Arginase Isoform Expression in Chronic Rhinosinusitis
Abstract
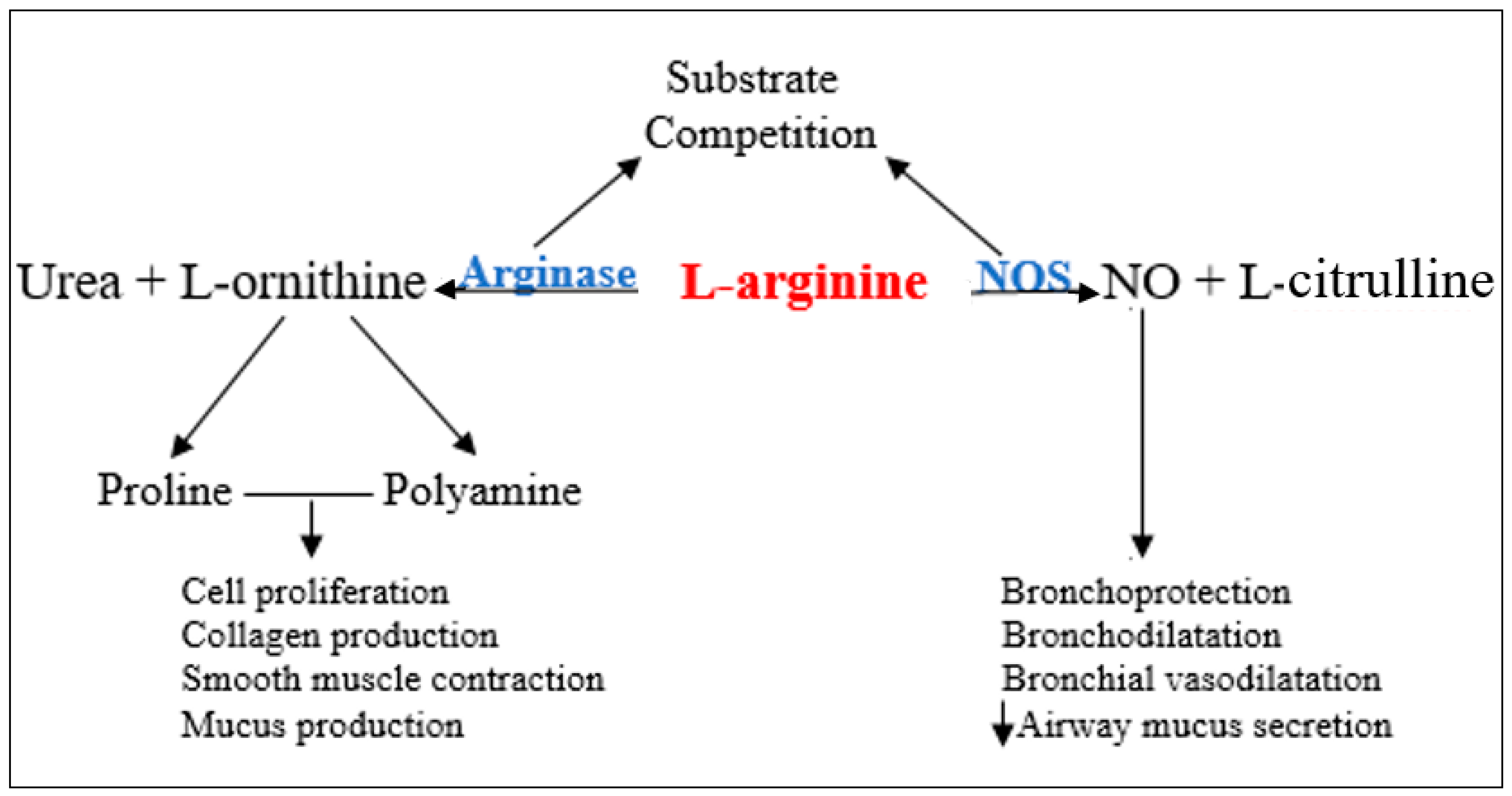
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Classification
2.3. Sample Collection
2.4. Genetic Assessment
2.5. Statistical Analysis
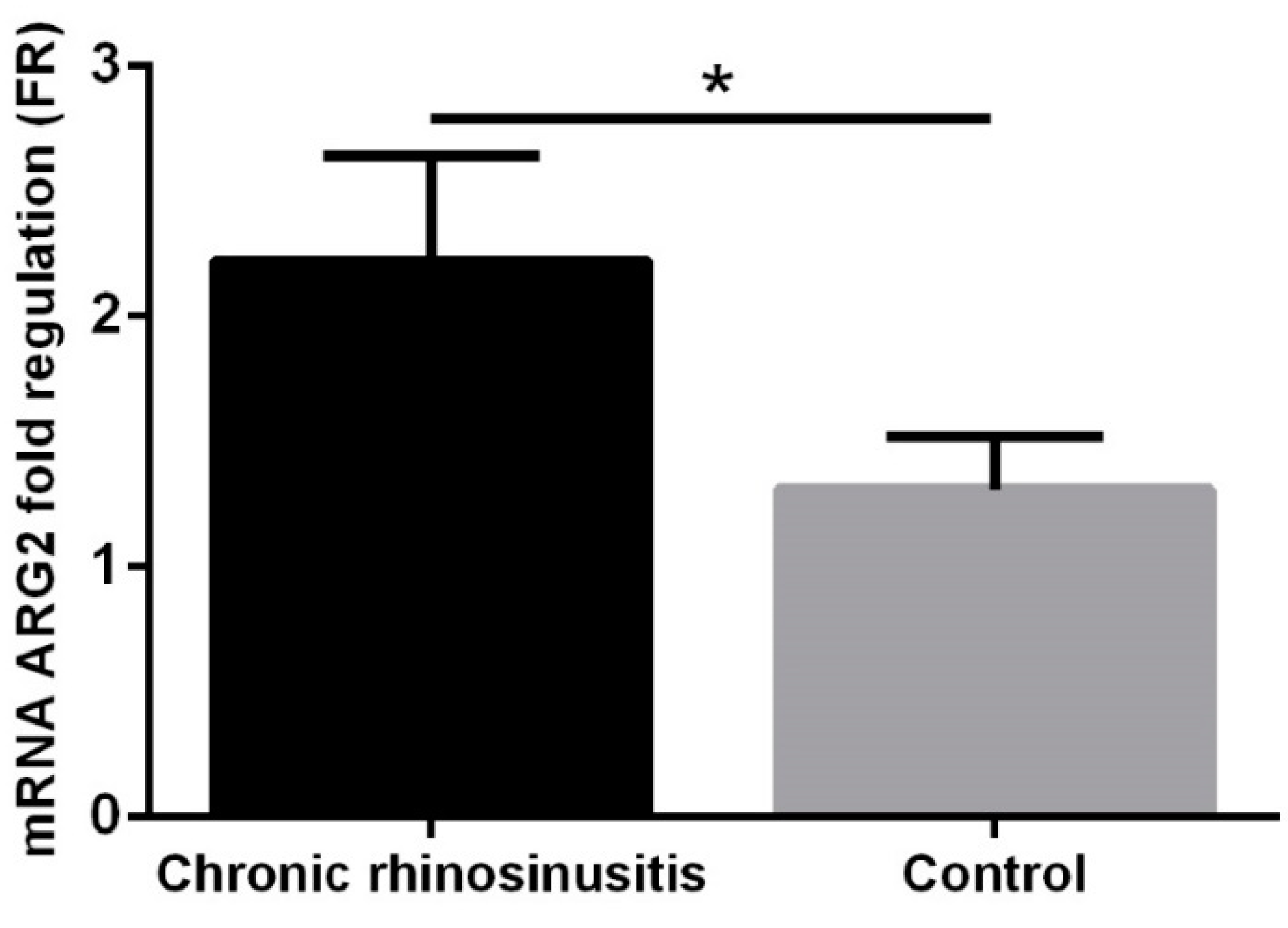
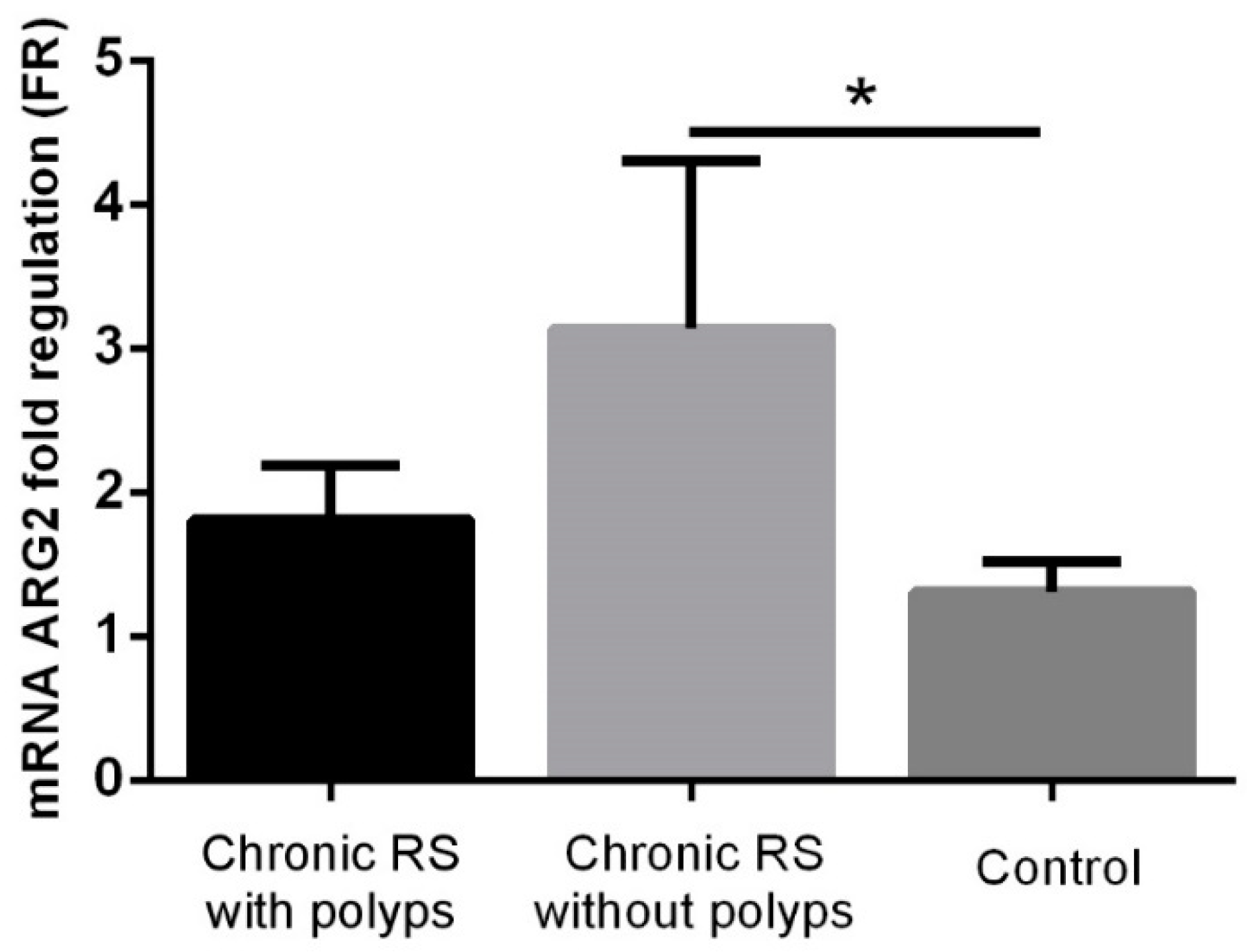
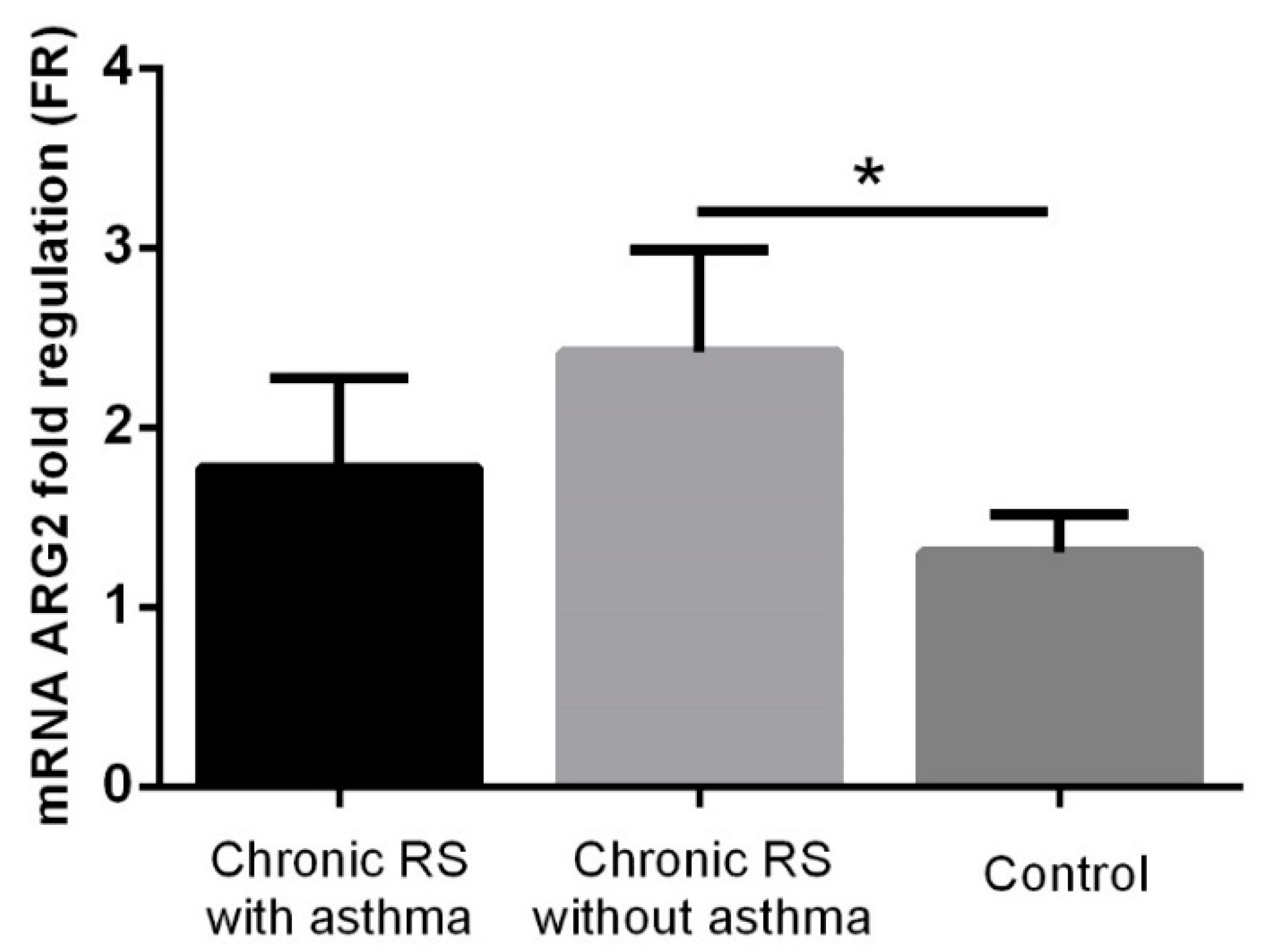
3. Results
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rosenfeld, R.M.; Piccirillo, J.F.; Chandrasekhar, S.S.; Brook, I.; Ashok Kumar, K.; Kramper, M.; Orlandi, R.R.; Palmer, J.N.; Patel, Z.M.; Peters, A.; et al. Clinical practice guideline (update): Adult sinusitis. Otolaryngol. Head Neck Surg. 2015, 152 (Suppl. 2), S1–S39. [Google Scholar] [CrossRef] [PubMed]
- Tomassen, P.; Zele, T.V.; Zhang, N.; Perez-Novo, C.; Bruaene, N.V.; Gevaert, P.; Bachert, C. Pathophysiology of chronic rhinosinusitis. Proc. Am. Thorac. Soc. 2011, 8, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Eby, G.A. Strong humming for one hour daily to terminate chronic rhinosinusitis in four days: A case report and hypothesis for action by stimulation of endogenous nasal nitric oxide production. Med. Hypotheses 2006, 66, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Taruya, T.; Takeno, S.; Kubota, K.; Sasaki, A.; Ishino, T.; Hirakawa, K. Comparison of arginase isoform expression in patients with different subtypes of chronic rhinosinusitis. J. Laryngol. Otol. 2015, 129, 1194–1200. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M. Multiple roles of nitric oxide in the airways. Thorax 2003, 58, 175–182. [Google Scholar] [CrossRef]
- Bommarito, L.; Guida, G.; Heffler, E.; Badiu, I.; Nebiolo, F.; Usai, A.; De Stefani, A.; Rolla, G. Nasal nitric oxide concentration in suspected chronic rhinosinusitis. Ann. Allergy Asthma Immunol. 2008, 101, 358–362. [Google Scholar] [CrossRef]
- Lanz, M.J.; Prendes, S.; Peyrou, N.; Toledo, G.; Ferrer, C.M. Nasal nitric oxide as a noninvasive marker in the antibiotic treatment of acute bacterial sinusitis. J. Allergy Clin. Immunol. 2008, 121, 530–531. [Google Scholar] [CrossRef]
- Woodehouse, T.; Kharitonov, S.A.; Mackay, I.S.; Barnes, P.J.; Wilson, R.; Cole, P.J. Nasal nitric oxide for the screening of primary ciliary dyskinesia. Eur. Respir. J. 2003, 21, 43–47. [Google Scholar] [CrossRef]
- Lee, K.J.; Cho, S.H.; Lee, S.H.; Tae, K.; Yoon, H.J.; Kim, S.H.; Jeong, J.H. Nasal and Exhaled Nitric Oxide in Allergic Rhinitis. Clin. Exp. Otorhinolaryngol. 2012, 5, 228–233. [Google Scholar] [CrossRef]
- Vlad, D.; Trombitas, V.; Capusan, M.; Albu, S. The role of nitric oxide in chronic rhinosinusitis. Rom. J. Rhinol. 2015, 5, 135–141. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Ten Have, G.A.; Wolfe, R.R.; Deutz, N.E. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef] [PubMed]
- Maarsingh, H.; Zaagsma, J.; Meurs, H. Arginine homeostasis in allergic asthma. Eur. J. Pharmacol. 2008, 585, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, N.; Rothenberg, M.E. The arginine-arginase balance in asthma and lung inflammation. Eur. J. Pharmacol. 2006, 533, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Munder, M. Arginase: An emerging key player in the mammalian immune system. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef]
- Lara, A.; Khatri, S.B.; Wang, Z.; Comhair, S.A.; Xu, W.; Dweik, R.A.; Bodine, M.; Levison, B.S.; Hammel, J.; Bleecker, E.; et al. Alterations of the arginine metabolome in asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 673–681. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef]
- Han, J.K. Subclassification of chronic rhinosinusitis. Laryngoscope 2013, 123 (Suppl. 2), S15–S27. [Google Scholar] [CrossRef]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitis. Rhinology 1993, 31, 183–184. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2017. Available online: www.ginaasthma.org (accessed on 20 July 2016).
- Wallace, D.V.; Bahna, S.L.; Goldstein, S.; Hamilton, R.G.; Cohn, J.R. American Academy of Allergy, Asthma & Immunology Work Group Report: Allergy diagnosis in clinical practice. J. Allergy Clin. Immunol. 2007, 120, 967–969. [Google Scholar]
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Duong-Quy, S.; Vu-Minh, T.; Hua-Huy, T.; Tang-Thi-Thao, T.; Le-Quang, K.; Tran-Thanh, D.; Doan-Thi-Quynh, N.; Le-Dong, N.N.; Craig, T.J.; Dinh-Xuan, A.T. Study of nasal exhaled nitric oxide levels in diagnosis of allergic rhinitis in subjects with and without asthma. J. Asthma Allergy 2017, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Runner, T.; Lindberg, S. Effects of nitric oxide on blood flow and mucociliary activity in the human nose. Ann. Otol. Rhinol. Laryngol. 1998, 107, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Runner, T.; Cervin, A.; Lindberg, S.; Uddman, R. Nitric oxide is a regulator of mucociliary activity in the upper respiratory tract. Otolaryngol. Head Neck Surg. 1998, 119, 278–287. [Google Scholar]
- McInnes, I.B.; Liew, F.Y. Immunomodulatory Actions of Nitric Oxide. In Nitric Oxide and Infection; Springer: Boston, MA, USA, 2002; Volume 10, pp. 199–213. [Google Scholar]
- Ibiza, S.; Serrador, J.M. The role of nitric oxide in the regulation of adaptive immune responses. Inmunología 2008, 27, 103–117. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Koyasu, S.; Moro, K. Type 2 innate immune responses and the natural helper cell. Immunology 2011, 132, 475–481. [Google Scholar] [CrossRef]
- Niedbala, W.; Cai, B.; Liew, F.Y. Role of nitric oxide in the regulation of T cell functions. Ann. Rheum. Dis. 2006, 65 (Suppl. 3), iii37–iii40. [Google Scholar] [CrossRef]
- Ragab, S.M.; Lund, V.J.; Saleh, H.A.; Scadding, G. Nasal nitric oxide in objective evaluation of chronic rhinosinusitis therapy. Allergy 2006, 61, 717–724. [Google Scholar] [CrossRef]
- Maniscalco, M.; Sofia, M.; Pelaia, G. Nitric oxide in upper airways inflammatory diseases. Inflamm. Res. 2007, 56, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Dabholkar, Y.G.; Saberwal, A.A.; Velankar, H.K.; Shetty, A.K.; Chordia, N.P.; Budhwani, S.R. Correlation of Nasal Nitric Oxide Measurement with Computed Tomography Findings in Chronic Rhinosinusitis. Indian J. Otolaryngol. Head Neck Surg. 2014, 66, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M., Jr. Recent advances in arginine metabolism: Roles and regulation of the arginases. Br. J. Pharmacol. 2009, 157, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Pera, T.; Zuidhof, A.B.; Smit, M.; Menzen, M.H.; Klein, T.; Flik, G.; Zaagsma, J.; Meurs, H.; Maarsingh, H. Arginase Inhibition Prevents Inflammation and Remodeling in a Guinea Pig Model of Chronic Obstructive Pulmonary Disease. J. Pharmacol. Exp. Ther. 2014, 349, 229–238. [Google Scholar] [CrossRef]
- Maarsingh, H.; Dekkers, B.G.; Zuidhof, A.B.; Bos, I.S.; Menzen, M.H.; Klein, T.; Flik, G.; Zaagsma, J.; Meurs, H. Increased arginase activity contributes to airway remodelling in chronic allergic asthma. Eur. Respir. J. 2011, 38, 318–328. [Google Scholar] [CrossRef]
- Bratt, J.M.; Zeki, A.A.; Last, J.A.; Kenyon, N.J. Competitive metabolism of L-arginine: Arginase as a therapeutic target in asthma. J. Biomed. Res. 2011, 25, 299–308. [Google Scholar] [CrossRef]
- Lee SLane, A.P. Chronic Rhinosinusitis as a Multifactorial Inflammatory Disorder. Curr. Infect. Dis. Rep. 2011, 13, 159–168. [Google Scholar] [CrossRef]
- Shah, S.A.; Ishinaga, H.; Takeuchi, K. Pathogenesis of eosinophilic chronic rhinosinusitis. J. Inflamm. 2016, 13, 11. [Google Scholar] [CrossRef]
- Kato, A. Immunopathology of chronic rhinosinusitis. Allergol. Int. 2015, 64, 121–130. [Google Scholar] [CrossRef]
- Jeong, J.H.; Yoo, H.S.; Lee, S.H.; Kim, K.R.; Yoon, H.J.; Kim, S.H. Nasal and exhaled nitric oxide in chronic rhinosinusitis with polyps. Am. J. Rhinol. Allergy 2014, 28, e11–e16. [Google Scholar] [CrossRef]
- Lee, J.M.; McKnight, C.L.; Aves, T.; Yip, J.; Grewal, A.S.; Gupta, S. Nasal nitric oxide as a marker of sinus mucosal health in patients with nasal polyposis. Int. Forum Allergy Rhinol. 2015, 5, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.N.; Yang, Y.F.; Ono, S.; Zhong, X.G.; Feng, Y.H.; Ren, Y.X.; Ni, J.; Fu, Y.F.; Tang, W.; Zuo, J.P. Differential expression of inducible nitric oxide synthase and IL-12 between peritoneal and splenic macrophages stimulated with LPS plus IFN-gamma is associated with the activation of extracellular signal-related kinase. Int. Immunol. 2006, 18, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Gannon, P.O.; Godin-Ethier, J.; Hassler, M.; Delvoye, N.; Aversa, M.; Poisson, A.O.; Péant, B.; Alam Fahmy, M.; Saad, F.; Lapointe, R.; et al. Androgen-regulated expression of arginase 1, arginase 2 and interleukin-8 in human prostate cancer. PLoS ONE 2010, 5, e12107. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, R.; Barisione, G.; Mastracci, L.; Grossi, F.; Orengo, A.M.; Costa, R.; Truini, M.; Fabbi, M.; Ferrini, S.; Barbieri, O. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int. J. Cancer 2009, 125, 887–893. [Google Scholar] [CrossRef]
- Colantonio, D.; Brouillette, L.; Parikh, A.; Scadding, G.K. Paradoxical low nasal nitric oxide in nasal polyposis. Clin. Exp. Allergy 2002, 32, 698–701. [Google Scholar] [CrossRef]

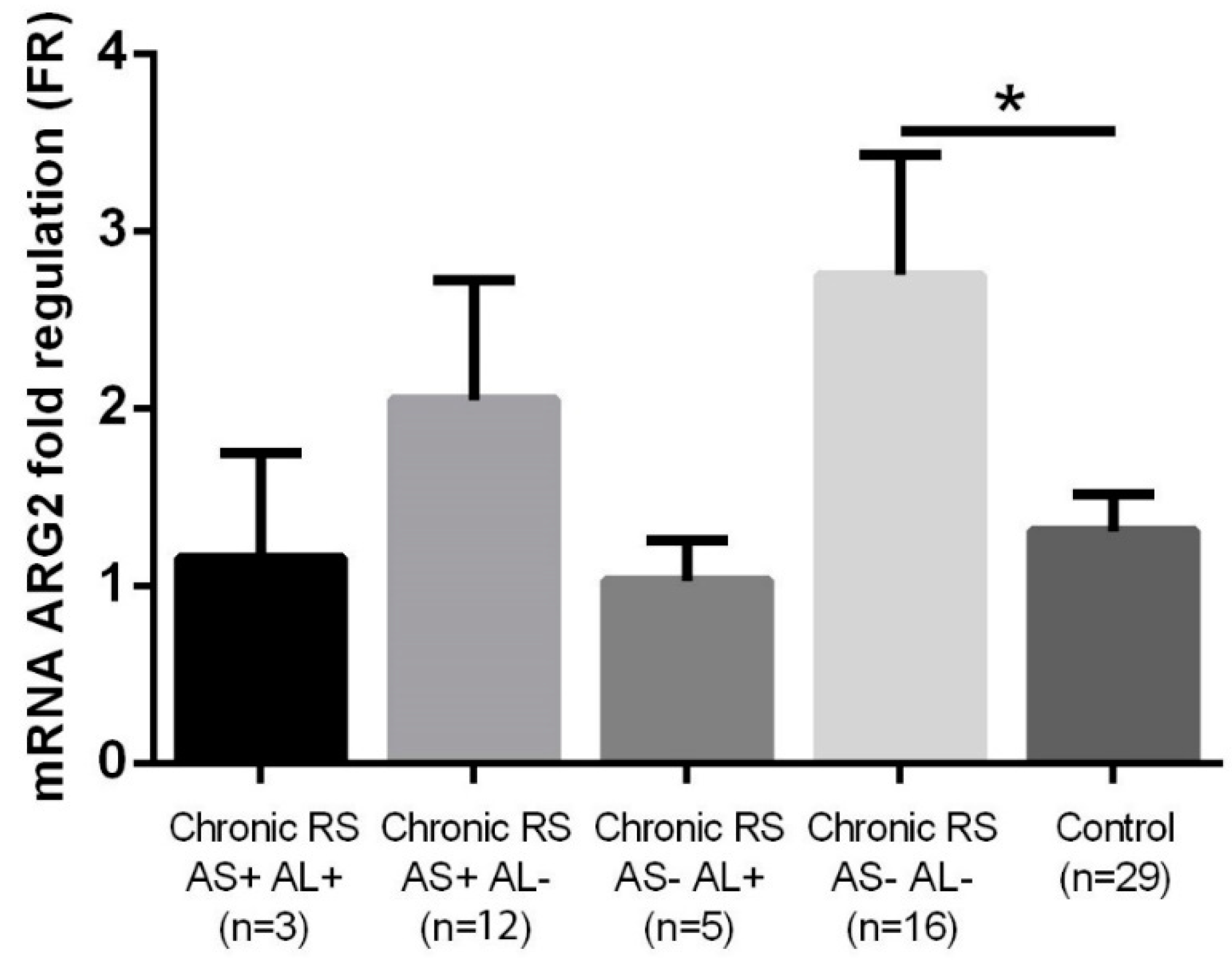

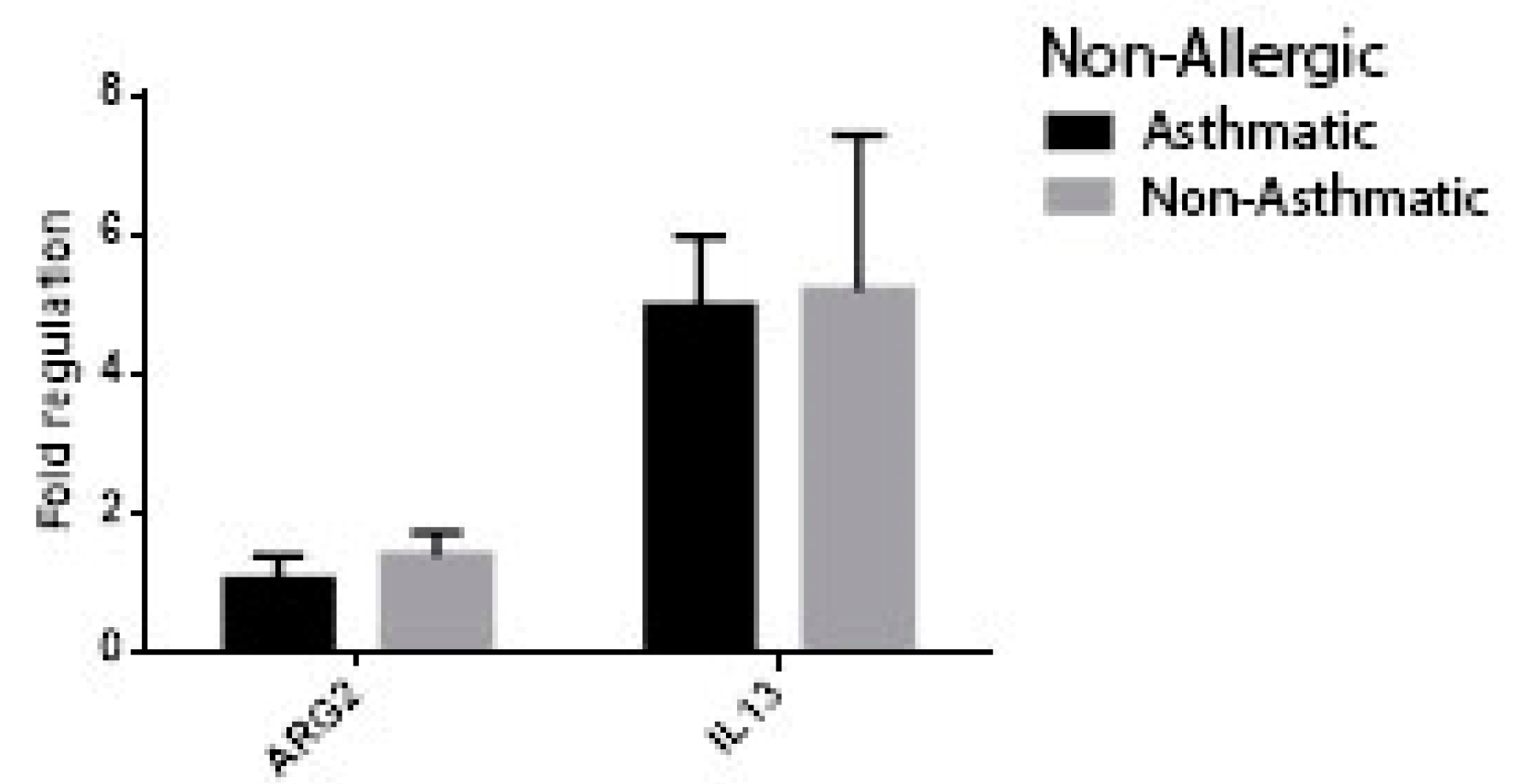

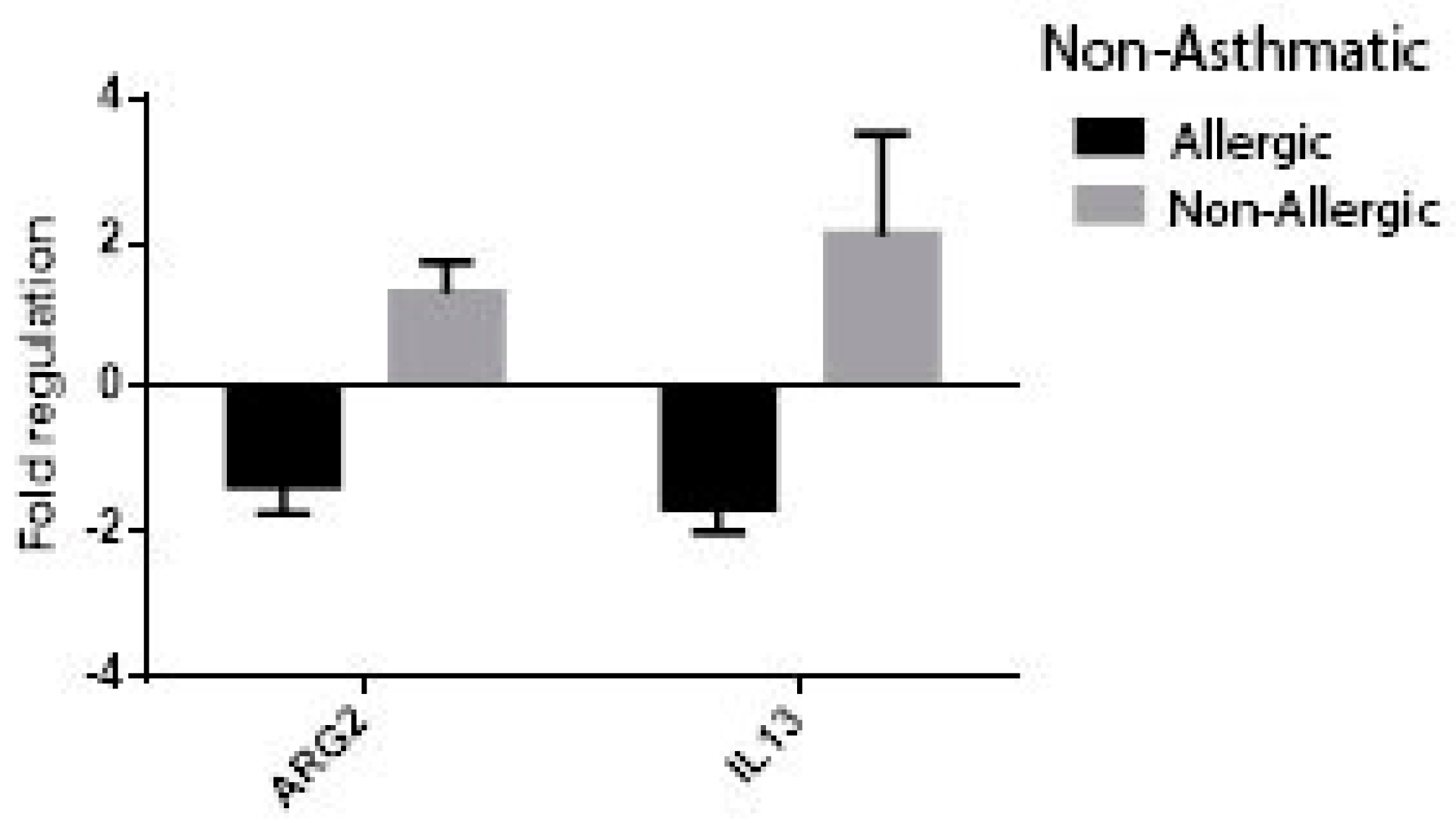
| Chronic Rhinosinusitis (n = 36) | Control (n = 29) | p-Value | |
|---|---|---|---|
| Age (years) | 45.8 ± 10.7 | 42.7 ± 15.4 | 0.35 |
| Male gender | 18 (50%) | 17 (58.6%) | 0.65 |
| Urban sample | 24 (66.6%) | 19 (65.5%) | 0.66 |
| Polyposis | 14 (38.8%) | - | - |
| Respiratory allergy | 8 (22.2%) | - | - |
| Asthma + (AL+ AS+) | 3 (8.3%) | - | - |
| Asthma − (AL+ AS−) | 5 (13.8%) | - | - |
| Asthma | |||
| Allergy + (AS+ AL+) | 15 (41.6%) | - | - |
| Allergy − (AS+ AL−) | 3 (8.3%) | - | - |
| Asthma − Allergy − | 12 (27.7%) | - | - |
| (AS − AL−) | 16 (44.4%) | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlad, D.; Albu, S. Arginase Isoform Expression in Chronic Rhinosinusitis. J. Clin. Med. 2019, 8, 1809. https://doi.org/10.3390/jcm8111809
Vlad D, Albu S. Arginase Isoform Expression in Chronic Rhinosinusitis. Journal of Clinical Medicine. 2019; 8(11):1809. https://doi.org/10.3390/jcm8111809
Chicago/Turabian StyleVlad, Diana, and Silviu Albu. 2019. "Arginase Isoform Expression in Chronic Rhinosinusitis" Journal of Clinical Medicine 8, no. 11: 1809. https://doi.org/10.3390/jcm8111809
APA StyleVlad, D., & Albu, S. (2019). Arginase Isoform Expression in Chronic Rhinosinusitis. Journal of Clinical Medicine, 8(11), 1809. https://doi.org/10.3390/jcm8111809






