scholar] [CrossRef]
- Mazio, C.; Casale, C.; Imparato, G.; Urciuolo, F.; Attanasio, C.; De Gregorio, M.; Rescigno, F.; Netti, P.A. Pre-vascularized dermis model for fast and functional anastomosis with host vasculature. Biomaterials 2019, 192, 159–170. [Google Scholar] [CrossRef]
- Casale, C.; Imparato, G.; Urciuolo, F.; Rescigno, F.; Scamardella, S.; Escolino, M.; Netti, P.A. Engineering a human skin equivalent to study dermis remodelling and epidermis senescence in vitro after UVA exposure. J. Tissue Eng. Regen. Med. 2018, 12, 1658–1669. [Google Scholar] [CrossRef]
- Totaro, A.; Salerno, A.; Imparato, G.; Domingo, C.; Urciuolo, F.; Netti, P.A. PCL-HA microscaffolds for in vitro modular bone tissue engineering: PCL-HA microscaffolds for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Urciuolo, F.; Imparato, G.; Netti, P.A. Engineered cardiac micromodules for the in vitro fabrication of 3D endogenous macro-tissues. Biofabrication 2016, 8, 025014. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Yamada, M.; Utoh, R.; Seki, M. Collagen Microparticle-Mediated 3D Cell Organization: A Facile Route to Bottom-up Engineering of Thick and Porous Tissues. ACS Biomater. Sci. Eng. 2017, 3, 2144–2154. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Ye, Z.; Zhang, Y.; Zhou, Y.; Tan, W.-S. A modular approach to the engineering of a centimeter-sized bone tissue construct with human amniotic mesenchymal stem cells-laden microcarriers. Biomaterials 2011, 32, 7532–7542. [Google Scholar] [CrossRef]
- Scott, E.A.; Nichols, M.D.; Kuntz-Willits, R.; Elbert, D.L. Modular scaffolds assembled around living cells using poly(ethylene glycol) microspheres with macroporation via a non-cytotoxic porogen. Acta Biomater. 2010, 6, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Tan, X.; Yeong, W.Y.; Tor, S.B. Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: A new biofabrication strategy. Sci. Rep. 2016, 6, 39140. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Xi, H.; Li, J.; Wei, D.; Li, B.; Liao, X.; Fan, H. Fabrication and assembly of porous micropatterned scaffolds for modular tissue engineering. Mater. Lett. 2018, 228, 360–364. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Lu, H.; Yang, Y.; Liu, Z.; Shang, W.; Shen, Y. Magnetically Actuated Heterogeneous Microcapsule-Robot for the Construction of 3D Bioartificial Architectures. ACS Appl. Mater. Interfaces 2019, 11, 25664–25673. [Google Scholar] [CrossRef]
- Gallego, D.; Ferrell, N.; Sun, Y.; Hansford, D.J. Multilayer micromolding of degradable polymer tissue engineering scaffolds. Mater. Sci. Eng. C 2008, 28, 353–358. [Google Scholar] [CrossRef]
- Sodha, S.; Wall, K.; Redenti, S.; Klassen, H.; Young, M.J.; Tao, S.L. Microfabrication of a Three-Dimensional Polycaprolactone Thin-Film Scaffold for Retinal Progenitor Cell Encapsulation. J. Biomater. Sci. Polym. Ed. 2011, 22, 443–456. [Google Scholar] [CrossRef]
- Rosellini, E.; Vozzi, G.; Barbani, N.; Giusti, P.; Cristallini, C. Three-dimensional microfabricated scaffolds with cardiac extracellular matrix-like architecture. Int. J. Artif. Organs 2010, 33, 885–894. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Hao, X.; Mao, M.; Zhu, L.; Li, D. Bottom-up generation of 3D silk fibroin–gelatin microfluidic scaffolds with improved structural and biological properties. Mater. Lett. 2012, 78, 102–105. [Google Scholar] [CrossRef]
- He, J.; Zhu, L.; Liu, Y.; Li, D.; Jin, Z. Sequential assembly of 3D perfusable microfluidic hydrogels. J. Mater. Sci. Mater. Electron. 2014, 25, 2491–2500. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Bae, C.Y.; Park, J.-K. Freestanding stacked mesh-like hydrogel sheets enable the creation of complex macroscale cellular scaffolds. Biotechnol. J. 2016, 11, 585–591. [Google Scholar] [CrossRef]
- Son, J.; Bang, M.S.; Park, J.-K. Hand-Maneuverable Collagen Sheet with Micropatterns for 3D Modular Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 339–345. [Google Scholar] [CrossRef]
- Zhang, B.; Lai, B.F.L.; Xie, R.; Huyer, L.D.; Montgomery, M.; Radisic, M. Microfabrication of AngioChip, a biodegradable polymer scaffold with microfluidic vasculature. Nat. Protoc. 2018, 13, 1793–1813. [Google Scholar] [CrossRef]
- Lee, B.-K.; Lee, B.-Y. Investigation of thermoplastic hot embossing process using soft polydimethylsiloxane (PDMS) micromold. J. Mech. Sci. Technol. 2015, 29, 5063–5067. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Kang, X.; Lee, L.J.; Kniss, D.A. Assembly of Three-Dimensional Polymeric Constructs Containing Cells/Biomolecules Using Carbon Dioxide. J. Am. Chem. Soc. 2006, 128, 14040–14041. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, Y.; Kang, X.; Li, R.; Volakis, L.I.; Zhang, X.; Lee, L.J.; Kniss, D.A. Bioassembly of three-dimensional embryonic stem cell-scaffold complexes using compressed gases. Biotechnol. Prog. 2009, 25, 535–542. [Google Scholar] [CrossRef]
- Wang, Y.; Balowski, J.; Phillips, C.; Phillips, R.; Sims, C.E.; Allbritton, N.L. Benchtop micromolding of polystyrene by soft lithography. Lab Chip 2011, 11, 3089–3097. [Google Scholar] [CrossRef]
- Papenburg, B.J.; Liu, J.; Higuera, G.A.; Barradas, A.M.; De Boer, J.; Van Blitterswijk, C.A.; Wessling, M.; Stamatialis, D. Development and analysis of multi-layer scaffolds for tissue engineering. Biomaterials 2009, 30, 6228–6239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, M.; Takeuchi, M.; Yue, T.; Hasegawa, Y.; Huang, Q.; Fukuda, T. In vitro mimicking the morphology of hepatic lobule tissue based on Ca-alginate cell sheets. Biomed. Mater. 2018, 13, 035004. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lu, L.; Kolewe, M.E.; Hearon, K.; Fischer, K.M.; Coppeta, J.; Freed, L.E. Scalable units for building cardiac tissue. Adv. Mater. 2014, 26, 7202–7208. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.M.; Morgan, K.Y.; Hearon, K.; Sklaviadis, D.; Tochka, Z.L.; Fenton, O.S.; Anderson, D.G.; Langer, R.; Freed, L.E. Poly (Limonene Thioether) Scaffold for Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.; Hammerick, K.E.; Kim, Y.B.; Kim, J.B.; Fasching, R.; Prinz, F.B. Three-dimensional biodegradable microscaffolding: Scaffold characterization and cell population at single cell resolution. Acta Biomater. 2011, 7, 3325–3335. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.J.; Pirraco, R.P.; Sousa, R.A.; Neves, N.M.; Marques, A.P.; Bhattacharya, M.; Correlo, V.M.; Reis, R.L. Bottom-up approach to construct microfabricated multi-layer scaffolds for bone tissue engineering. Biomed. Microdevices 2014, 16, 69–78. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Chen, G.; Sun, Y.; Lu, F.; Jiang, A.; Subedi, D.; Kong, P.; Wang, X.; Yu, T.; Chi, H.; Song, C.; et al. A three-dimensional (3D) printed biomimetic hierarchical scaffold with a covalent modular release system for osteogenesis. Mater. Sci. Eng. C 2019, 104, 109842. [Google Scholar] [CrossRef]
- Moncal, K.K.; Aydin, R.S.T.; Abu-Laban, M.; Heo, D.N.; Rizk, E.; Tucker, S.M.; Lewis, G.S.; Hayes, D.; Ozbolat, I.T. Collagen-infilled 3D printed scaffolds loaded with miR-148b-transfected bone marrow stem cells improve calvarial bone regeneration in rats. Mater. Sci. Eng. C 2019, 105, 110128. [Google Scholar] [CrossRef]
- Mekhileri, N.V.; Lim, K.S.; Brown, G.C.J.; Mutreja, I.; Schon, B.S.; Hooper, G.J.; Woodfield, T.B.F.; Lim, K. Automated 3D bioassembly of micro-tissues for biofabrication of hybrid tissue engineered constructs. Biofabrication 2018, 10, 024103. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C.; et al. Direct 3D Printing of High Strength Biohybrid Gradient Hydrogel Scaffolds for Efficient Repair of Osteochondral Defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Deng, C.; Yang, Q.; Sun, X.; Chen, L.; Feng, C.; Chang, J.; Wu, C. Bioactive scaffolds with Li and Si ions-synergistic effects for osteochondral defects regeneration. Appl. Mater. Today 2018, 10, 203–216. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, D.; Huang, S.; Yang, Q.; Song, B.; Guo, Y.; Shen, A.; Yuan, Z.; Li, S.; Qing, F.; et al. Elastic 3D-Printed Hybrid Polymeric Scaffold Improves Cardiac Remodeling after Myocardial Infarction. Adv. Healthc. Mater. 2019, 8, e1900065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Seong, B.; Nguyen, V.; Byun, D. 3D printing of high-resolution PLA-based structures by hybrid electrohydrodynamic and fused deposition modeling techniques. J. Micromech. Microeng. 2016, 26, 25015. [Google Scholar] [CrossRef]
- Boffito, M.; Di Meglio, F.; Mozetic, P.; Giannitelli, S.M.; Carmagnola, I.; Castaldo, C.; Nurzynska, D.; Sacco, A.M.; Miraglia, R.; Montagnani, S.; et al. Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLoS ONE 2018, 13, e0199896. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.-Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M.; et al. 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Li, X.; Xiao, Z.; Yao, Y.; Chu, Y.; Farkas, B.; Romano, I.; Brandi, F.; Dai, J. Functional Multichannel Poly (Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv. Healthc. Mater. 2018, 7, 1800315. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salerno, A.; Cesarelli, G.; Pedram, P.; Netti, P.A. Modular Strategies to Build Cell-Free and Cell-Laden Scaffolds towards Bioengineered Tissues and Organs. J. Clin. Med. 2019, 8, 1816. https://doi.org/10.3390/jcm8111816
Salerno A, Cesarelli G, Pedram P, Netti PA. Modular Strategies to Build Cell-Free and Cell-Laden Scaffolds towards Bioengineered Tissues and Organs. Journal of Clinical Medicine. 2019; 8(11):1816. https://doi.org/10.3390/jcm8111816
Chicago/Turabian StyleSalerno, Aurelio, Giuseppe Cesarelli, Parisa Pedram, and Paolo Antonio Netti. 2019. "Modular Strategies to Build Cell-Free and Cell-Laden Scaffolds towards Bioengineered Tissues and Organs" Journal of Clinical Medicine 8, no. 11: 1816. https://doi.org/10.3390/jcm8111816
APA StyleSalerno, A., Cesarelli, G., Pedram, P., & Netti, P. A. (2019). Modular Strategies to Build Cell-Free and Cell-Laden Scaffolds towards Bioengineered Tissues and Organs. Journal of Clinical Medicine, 8(11), 1816. https://doi.org/10.3390/jcm8111816






