Characterization and Potential Applications of Dog Natural Killer Cells in Cancer Immunotherapy
Abstract
1. Introduction
2. The Genetic Basis of Canine NK Cells
3. Phenotypic Identification of Canine NK Cells with Surface Markers
4. Ex vivo Manipulation and Expansion of Canine NK Cells
5. Clinical Applications of Canine NK Cells
Author Contributions
Funding
Conflicts of Interest
References
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Vallentin, B.; Barlogis, V.; Piperoglou, C.; Cypowyj, S.; Zucchini, N.; Chene, M.; Navarro, F.; Farnarier, C.; Vivier, E.; Vely, F. Innate Lymphoid Cells in Cancer. Cancer Immunol. Res. 2015, 3, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef]
- Suen, W.C.; Lee, W.Y.; Leung, K.T.; Pan, X.H.; Li, G. Natural Killer Cell-Based Cancer Immunotherapy: A Review on 10 Years Completed Clinical Trials. Cancer Investig. 2018, 36, 431–457. [Google Scholar] [CrossRef]
- Li, Y.; Yin, J.; Li, T.; Huang, S.; Yan, H.; Leavenworth, J.; Wang, X. NK cell-based cancer immunotherapy: From basic biology to clinical application. Sci. China Life Sci. 2015, 58, 1233–1245. [Google Scholar] [CrossRef]
- Habif, G.; Crinier, A.; Andre, P.; Vivier, E.; Narni-Mancinelli, E. Targeting natural killer cells in solid tumors. Cell. Mol. Immunol. 2019, 16, 415–422. [Google Scholar] [CrossRef]
- Chiossone, L.; Vienne, M.; Kerdiles, Y.M.; Vivier, E. Natural killer cell immunotherapies against cancer: Checkpoint inhibitors and more. Semin. Immunol. 2017, 31, 55–63. [Google Scholar] [CrossRef]
- Becker, P.S.; Suck, G.; Nowakowska, P.; Ullrich, E.; Seifried, E.; Bader, P.; Tonn, T.; Seidl, C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016, 65, 477–484. [Google Scholar] [CrossRef]
- Grossenbacher, S.K.; Aguilar, E.G.; Murphy, W.J. Leveraging natural killer cells for cancer immunotherapy. Immunotherapy 2017, 9, 487–497. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Lanier, L.L.; Phillips, J.H. Development of human T and natural killer cells. Blood 1995, 85, 2654–2670. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Nunes, J.A.; Vely, F. Natural killer cell signaling pathways. Science 2004, 306, 1517–1519. [Google Scholar] [CrossRef]
- Goh, W.; Huntington, N.D. Regulation of Murine Natural Killer Cell Development. Front. Immunol. 2017, 8, 130. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Thielens, A.; Marabelle, A.; Sagiv-Barfi, I.; Sola, C.; Chanuc, F.; Fuseri, N.; Bonnafous, C.; Czerwinski, D.; Rajapaksa, A.; et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014, 123, 678–686. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Leitman, S.; Chang, A.E.; Ettinghausen, S.E.; Matory, Y.L.; Skibber, J.M.; Shiloni, E.; Vetto, J.T.; et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, W.; Tian, Z. NK cell-based immunotherapy for cancer. Semin. Immunol. 2017, 31, 37–54. [Google Scholar] [CrossRef]
- Luna, J.I.; Grossenbacher, S.K.; Murphy, W.J.; Canter, R.J. Targeting Cancer Stem Cells with Natural Killer Cell Immunotherapy. Expert Opin. Biol. Ther. 2017, 17, 313–324. [Google Scholar] [CrossRef]
- Sungur, C.M.; Murphy, W.J. Positive and negative regulation by NK cells in cancer. Crit. Rev. Oncog. 2014, 19, 57–66. [Google Scholar] [CrossRef]
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Chen, M.; Smith, R.C.; Hagino, T.; Perez-Cunningham, J.; Sckisel, G.D.; Urayama, S.; et al. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J. Immunol. 2015, 195, 4010–4019. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.W.; Wagner, J.A.; Ireland, A.R.; Fehniger, T.A. Transcriptional and post-transcriptional regulation of NK cell development and function. Clin. Immunol. 2017, 177, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Childs, R. Ex-vivo expansion of NK cells: What is the priority--high yield or high purity? Cytotherapy 2010, 12, 969–970. [Google Scholar] [CrossRef]
- Berg, M.; Lundqvist, A.; McCoy, P., Jr.; Samsel, L.; Fan, Y.; Tawab, A.; Childs, R. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 2009, 11, 341–355. [Google Scholar] [CrossRef]
- Childs, R.W.; Berg, M. Bringing natural killer cells to the clinic: Ex vivo manipulation. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 234–246. [Google Scholar] [CrossRef]
- Denman, C.J.; Senyukov, V.V.; Somanchi, S.S.; Phatarpekar, P.V.; Kopp, L.M.; Johnson, J.L.; Singh, H.; Hurton, L.; Maiti, S.N.; Huls, M.H.; et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 2012, 7, e30264. [Google Scholar] [CrossRef]
- Dhupkar, P.; Gordon, N. Interleukin-2: Old and New Approaches to Enhance Immune-Therapeutic Efficacy. Adv. Exp. Med. Biol. 2017, 995, 33–51. [Google Scholar] [CrossRef]
- Fujisaki, H.; Kakuda, H.; Shimasaki, N.; Imai, C.; Ma, J.; Lockey, T.; Eldridge, P.; Leung, W.H.; Campana, D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009, 69, 4010–4017. [Google Scholar] [CrossRef]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef]
- Burga, R.A.; Nguyen, T.; Zulovich, J.; Madonna, S.; Ylisastigui, L.; Fernandes, R.; Yvon, E. Improving efficacy of cancer immunotherapy by genetic modification of natural killer cells. Cytotherapy 2016, 18, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.H. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front. Immunol. 2015, 6, 578. [Google Scholar] [CrossRef] [PubMed]
- Dunai, C.; Murphy, W.J. NK cells for PD-1/PD-L1 blockade immunotherapy: Pinning down the NK cell. J. Clin. Investig. 2018, 128, 4251–4253. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Withers, S.S.; Modiano, J.F.; Kent, M.S.; Chen, M.; Luna, J.I.; Culp, W.T.N.; Sparger, E.E.; Rebhun, R.B.; Monjazeb, A.M.; et al. Canine cancer immunotherapy studies: Linking mouse and human. J. Immunother. Cancer 2016, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Addissie, S.; Klingemann, H. Cellular Immunotherapy of Canine Cancer. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.E. Naturally occurring cancers in dogs: Insights for translational genetics and medicine. Ilar J. 2014, 55, 16–45. [Google Scholar] [CrossRef]
- Paoloni, M.; Khanna, C. Translation of new cancer treatments from pet dogs to humans. Nat. Rev. Cancer 2008, 8, 147–156. [Google Scholar] [CrossRef]
- Paoloni, M.; Davis, S.; Lana, S.; Withrow, S.; Sangiorgi, L.; Picci, P.; Hewitt, S.; Triche, T.; Meltzer, P.; Khanna, C. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genom. 2009, 10, 625. [Google Scholar] [CrossRef]
- Christopher, M.M. One health, one literature: Weaving together veterinary and medical research. Sci. Transl. Med. 2015, 7, 303fs36. [Google Scholar] [CrossRef]
- Kol, A.; Arzi, B.; Athanasiou, K.A.; Farmer, D.L.; Nolta, J.A.; Rebhun, R.B.; Chen, X.; Griffiths, L.G.; Verstraete, F.J.; Murphy, C.J.; et al. Companion animals: Translational scientist’s new best friends. Sci. Transl. Med. 2015, 7, 308ps21. [Google Scholar] [CrossRef]
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Lauber, C.; Costello, E.K.; Lozupone, C.A.; Humphrey, G.; Berg-Lyons, D.; Caporaso, J.G.; Knights, D.; Clemente, J.C.; Nakielny, S.; et al. Cohabiting family members share microbiota with one another and with their dogs. eLife 2013, 2, e00458. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.J.; Grossenbacher, S.K.; Foltz, J.A.; Sturgill, I.R.; Park, J.S.; Luna, J.I.; Kent, M.S.; Culp, W.T.N.; Chen, M.; Modiano, J.F.; et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J. Immunother. Cancer 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Mace, E.M.; Hsu, A.P.; Monaco-Shawver, L.; Makedonas, G.; Rosen, J.B.; Dropulic, L.; Cohen, J.I.; Frenkel, E.P.; Bagwell, J.C.; Sullivan, J.L.; et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood 2013, 121, 2669–2677. [Google Scholar] [CrossRef]
- Mace, E.M.; Orange, J.S. Genetic Causes of Human NK Cell Deficiency and Their Effect on NK Cell Subsets. Front. Immunol. 2016, 7, 545. [Google Scholar] [CrossRef]
- Wilk, A.J.; Blish, C.A. Diversification of human NK cells: Lessons from deep profiling. J. Leukoc. Biol. 2018, 103, 629–641. [Google Scholar] [CrossRef]
- Strauss-Albee, D.M.; Fukuyama, J.; Liang, E.C.; Yao, Y.; Jarrell, J.A.; Drake, A.L.; Kinuthia, J.; Montgomery, R.R.; John-Stewart, G.; Holmes, S.; et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci. Transl. Med. 2015, 7, 297ra115. [Google Scholar] [CrossRef]
- Jacob, J.A. Researchers Turn to Canine Clinical Trials to Advance Cancer Therapies. JAMA 2016, 315, 1550–1552. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Scoville, S.D.; Freud, A.G.; Caligiuri, M.A. Cellular pathways in the development of human and murine innate lymphoid cells. Curr. Opin. Immunol. 2019, 56, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Back to the future--defining NK cells and T cells. Eur. J. Immunol. 2007, 37, 1424–1426. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Elliott, J.M.; Keyel, P.A.; Yang, L.; Carrero, J.A.; Yokoyama, W.M. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Paust, S.; Blish, C.A.; Reeves, R.K. Redefining Memory: Building the Case for Adaptive NK Cells. J. Virol. 2017, 91, e00169-17. [Google Scholar] [CrossRef]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Immune memory redefined: Characterizing the longevity of natural killer cells. Immunol. Rev. 2010, 236, 83–94. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, D.J.; Kim, Y.; Kim, C.J.; Lee, J.J.; Yoon, M.S.; Uong, T.N.T.; Yu, D.; Jung, J.Y.; Cho, D.; et al. Comparison of Phenotypic and Functional Characteristics Between Canine Non-B, Non-T Natural Killer Lymphocytes and CD3(+)CD5(dim)CD21(-) Cytotoxic Large Granular Lymphocytes. Front. Immunol. 2018, 9, 841. [Google Scholar] [CrossRef]
- Shin, D.J.; Park, J.Y.; Jang, Y.Y.; Lee, J.J.; Lee, Y.K.; Shin, M.G.; Jung, J.Y.; Carson, W.E., 3rd; Cho, D.; Kim, S.K. Ex vivo expansion of canine cytotoxic large granular lymphocytes exhibiting characteristics of natural killer cells. Vet. Immunol. Immunopathol. 2013, 153, 249–259. [Google Scholar] [CrossRef]
- Shin, D.J.; Lee, S.H.; Park, J.Y.; Kim, J.S.; Lee, J.J.; Suh, G.H.; Lee, Y.K.; Cho, D.; Kim, S.K. Interleukin-21 induces proliferation and modulates receptor expression and effector function in canine natural killer cells. Vet. Immunol. Immunopathol. 2015, 165, 22–33. [Google Scholar] [CrossRef]
- Schmitz, G.; Armien, A.G.; Fonfara, S.; Teifke, J.P.; Burkhardt, E. Induction of apoptosis by canine natural killer cells. J. Vet. Med. Ser. A 2003, 50, 156–159. [Google Scholar] [CrossRef]
- Lin, C.S.; Chang, C.P.; Chiang, H.C.; Chuang, T.F.; Hsu, C.H.; Liu, C.C. Activating natural killer (NK) cytotoxicity of canine CD5(-)CD21(-) cells requires low surface CD5 density NK cells. Iran. J. Vet. Res. 2018, 19, 87–95. [Google Scholar] [PubMed]
- Park, J.Y.; Shin, D.J.; Lee, S.H.; Lee, J.J.; Suh, G.H.; Cho, D.; Kim, S.K. The anti-canine distemper virus activities of ex vivo-expanded canine natural killer cells. Vet. Microbiol. 2015, 176, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.G.; Scalzo, A.A. NK gene complex dynamics and selection for NK cell receptors. Semin. Immunol. 2008, 20, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Foley, B.; Cooley, S.; Verneris, M.R.; Pitt, M.; Curtsinger, J.; Luo, X.; Lopez-Verges, S.; Lanier, L.L.; Weisdorf, D.; Miller, J.S. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012, 119, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Guma, M.; Angulo, A.; Vilches, C.; Gomez-Lozano, N.; Malats, N.; Lopez-Botet, M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Verges, S.; Milush, J.M.; Schwartz, B.S.; Pando, M.J.; Jarjoura, J.; York, V.A.; Houchins, J.P.; Miller, S.; Kang, S.M.; Norris, P.J.; et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 14725–14732. [Google Scholar] [CrossRef] [PubMed]
- Ringler, S.S.; Krakowka, S. Effects of canine distemper virus on natural killer cell activity in dogs. Am. J. Vet. Res. 1985, 46, 1781–1786. [Google Scholar]
- Shek, W.R.; Schultz, R.D.; Appel, M.J. Natural and immune cytolysis of canine distemper virus-infected target cells. Infect. Immun. 1980, 28, 724–734. [Google Scholar]
- Foltz, J.A.; Somanchi, S.S.; Yang, Y.; Aquino-Lopez, A.; Bishop, E.E.; Lee, D.A. NCR1 Expression Identifies Canine Natural Killer Cell Subsets with Phenotypic Similarity to Human Natural Killer Cells. Front. Immunol. 2016, 7, 521. [Google Scholar] [CrossRef]
- Grondahl-Rosado, C.; Bonsdorff, T.B.; Brun-Hansen, H.C.; Storset, A.K. NCR1+ cells in dogs show phenotypic characteristics of natural killer cells. Vet. Res. Commun. 2015, 39, 19–30. [Google Scholar] [CrossRef]
- Grondahl-Rosado, C.; Boysen, P.; Johansen, G.M.; Brun-Hansen, H.; Storset, A.K. NCR1 is an activating receptor expressed on a subset of canine NK cells. Vet. Immunol. Immunopathol. 2016, 177, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.S.; Gyurkocza, B.; Stone, D.M.; Parker, M.H.; Abrams, K.; Jochum, C.; Gallo, S.; Saad, M.; Johnson, M.M.; Rosinski, S.L.; et al. Development and characterization of a canine-specific anti-CD94 (KLRD-1) monoclonal antibody. Vet. Immunol. Immunopathol. 2019, 211, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Dimasi, N.; Wang, J.; Margulies, D.H.; Mariuzza, R.A. MHC class I recognition by Ly49 natural killer cell receptors. Mol. Immunol. 2002, 38, 1023–1027. [Google Scholar] [CrossRef]
- Tough, D.F.; Sprent, J. Bystander stimulation of T cells in vivo by cytokines. Vet. Immunol. Immunopathol. 1998, 63, 123–129. [Google Scholar] [CrossRef]
- Nakada, Y.; Tsukatani, Y.; Kosaka, T.; Miyamori, M.; Kuwabara, M.; Tanaka, S.; Koide, F. Release of natural killer cytotoxic factor (NKCF) from canine natural killer (NK) cells stimulated with cytoplasmic membrane of target cells. J. Vet. Med. Sci. 1995, 57, 165–167. [Google Scholar] [CrossRef][Green Version]
- Yokoyama, W.M.; Plougastel, B.F. Immune functions encoded by the natural killer gene complex. Nat. Rev. Immunol. 2003, 3, 304–316. [Google Scholar] [CrossRef]
- Hao, L.; Klein, J.; Nei, M. Heterogeneous but conserved natural killer receptor gene complexes in four major orders of mammals. Proc. Natl. Acad. Sci. USA 2006, 103, 3192–3197. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef]
- Gagnier, L.; Wilhelm, B.T.; Mager, D.L. Ly49 genes in non-rodent mammals. Immunogenetics 2003, 55, 109–115. [Google Scholar] [CrossRef]
- Hammond, J.A.; Guethlein, L.A.; Abi-Rached, L.; Moesta, A.K.; Parham, P. Evolution and survival of marine carnivores did not require a diversity of killer cell Ig-like receptors or Ly49 NK cell receptors. J. Immunol. 2009, 182, 3618–3627. [Google Scholar] [CrossRef]
- Walzer, T.; Jaeger, S.; Chaix, J.; Vivier, E. Natural killer cells: From CD3(-)NKp46(+) to post-genomics meta-analyses. Curr. Opin. Immunol. 2007, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Yudanin, N.A.; Schmitz, F.; Flamar, A.L.; Thome, J.J.C.; Tait Wojno, E.; Moeller, J.B.; Schirmer, M.; Latorre, I.J.; Xavier, R.J.; Farber, D.L.; et al. Spatial and Temporal Mapping of Human Innate Lymphoid Cells Reveals Elements of Tissue Specificity. Immunity 2019, 50, 505–519.e4. [Google Scholar] [CrossRef] [PubMed]
- McDonough, S.P.; Moore, P.F. Clinical, hematologic, and immunophenotypic characterization of canine large granular lymphocytosis. Vet. Pathol. 2000, 37, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Huang, Y.C.; Wang, Y.S.; Juang, R.H.; Liao, K.W.; Chu, R.M. Canine CD8 T cells showing NK cytotoxic activity express mRNAs for NK cell-associated surface molecules. Vet. Immunol. Immunopathol. 2010, 133, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Hung, S.W.; Jan, T.R.; Liao, K.W.; Cheng, C.H.; Wang, Y.S.; Chu, R.M. CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells. J. Leukoc. Biol. 2008, 84, 1501–1510. [Google Scholar] [CrossRef]
- Sarrias, M.R.; Gronlund, J.; Padilla, O.; Madsen, J.; Holmskov, U.; Lozano, F. The Scavenger Receptor Cysteine-Rich (SRCR) domain: An ancient and highly conserved protein module of the innate immune system. Crit. Rev. Immunol. 2004, 24. [Google Scholar] [CrossRef]
- Yasuda, N.; Masuda, K.; Tsukui, T.; Teng, A.; Ishii, Y. Identification of canine natural CD3-positive T cells expressing an invariant T-cell receptor alpha chain. Vet. Immunol. Immunopathol. 2009, 132, 224–231. [Google Scholar] [CrossRef]
- Pessino, A.; Sivori, S.; Bottino, C.; Malaspina, A.; Morelli, L.; Moretta, L.; Biassoni, R.; Moretta, A. Molecular cloning of NKp46: A novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 1998, 188, 953–960. [Google Scholar] [CrossRef]
- Mair, K.H.; Essler, S.E.; Patzl, M.; Storset, A.K.; Saalmuller, A.; Gerner, W. NKp46 expression discriminates porcine NK cells with different functional properties. Eur. J. Immunol. 2012, 42, 1261–1271. [Google Scholar] [CrossRef]
- Schuberth, H.J.; Kucinskiene, G.; Chu, R.M.; Faldyna, M. Reactivity of cross-reacting monoclonal antibodies with canine leukocytes, platelets and erythrocytes. Vet. Immunol. Immunopathol. 2007, 119, 47–55. [Google Scholar] [CrossRef]
- Biassoni, R.; Cantoni, C.; Pende, D.; Sivori, S.; Parolini, S.; Vitale, M.; Bottino, C.; Moretta, A. Human natural killer cell receptors and co-receptors. Immunol. Rev. 2001, 181, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Daeron, M. Fc receptor biology. Annu. Rev. Immunol. 1997, 15, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Mechetina, L.V.; Najakshin, A.M.; Alabyev, B.Y.; Chikaev, N.A.; Taranin, A.V. Identification of CD16-2, a novel mouse receptor homologous to CD16/Fc gamma RIII. Immunogenetics 2002, 54, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Helfand, S.C.; Soergel, S.A.; Modiano, J.F.; Hank, J.A.; Sondel, P.M. Induction of lymphokine-activated killer (LAK) activity in canine lymphocytes with low dose human recombinant interleukin-2 in vitro. Cancer Biother. 1994, 9, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.T.; Ito, D.; McCullar, V.; Zhang, B.; Miller, J.S.; Modiano, J.F. Isolation and characterization of canine natural killer cells. Vet. Immunol. Immunopathol. 2013, 155, 211–217. [Google Scholar] [CrossRef]
- Evans, D.L.; Jaso-Friedmann, L. Natural killer (NK) cells in domestic animals: Phenotype, target cell specificity and cytokine regulation. Vet. Res. Commun. 1993, 17, 429–447. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, D.J.; Kim, S.K. Generation of recombinant canine interleukin-15 and evaluation of its effects on the proliferation and function of canine NK cells. Vet. Immunol. Immunopathol. 2015, 165, 1–13. [Google Scholar] [CrossRef]
- Funk, J.; Schmitz, G.; Bach, U.; Failing, K.; Burkhardt, E. Influence of different tumour types on natural cytotoxicity (NK cell activity) and mitogen-induced lymphocyte proliferation in isolated blood lymphocytes from 110 dogs with tumours. Res. Vet. Sci. 2003, 74, 129–135. [Google Scholar] [CrossRef]
- Funk, J.; Schmitz, G.; Failing, K.; Burkhardt, E. Natural killer (NK) and lymphokine-activated killer (LAK) cell functions from healthy dogs and 29 dogs with a variety of spontaneous neoplasms. Cancer Immunol. Immunother. 2005, 54, 87–92. [Google Scholar] [CrossRef]
- Park, K.U.; Jin, P.; Sabatino, M.; Feng, J.; Civini, S.; Khuu, H.; Berg, M.; Childs, R.; Stroncek, D. Gene expression analysis of ex vivo expanded and freshly isolated NK cells from cancer patients. J. Immunother. 2010, 33, 945–955. [Google Scholar] [CrossRef]
- Dow, S.; Elmslie, R.; Kurzman, I.; MacEwen, G.; Pericle, F.; Liggitt, D. Phase I study of liposome-DNA complexes encoding the interleukin-2 gene in dogs with osteosarcoma lung metastases. Hum. Gene Ther. 2005, 16, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Khanna, C.; Anderson, P.M.; Hasz, D.E.; Katsanis, E.; Neville, M.; Klausner, J.S. Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer 1997, 79, 1409–1421. [Google Scholar] [CrossRef]
- Cifaldi, L.; Locatelli, F.; Marasco, E.; Moretta, L.; Pistoia, V. Boosting Natural Killer Cell-Based Immunotherapy with Anticancer Drugs: A Perspective. Trends Mol. Med. 2017, 23, 1156–1175. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, M.M.; Gillgrass, A.; Nham, T.; Hogg, R.; Lee, A.J.; Chew, M.V.; Shafaei, M.; Aarts, C.; Lee, D.A.; Hassell, J.; et al. Ex vivo expanded natural killer cells from breast cancer patients and healthy donors are highly cytotoxic against breast cancer cell lines and patient-derived tumours. Breast Cancer Res. 2017, 19, 76. [Google Scholar] [CrossRef]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-man clinical trial of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018, 8, 1083–1089. [Google Scholar]
- Romanski, A.; Uherek, C.; Bug, G.; Seifried, E.; Klingemann, H.; Wels, W.S.; Ottmann, O.G.; Tonn, T. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J. Cell. Mol. Med. 2016, 20, 1287–1294. [Google Scholar] [CrossRef]
- Angka, L.; Khan, S.T.; Kilgour, M.K.; Xu, R.; Kennedy, M.A.; Auer, R.C. Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery. Int. J. Mol. Sci. 2017, 18, 1787. [Google Scholar] [CrossRef]
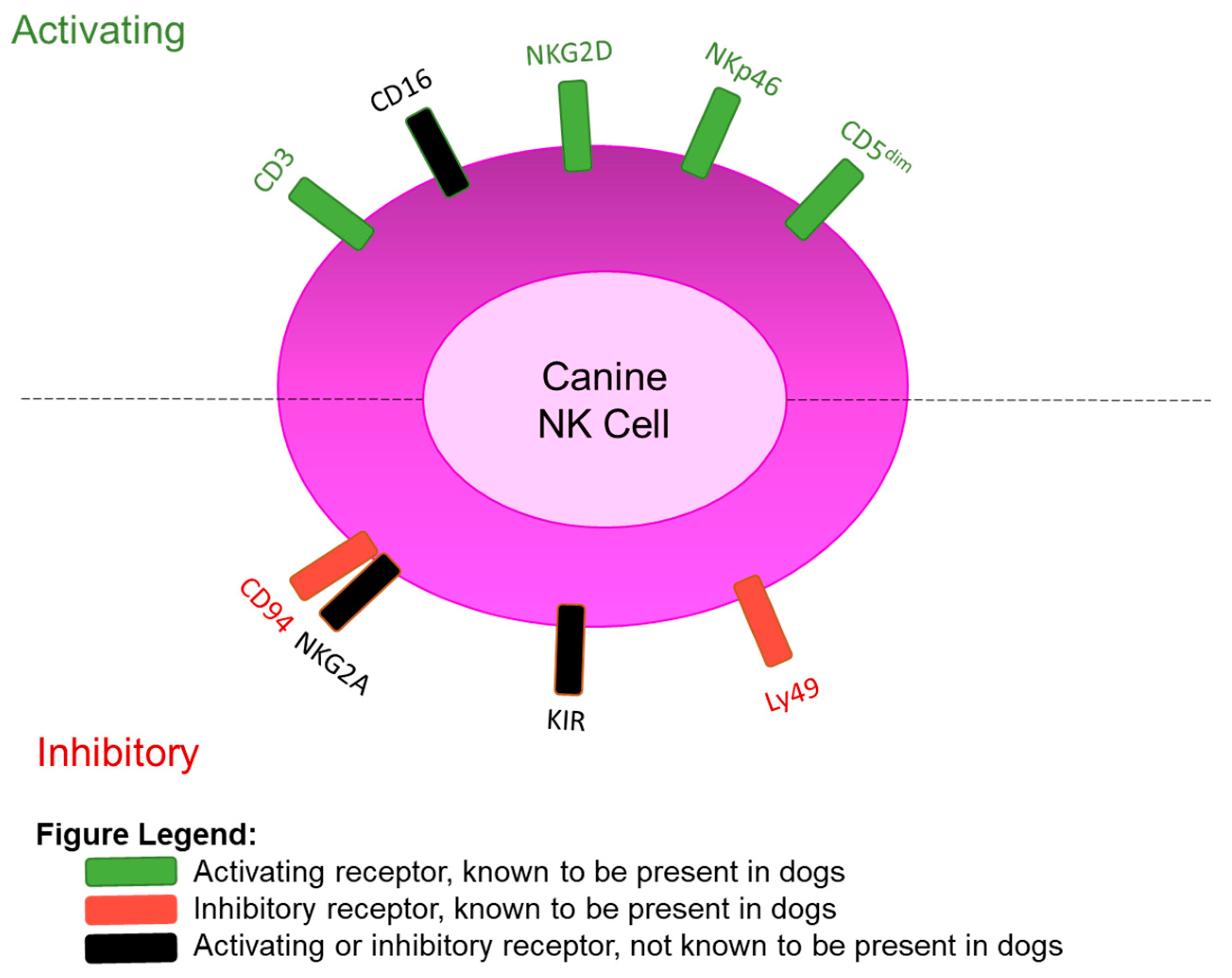
| Known Canine NK Cell Activating Receptors | ||||
| Receptor | Gene | Verified by | Additional info | Homology |
| CD5dim | CD5 | Flow cytometry | 15% of PBMCs | Human, mouse |
| NKp46 | NCR1 | Flow cytometry | 2.5% of PBMCs | Human, mouse |
| CD16 | FCGR3A | DNA Sequencing | Absent/not annotated on CanFam3.1 assembly | Human, mouse |
| NKG2D | KLRK1 | DNA Sequencing | Annotated on CanFam3.1 assembly | Human, mouse |
| CD3 | CD3E | Flow cytometry | Typically a T-cell marker, persists in candidate populations of canine NK cells | Human, mouse |
| Known Canine NK Cell MHC-I Inhibitory Receptors | ||||
| Receptor | Gene | Verified by | Additional info | Homology |
| Ly49 | Ly49 | DNA sequencing, Southern blot | Cysteine-to-tyrosine mutation present, function unknown | Mouse |
| CD94 | KLRD1 | Flow cytometry | 7% of PBMCs. Function unknown, lack of NKG2A to form heterodimer | Human, mouse |
| KIR | Absent | DNA sequencing | LRC appears to be truncated prior to KIR gene locations | Human |
| Study | Year | Starting Population | Feeder Cells | Media | Additives | Human Cytokines | Canine Cytokine | Days | |
|---|---|---|---|---|---|---|---|---|---|
| Foltz | 2016 | CD3−/ NKp46+ cells from healthy dog PBMCs | Irradiated K562 Clone9. mbIL-21 cells | 100 IU/mL rh-IL2 * | 6.1 ng/ mL rcIL-2 * | Not used in combination with human cytokines | 21 days | ||
| Lee | 2015 | Healthy dog isolated PBMCs | 100-Gy-irradiated K562 cells | RPMI | fetal bovine serum, penicillin, streptomycin | 100 IU/mL rhIL-2 10 IU/ml rhIL-15 | 10 IU/mL rcIL-15 | Used in combination with rhIL-2 | 21 days |
| Shin | 2015 | Healthy dog isolated PBMCs | 100-Gy-irradiated K562 cells | RPMI 1640 | fetal bovine serum, penicillin, streptomycin | 100 IU/mL rhIL-2 10 IU/mL rhIL-15 | 5 ng/mL rcIL-21 | Used in combination with rhIL-2 and rhIL-15 | 21 days |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gingrich, A.A.; Modiano, J.F.; Canter, R.J. Characterization and Potential Applications of Dog Natural Killer Cells in Cancer Immunotherapy. J. Clin. Med. 2019, 8, 1802. https://doi.org/10.3390/jcm8111802
Gingrich AA, Modiano JF, Canter RJ. Characterization and Potential Applications of Dog Natural Killer Cells in Cancer Immunotherapy. Journal of Clinical Medicine. 2019; 8(11):1802. https://doi.org/10.3390/jcm8111802
Chicago/Turabian StyleGingrich, Alicia A., Jaime F. Modiano, and Robert J. Canter. 2019. "Characterization and Potential Applications of Dog Natural Killer Cells in Cancer Immunotherapy" Journal of Clinical Medicine 8, no. 11: 1802. https://doi.org/10.3390/jcm8111802
APA StyleGingrich, A. A., Modiano, J. F., & Canter, R. J. (2019). Characterization and Potential Applications of Dog Natural Killer Cells in Cancer Immunotherapy. Journal of Clinical Medicine, 8(11), 1802. https://doi.org/10.3390/jcm8111802





