Efficacy of HE4, CA125, Risk of Malignancy Index and Risk of Ovarian Malignancy Index to Detect Ovarian Cancer in Women with Presumed Benign Ovarian Tumours: A Prospective, Multicentre Trial
Abstract
1. Background
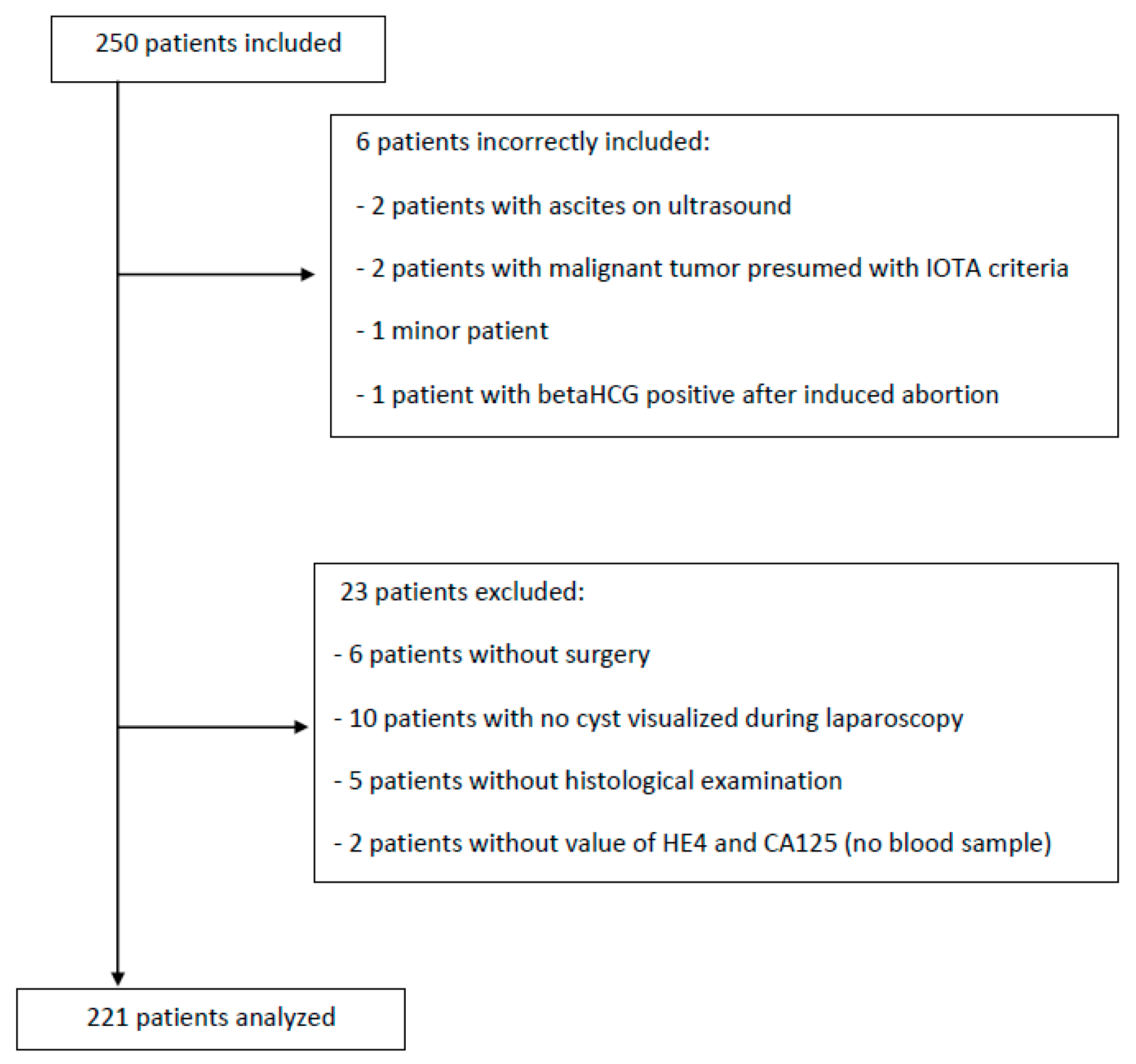
2. Materials and Methods
2.1. Sample Size
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CA125 | Carbohydrate Antigen 125 |
| HE4 | Human Epididymis Protein 4 |
| IOTA | International Ovarian Tumour Analysis |
| PBOT | Presumed Benign Ovarian Tumour |
| RMI | Risk of Malignancy Index |
| ROC | Receiver Operating Characteristic |
| ROMA | Risk of Ovarian Malignancy Index |
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics. Cancer J. Clin. 2008, 58, 71–96. [Google Scholar]
- Heintz, A.P.M.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Benedet, J.L.; Creasman, W.T.; Ngan, H.Y.S.; Pecorelli, S.; Beller, U. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95, 161–192. [Google Scholar] [CrossRef]
- Enakpene, C.A.; Omigbodun, A.O.; Goecke, T.W.; Odukogbe, A.-T.; Beckmann, M.W. Preoperative evaluation and triage of women with suspicious adnexal masses using risk of malignancy index. J. Obstet. Gynaecol. Res. 2009, 35, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ameye, L.; Jurkovic, D.; Van Holsbeke, C.; Paladini, D.; Van Calster, B.; Vergote, I.; Van Huffel, S.; et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. 2008, 31, 681–690. [Google Scholar] [CrossRef]
- Brun, J.-L.; Fritel, X.; Aubard, Y.; Borghese, B.; Bourdel, N.; Chabbert-Buffet, N.; Collinet, P.; Deffieux, X.; Dubernard, G.; Huchon, C.; et al. Management of presumed benign ovarian tumors: Updated French guidelines. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 183, 52–58. [Google Scholar] [CrossRef]
- Brun, J.-L.; Fritel, X.; Levêque, J. Recommandations pour la pratique clinique: Tumeurs ovariennes présumées bénignes – Objectifs, méthodes et organisation. J. Gynecol. Obstet. Biol. Reprod. 2013, 42, 710–714. [Google Scholar] [CrossRef]
- Lahlou, N.; Brun, J.-L. Marqueurs sériques et tumoraux ovariens dans le diagnostic des tumeurs ovariennes présumées bénignes. J. Gynecol. Obstet. Biol. Reprod. 2013, 42, 752–759. [Google Scholar] [CrossRef]
- Hellström, I.; Raycraft, J.; Hayden-Ledbetter, M.; Ledbetter, J.A.; Schummer, M.; McIntosh, M.; Drescher, C.; Urban, N.; Hellström, K.E. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003, 63, 3695–3700. [Google Scholar]
- Buamah, P. Benign conditions associated with raised serum CA-125 concentration. J. Surg. Oncol. 2000, 75, 264–265. [Google Scholar] [CrossRef]
- Drapkin, R.; von Horsten, H.H.; Lin, Y.; Mok, S.C.; Crum, C.P.; Welch, W.R.; Hecht, J.L. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005, 65, 2162–2169. [Google Scholar] [CrossRef]
- Yanaranop, M.; Anakrat, V.; Siricharoenthai, S.; Nakrangsee, S.; Thinkhamrop, B. Is the Risk of Ovarian Malignancy Algorithm Better Than Other Tests for Predicting Ovarian Malignancy in Women with Pelvic Masses? Gynecol. Obstet. Invest. 2017, 82, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wilailak, S.; Chan, K.K.; Chen, C.A.; Nam, J.H.; Ochiai, K.; Aw, T.C.; Sabaratnam, S.; Hebbar, S.; Sickan, J.; Schodin, B.A.; et al. Distinguishing benign from malignant pelvic mass utilizing an algorithm with HE4, menopausal status, and ultrasound findings. J. Gynecol. Oncol. 2015, 26, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br. J. Obstet. Gynaecol. 1990, 97, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C., Jr.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Qin, J.; Sangvatanakul, V. Human epididymis protein 4 for differential diagnosis between benign gynecologic disease and ovarian cancer: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 81–85. [Google Scholar] [CrossRef]
- Ferraro, S.; Braga, F.; Lanzoni, M.; Boracchi, P.; Biganzoli, E.M.; Panteghini, M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: A systematic review. J. Clin. Pathol. 2013, 66, 273–281. [Google Scholar] [CrossRef]
- Bast, R.C.; Klug, T.L.; St John, E.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med. 1983, 309, 883–887. [Google Scholar] [CrossRef]
- Karlsen, M.A.; Sandhu, N.; Høgdall, C.; Christensen, I.J.; Nedergaard, L.; Lundvall, L.; Engelholm, S.A.; Pedersen, A.T.; Hartwell, D.; Lydolph, M.; et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2012, 127, 379–383. [Google Scholar] [CrossRef]
- Gizzo, S.; Berretta, R.; Di Gangi, S.; Guido, M.; Zanni, G.C.; Franceschetti, I.; Quaranta, M.; Plebani, M.; Nardelli, G.B.; Patrelli, T.S. Borderline ovarian tumors and diagnostic dilemma of intraoperative diagnosis: Could preoperative He4 assay and ROMA score assessment increase the frozen section accuracy? A multicenter case-control study. Biomed. Res. Int. 2014, 2014, 803598. [Google Scholar] [CrossRef]
- Richards, A.; Herbst, U.; Manalang, J.; Pather, S.; Saidi, S.; Tejada-Berges, T.; Tan, K.; Williams, P.; Carter, J. HE4, CA125, the Risk of Malignancy Algorithm and the Risk of Malignancy Index and complex pelvic masses—a prospective comparison in the pre-operative evaluation of pelvic masses in an Australian population. J. Obstet. Gynaecol. 2015, 55, 493–497. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Van Calster, B.; Bourne, T.; Ajossa, S.; Testa, A.C.; Guerriero, S.; Fruscio, R.; Lissoni, A.A.; Czekierdowski, A.; Savelli, L.; et al. External validation of diagnostic models to estimate the risk of malignancy in adnexal masses. Clin. Cancer Res. 2012, 18, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Chudecka-Głaz, A.M. ROMA, an algorithm for ovarian cancer. Clin. Chim. Acta 2014, 440C, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tie, R.; Chang, K.; Wang, F.; Deng, S.; Lu, W.; Yu, L.; Chen, M. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and CA125 in predicting epithelial ovarian cancer: A meta-analysis. BMC Cancer 2012, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Van Gorp, T.; Cadron, I.; Despierre, E.; Daemen, A.; Leunen, K.; Amant, F.; Timmerman, D.; De Moor, B.; Vergote, I. HE4 and CA125 as a diagnostic test in ovarian cancer: Prospective validation of the Risk of Ovarian Malignancy Algorithm. Br. J. Cancer 2011, 104, 863–870. [Google Scholar] [CrossRef]
- Montagnana, M.; Danese, E.; Ruzzenente, O.; Bresciani, V.; Nuzzo, T.; Gelati, M.; Salvagno, G.L.; Franchi, M.; Lippi, G.; Guidi, G.C. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: Is it really useful? Clin. Chem. Lab. Med. 2011, 49, 521–525. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.; Yao, H.; Wu, Z.; Wang, M.; Qi, J. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: A meta-analysis. Tumour Biol. 2014, 35, 6127–6138. [Google Scholar] [CrossRef]
- Zhen, S.; Bian, L.-H.; Chang, L.-L.; Gao, X. Comparison of serum human epididymis protein 4 and carbohydrate antigen 125 as markers in ovarian cancer: A meta-analysis. Mol Clin Oncol 2014, 2, 559–566. [Google Scholar] [CrossRef]
- Bolstad, N.; Øijordsbakken, M.; Nustad, K.; Bjerner, J. Human epididymis protein 4 reference limits and natural variation in a Nordic reference population. Tumour Biol. 2012, 33, 141–148. [Google Scholar] [CrossRef]
- Moore, R.G.; Miller, M.C.; Eklund, E.E.; Lu, K.H.; Bast, R.C.; Lambert-Messerlian, G. Serum levels of the ovarian cancer biomarker HE4 are decreased in pregnancy and increase with age. Am. J. Obstet. Gynecol. 2012, 206, 349.e1–349.e7. [Google Scholar] [CrossRef]
- Goff, B.A.; Agnew, K.; Neradilek, M.B.; Gray, H.J.; Liao, J.B.; Urban, R.R. Combining a symptom index, CA125 and HE4 (triple screen) to detect ovarian cancer in women with a pelvic mass. Gynecol. Oncol. 2017, 147, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, H.; Chen, R.; He, J.; Wang, Y.; Huang, L.; Sun, L.; Duan, C.; Luo, X.; Yan, H. Development of a multimarker assay for differential diagnosis of benign and malignant pelvic masses. Clin. Chim. Acta 2014, 440C, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.O.; Ahmed, H.; Sedeek, O.B.; Mohammed, A.M.; Abd-Alla, A.A.; Abdel Ghaffar, H.M. Significance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment response. Diagn. Pathol. 2013, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Brown, A.K.; Miller, M.C.; Skates, S.; Allard, W.J.; Verch, T.; Steinhoff, M.; Messerlian, G.; DiSilvestro, P.; Granai, C.O.; et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol. Oncol. 2008, 108, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.A.; Schulz, K.F. Refining clinical diagnosis with likelihood ratios. Lancet 2005, 365, 1500–1505. [Google Scholar] [CrossRef]
- Romagnolo, C.; Leon, A.E.; Fabricio, A.S.C.; Taborelli, M.; Polesel, J.; Del Pup, L.; Steffan, A.; Cervo, S.; Ravaggi, A.; Zanotti, L.; et al. HE4, CA125 and risk of ovarian malignancy algorithm (ROMA) as diagnostic tools for ovarian cancer in patients with a pelvic mass: An Italian multicenter study. Gynecol. Oncol. 2016, 141, 303–311. [Google Scholar] [CrossRef]
- Meys, E.M.J.; Kaijser, J.; Kruitwagen, R.F.P.M.; Slangen, B.F.M.; Van Calster, B.; Aertgeerts, B.; Verbakel, J.Y.; Timmerman, D.; Van Gorp, T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 17–29. [Google Scholar] [CrossRef]
- Van Gorp, T.; Veldman, J.; Van Calster, B.; Cadron, I.; Leunen, K.; Amant, F.; Timmerman, D.; Vergote, I. Subjective assessment by ultrasound is superior to the risk of malignancy index (RMI) or the risk of ovarian malignancy algorithm (ROMA) in discriminating benign from malignant adnexal masses. Eur. J. Cancer 2012, 48, 1649–1656. [Google Scholar] [CrossRef]
- Kaijser, J.; Van Belle, V.; Van Gorp, T.; Sayasneh, A.; Vergote, I.; Bourne, T.; Van Calster, B.; Timmerman, D. Prognostic value of serum HE4 levels and risk of ovarian malignancy algorithm scores at the time of ovarian cancer diagnosis. Int. J. Gynecol. Cancer 2014, 24, 1173–1180. [Google Scholar] [CrossRef]
- Vessey, M.; Metcalfe, A.; Wells, C.; McPherson, K.; Westhoff, C.; Yeates, D. Ovarian neoplasms, functional ovarian cysts, and oral contraceptives. Br. Med. J. (Clin. Res. Ed.) 1987, 294, 1518–1520. [Google Scholar] [CrossRef]
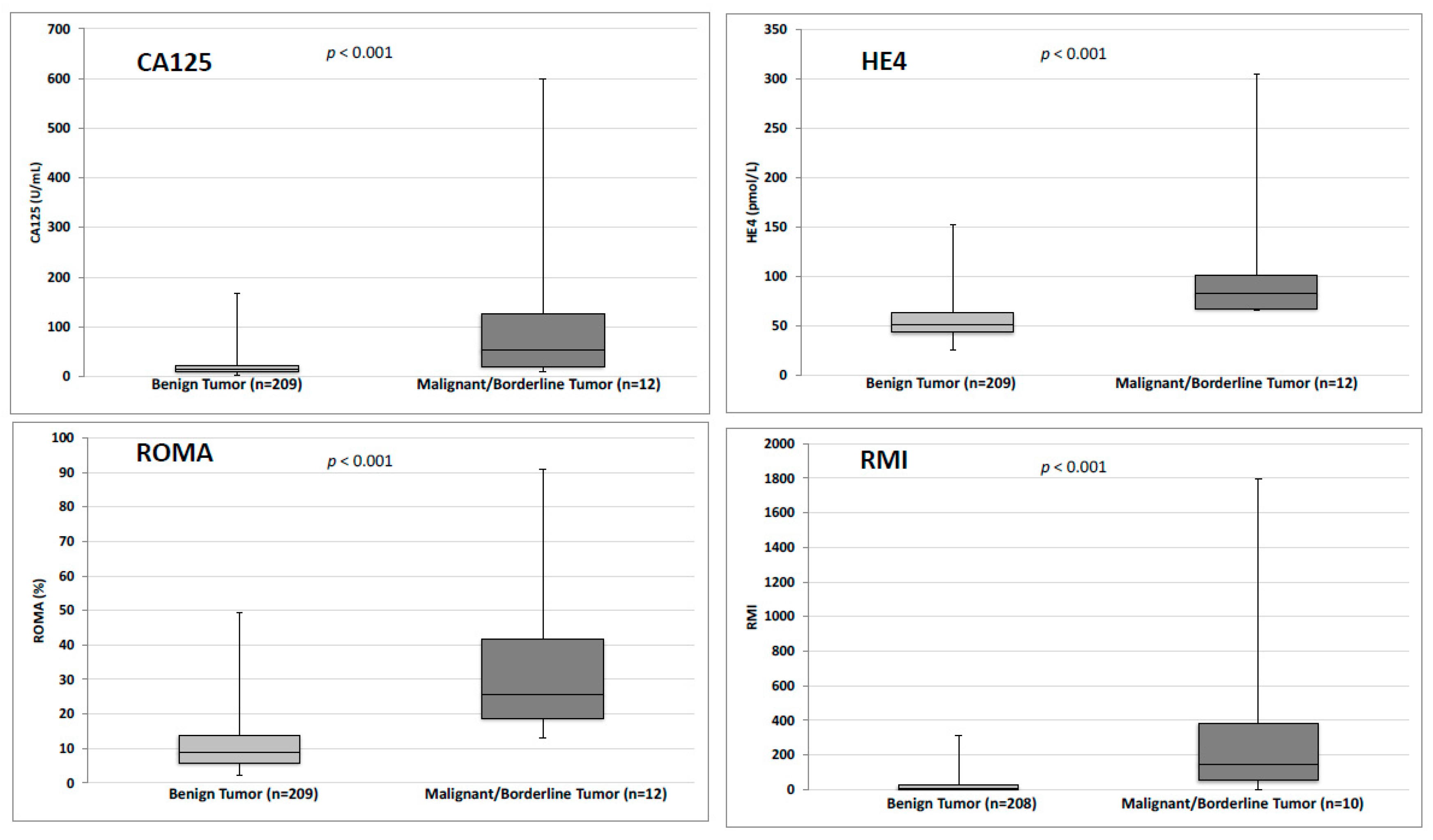
| Benign Tumor (n = 209) | Borderline/Malign Tumor (n = 12) | Total n (%) | p | |
|---|---|---|---|---|
| Age (years) | 46.2 ± 15.1 | 53.1 ± 16.3 | 46.5 ± 15.2 | 0.14 |
| (Minimum-Maximum) | (19–88) | (25–79) | (19–88) | |
| Body Mass Index (kg/m2) | 24.6 ± 4.8 | 24.8 ± 5.7 | 24.6 ± 4.9 | 0.95 |
| (Minimum-Maximum) | (14.9–39.8) | (17.3–35.5) | (14.9–39.8) | |
| CA125 < 35 U/mL | 189 (90.4) | 4 (33.3) | 193 (87.3) | <0.001 |
| HE4 < 70 pmol/L for premenopausal women OR < 140 pmol/L for postmenopausal women | 191 (91.4) | 6 (50) | 197 (89.1) | <0.001 |
| RMI score > 200 | 2 (1) | 4 (40) | 6 (2.8) | <0.001 |
| ROMA score > 11.4% for premenopausal women OR > 29.9% for postmenopausal women | 35 (16.7) | 9 (75) | 44 (19.9) | <0.001 |
| CA125 (Sp = 90.4%) | HE4 (Sp = 91.4%) | CA125 + HE4 (Sp = 99.5%) | RMI (Sp = 99.0%) | ROMA (Sp = 83.3%) | |
|---|---|---|---|---|---|
| CA125 (Sp = 90.4%) | |||||
| HE4 (Sp = 91.4%) | 0.87 | ||||
| CA125 + HE4 (Sp = 99.5%) | < 0.001 | < 0.001 | |||
| RMI (Sp = 99.0%) | < 0.001 | < 0.001 | 1 | ||
| ROMA (Sp = 83.3%) | 0.04 | < 0.001 | < 0.001 | < 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dochez, V.; Randet, M.; Renaudeau, C.; Dimet, J.; Le Thuaut, A.; Winer, N.; Thubert, T.; Vaucel, E.; Caillon, H.; Ducarme, G. Efficacy of HE4, CA125, Risk of Malignancy Index and Risk of Ovarian Malignancy Index to Detect Ovarian Cancer in Women with Presumed Benign Ovarian Tumours: A Prospective, Multicentre Trial. J. Clin. Med. 2019, 8, 1784. https://doi.org/10.3390/jcm8111784
Dochez V, Randet M, Renaudeau C, Dimet J, Le Thuaut A, Winer N, Thubert T, Vaucel E, Caillon H, Ducarme G. Efficacy of HE4, CA125, Risk of Malignancy Index and Risk of Ovarian Malignancy Index to Detect Ovarian Cancer in Women with Presumed Benign Ovarian Tumours: A Prospective, Multicentre Trial. Journal of Clinical Medicine. 2019; 8(11):1784. https://doi.org/10.3390/jcm8111784
Chicago/Turabian StyleDochez, Vincent, Mélanie Randet, Céline Renaudeau, Jérôme Dimet, Aurélie Le Thuaut, Norbert Winer, Thibault Thubert, Edouard Vaucel, Hélène Caillon, and Guillaume Ducarme. 2019. "Efficacy of HE4, CA125, Risk of Malignancy Index and Risk of Ovarian Malignancy Index to Detect Ovarian Cancer in Women with Presumed Benign Ovarian Tumours: A Prospective, Multicentre Trial" Journal of Clinical Medicine 8, no. 11: 1784. https://doi.org/10.3390/jcm8111784
APA StyleDochez, V., Randet, M., Renaudeau, C., Dimet, J., Le Thuaut, A., Winer, N., Thubert, T., Vaucel, E., Caillon, H., & Ducarme, G. (2019). Efficacy of HE4, CA125, Risk of Malignancy Index and Risk of Ovarian Malignancy Index to Detect Ovarian Cancer in Women with Presumed Benign Ovarian Tumours: A Prospective, Multicentre Trial. Journal of Clinical Medicine, 8(11), 1784. https://doi.org/10.3390/jcm8111784





