Comparison of Orthognathic Surgery Outcomes Between Patients With and Without Underlying High-Risk Conditions: A Multidisciplinary Team-Based Approach and Practical Guidelines
Abstract
1. Introduction
2. Methods
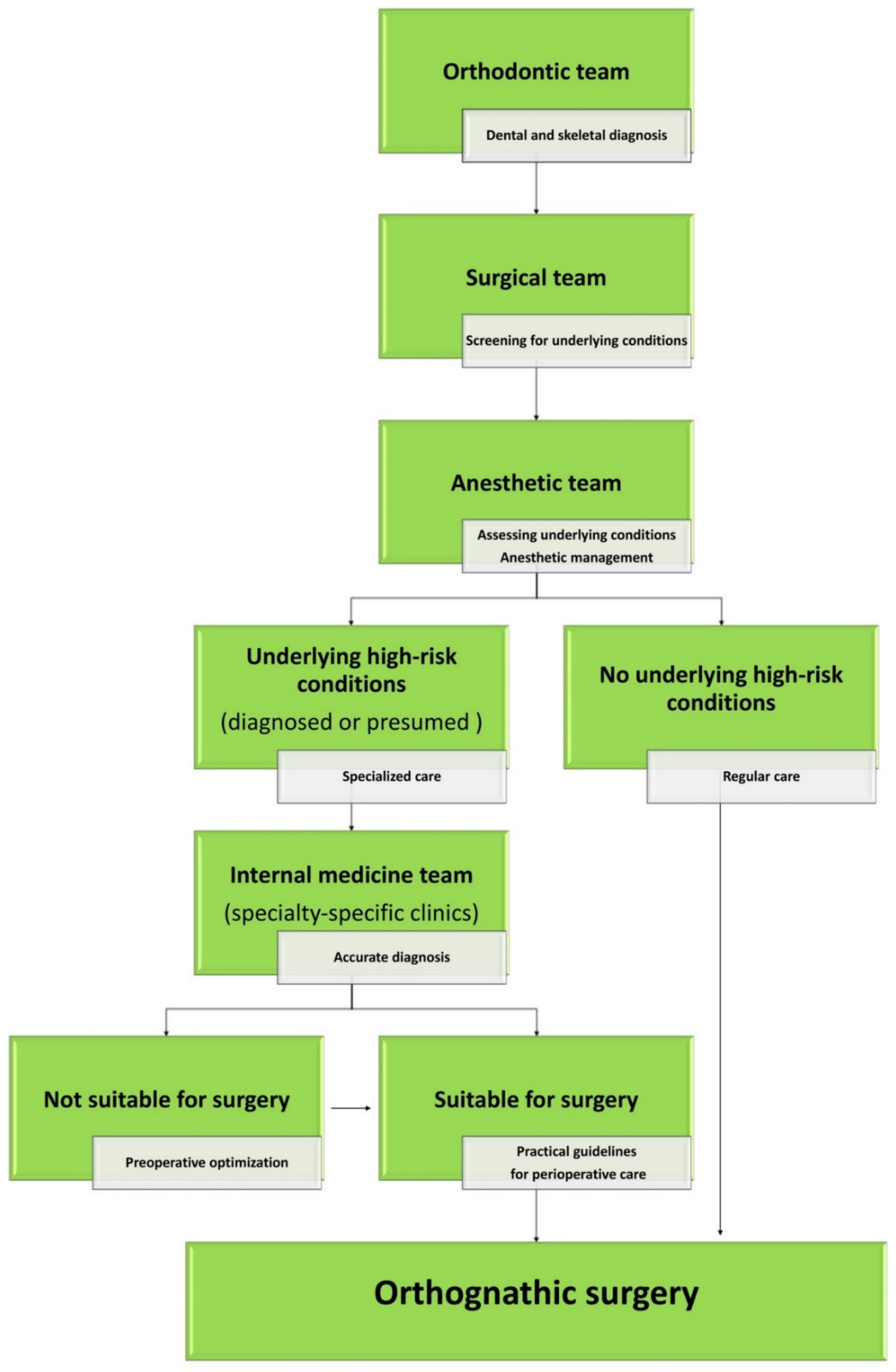
2.1. Multidisciplinary Approach
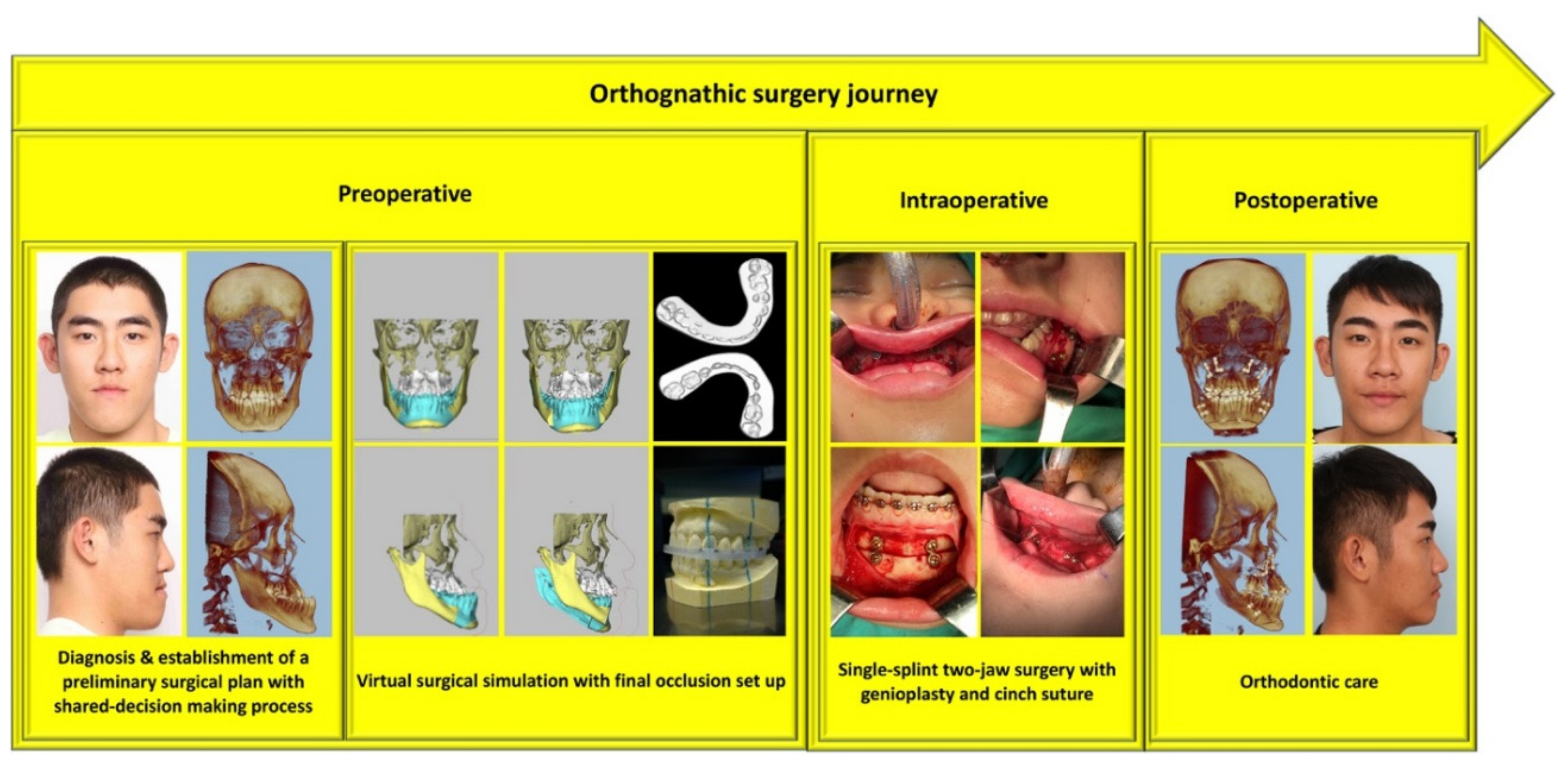
2.2. Practical Guidelines for Perioperative Care
2.3. Outcome Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Su, Y.Y.; Denadai, R.; Ho, C.T.; Lai, B.R.; Lo, L.J. Measuring patient-reported outcomes in orthognathic surgery: Linguistic and psychometric validation of the Mandarin Chinese version of FACE-Q. Biomed. J. 2019. [Google Scholar] [CrossRef]
- Denadai, R.; Chou, P.Y.; Pai, B.C.J.; Chen, C.; Lin, C.C.H.; Huang, C.S.; Chen, Y.R.; Lo, L.J. Skeletofacial reconstruction for cleft-related deformities: Four decades of evolving cleft care. Ann. Plast. Surg. 2019. in publication. [Google Scholar]
- Wu, R.T.; Wilson, A.T.; Gary, C.S.; Steinbacher, D.M. Complete reoperation in orthognathic surgery. Plast. Reconstr. Surg. 2019, 143, 1053e–1059e. [Google Scholar] [CrossRef] [PubMed]
- Bacos, J.; Turin, S.Y.; Vaca, E.E.; Gosain, A.K. Major Complications and 30-day morbidity for single jaw versus bimaxillary orthognathic surgery as reported by NSQIP. Cleft Palate Craniofac. J. 2019, 56, 705–710. [Google Scholar] [CrossRef]
- Kantar, R.S.; Cammarata, M.J.; Rifkin, W.J.; Alfonso, A.R.; DeMitchell-Rodriguez, E.M.; Noel, D.Y.; Rodriguez, E.D.; Greefield, J.A.; Levy-Lambert, D. Bimaxillary orthognathic surgery is associated with an increased risk of early complications. J. Craniofac. Surg. 2019, 30, 352–357. [Google Scholar] [CrossRef]
- Posnick, J.C.; Choi, E.; Chavda, A. Operative time, airway management, need for blood transfusions, and in-hospital stay for bimaxillary, intranasal, and osseous genioplasty surgery: Current clinical practices. J. Oral Maxillofac. Surg. 2016, 74, 590–600. [Google Scholar] [CrossRef]
- Lin, M.L.; Huang, C.T.; Chiang, H.H.; Chen, C.H. Exploring ethical aspects of elective surgery patients’ decision-making experiences. Nurs. Ethics 2013, 20, 672–683. [Google Scholar] [CrossRef]
- Leeds, I.L.; Efron, D.T.; Lehmann, L.S. Surgical gatekeeping-modifiable risk factors and ethical decision making. N. Engl. J. Med. 2018, 379, 389–394. [Google Scholar] [CrossRef]
- Liao, Y.F.; Chen, Y.F.; Yao, C.F.; Chen, Y.A.; Chen, Y.R. Long-term outcomes of bimaxillary surgery for treatment of asymmetric skeletal class III deformity using surgery-first approach. Clin. Oral Investig. 2019, 23, 1685–1693. [Google Scholar] [CrossRef]
- Ho, C.T.; Lin, H.H.; Lo, L.J. Intraoral scanning and setting up the digital final occlusion in 3D planning of orthognathic surgery: Its comparison with the dental model approach. Plast. Reconstr. Surg. 2019, 143, 1027e–1036e. [Google Scholar] [CrossRef]
- Wu, T.Y.; Denadai, R.; Lin, H.H.; Ho, C.T.; Lo, L.J. The outcome of skeletofacial reconstruction with mandibular rotation for management of asymmetric skeletal class III deformity: A three-dimensional computer-assisted investigation. Sci. Rep. 2019, 9, 13337. [Google Scholar] [CrossRef]
- Ho, C.T.; Lin, H.H.; Liou, E.J.; Lo, L.J. Three-dimensional surgical simulation improves the planning for correction of facial prognathism and asymmetry: A qualitative and quantitative study. Sci. Rep. 2017, 7, 40423. [Google Scholar] [CrossRef] [PubMed]
- Lonic, D.; Pai, B.C.J.; Yamaguchi, K.; Chortrakarnkij, P.; Lin, H.H.; Lo, L.J. Computer-assisted orthognathic surgery for patients with cleft lip/palate: From traditional planning to three-dimensional surgical simulation. PLoS ONE 2016, 11, e0152014. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Lonic, D.; Ko, E.W.C.; Lo, L.J. An integrated surgical protocol for adult patients with hemifacial microsomia: Methods and outcome. PLoS ONE 2017, 12, e0177223. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Rivera-Serrano, C.M.; Chen, C.; Chen, Y.R. Pre-surgical regional blocks in orthognathic surgery: Prospective study evaluating their influence on the intraoperative use of anaesthetics and blood pressure control. Int. J. Oral Maxillofac. Surg. 2016, 45, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, C.; Yao, C.F.; Chen, Y.A.; Chen, Y.R. Comparison of different hypotensive anaesthesia techniques in orthognathic surgery with regard to intraoperative blood loss, quality of the surgical field, and postoperative nausea and vomiting. Int. J. Oral Maxillofac. Surg. 2016, 45, 1526–1530. [Google Scholar] [CrossRef]
- Seo, H.J.; Denadai, R.; Pai, B.C.J.; Lo, L.J. Digital occlusion setup is quantitatively comparable with the conventional dental model approach: Characteristics and guidelines for orthognathic surgery in patients with unilateral cleft lip and palate. Ann. Plast. Surg. 2019. [Google Scholar] [CrossRef]
- Liao, Y.F.; Chiu, Y.T.; Huang, C.S.; Ko, E.W.C.; Chen, Y.R. Presurgical orthodontics versus no presurgical orthodontics: Treatment outcome of surgical-orthodontic correction for skeletal class III open bite. Plast. Reconstr. Surg. 2010, 126, 2074–2083. [Google Scholar] [CrossRef]
- Committee on Standards and Practice Parameters; Apfelbaum, J.L.; Connis, R.T.; Nickinovich, D.G.; Pasternak, L.R.; Arens, J.F.; Caplan, R.A.; Rice, L.J.; Fleisher, L.A.; Gold, B.S.; et al. Practice advisory for preanesthesia evaluation: An updated report by the American Society of Anesthesiologists Task Force on preanesthesia evaluation. Anesthesiology 2012, 116, 522–538. [Google Scholar]
- Armitage, J.N.; van der Meulen, J.H.; Royal College of Surgeons Co-morbidity Consensus Group. Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br. J. Surg. 2010, 97, 772–781. [Google Scholar] [CrossRef]
- Van Walraven, C.; Austin, P.C.; Jennings, A.; Quan, H.; Forster, A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med. Care 2009, 47, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Sinko, K.; Jagsch, R.; Benes, B.; Millesi, G.; Fischmeister, F.; Ewers, R. Facial aesthetics and the assignment of personality traits before and after orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2012, 41, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Denadai, R.; Chou, P.Y.; Su, Y.Y.; Lo, C.C.; Lin, H.H.; Ho, C.T.; Lo, L.J. Facial appearance and psychosocial features in orthognathic surgery: A FACE-Q-and 3D facial image-based comparative study of patient-, clinician-, and lay-observer-reported outcomes. J. Clin. Med. 2019, 8, 909. [Google Scholar] [CrossRef] [PubMed]
- Denadai, R.; Chou, P.Y.; Su, Y.Y.; Lin, H.H.; Ho, C.T.; Lo, L.J. The impacts of orthognathic surgery on the facial appearance and age perception of patients presenting skeletal class III deformity: An outcome study using the FACE-Q report and surgical professional-based panel assessment. Plast. Reconstr. Surg. 2019. in publication. [Google Scholar]
- Bose, S.; Talmor, D. Who is a high-risk surgical patient? Curr. Opin. Crit. Care 2018, 24, 547–553. [Google Scholar] [CrossRef]
- Selwood, A.; Senthuran, S.; Blakely, B.; Lane, P.; North, J.; Clay-Williams, R. Improving outcomes from high-risk surgery: A multimethod evaluation of a patient-centred advanced care planning intervention. BMJ Open 2017, 7, e014906. [Google Scholar] [CrossRef]
- Whiteman, A.R.; Dhesi, J.K.; Walker, D. The high-risk surgical patient: A role for a multi-disciplinary team approach? Br. J. Anaesth. 2016, 116, 311–314. [Google Scholar] [CrossRef]
- Fry, D.E.; Pine, M.; Jordan, H.S.; Elixhauser, A.; Hoaglin, D.C.; Jones, B.; Meimban, R.; Warner, D. Combining administrative and clinical data to stratify surgical risk. Ann. Surg. 2007, 246, 875–885. [Google Scholar] [CrossRef]
- Gupta, A.; Chowdhury, R.; Haring, R.S.; Leinbach, L.I.; Petrone, J.; Spitzer, M.J.; Schneider, E.B. Length of stay and cost in patients undergoing orthognathic surgery: Does surgeon volume matter? J. Oral Maxillofac. Surg. 2017, 75, 1948–1957. [Google Scholar] [CrossRef]
- Denson, D.A.; Waite, P.D.; Digumarthi, H.; Everts, J.E. Does practice type determine the complexity of patients encountered for orthognathic surgery? J. Oral Maxillofac. Surg. 2016, 74, 1643–1648. [Google Scholar] [CrossRef]
- Berlin, N.L.; Tuggle, C.T.; Steinbacher, D.M. Improved short-term outcomes following orthognathic surgery are associated with high-volume centers. Plast. Reconstr. Surg. 2016, 138, 273e–281e. [Google Scholar] [CrossRef] [PubMed]
- Allareddy, V. Orthognathic surgeries in patients with congenital craniofacial anomalies: Profile and hospitalization outcomes. Cleft Palate Craniofac. J. 2015, 52, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Lonic, D.; Chen, C.; Lo, L.J. Correction of facial deformity in Sturge-Weber syndrome. Plast. Reconstr. Surg. Glob. Open 2016, 4, e843. [Google Scholar] [CrossRef] [PubMed]
- Rosén, A.; Modig, M.; Larson, O. Orthognathic bimaxillary surgery in two patients with osteogenesis imperfecta and a review of the literature. Int. J. Oral Maxillofac. Surg. 2011, 40, 866–873. [Google Scholar] [CrossRef]
- Van Belois, H.J., Jr.; Oatis, G.W., Jr.; Sugg, W.E., Jr. Orthognathic surgery in the diabetic patient: Case report. Mil. Med. 1977, 142, 40–44. [Google Scholar] [CrossRef]
- El Amrani, M.; Clement, G.; Lenne, X.; Rogosnitzky, M.; Theis, D.; Pruvot, F.R.; Zerbib, P. The impact of hospital volume and Charlson score on postoperative mortality of proctectomy for rectal cancer: A nationwide study of 45,569 patients. Ann. Surg. 2018, 268, 854–860. [Google Scholar] [CrossRef]
- Moore, B.J.; White, S.; Washington, R.; Coenen, N.; Elixhauser, A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ Elixhauser Comorbidity Index. Med. Care 2017, 55, 698–705. [Google Scholar] [CrossRef]
- Mehta, H.B.; Dimou, F.; Adhikari, D.; Tamirisa, N.P.; Sieloff, E.; Williams, T.P.; Riall, T.S. Comparison of comorbidity scores in predicting surgical outcomes. Med. Care 2016, 54, 180–187. [Google Scholar] [CrossRef]
- Gutacker, N.; Bloor, K.; Cookson, R. Comparing the performance of the Charlson/Deyo and Elixhauser comorbidity measures across five European countries and three conditions. Eur. J. Public Health 2015, 25, 15–20. [Google Scholar] [CrossRef]
- Austin, S.R.; Wong, Y.N.; Uzzo, R.G.; Beck, J.R.; Egleston, B.L. Why summary comorbidity measures such as the Charlson comorbidity index and Elixhauser score work. Med. Care 2015, 53, e65. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Protopapa, K.L.; Simpson, J.C.; Smith, N.C.E.; Moonesinghe, S.R. Development and validation of the surgical outcome risk tool (SORT). Br. J. Surg. 2014, 101, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Liu, Y.; Paruch, J.L.; Zhou, L.; Kmiecik, T.E.; Ko, C.Y.; Cohen, M.E. Development and evaluation of the universal ACS NSQIP surgical risk calculator: A decision aid and informed consent tool for patients and surgeons. J. Am. Coll. Surg. 2013, 217, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Flierler, W.J.; Nübling, M.; Kasper, J.; Heidegger, T. Implementation of shared decision making in anaesthesia and its influence on patient satisfaction. Anaesthesia 2013, 68, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, V.A.; Watt, I.S. Treating patients as persons: A capabilities approach to support delivery of person-centered care. Am. J. Bioeth. 2013, 13, 29–39. [Google Scholar] [CrossRef]
- Donati, A.; Ruzzi, M.; Adrario, E.; Pelaia, P.; Coluzzi, F.; Gabbanelli, V.; Pietropaoli, P. A new and feasible model for predicting operative risk. Br. J. Anaesth. 2004, 93, 393–399. [Google Scholar] [CrossRef]
- Choi, B.K.; Yang, E.J.; Oh, K.S.; Lo, L.J. Assessment of blood loss and need for transfusion during bimaxillary surgery with or without maxillary setback. J. Oral Maxillofac. Surg. 2013, 71, 358–365. [Google Scholar] [CrossRef]
- Smith, M.E.; Shubeck, S.P.; Nuliyalu, U.; Dimick, J.B.; Nathan, H. Local referral of high-risk patients to high-quality hospitals: Surgical outcomes, cost savings, and travel burdens. Ann. Surg. 2019. [Google Scholar] [CrossRef]
- Vonlanthen, R.; Lodge, P.; Barkun, J.S.; Farges, O.; Rogiers, X.; Soreide, K.; Borel-Rinkes, I.; Kehlet, H.; Reynolds, J.; Naredi, P.; et al. Toward a consensus on centralization in surgery. Ann. Surg. 2018, 268, 712–724. [Google Scholar] [CrossRef]
- Isola, G.; Ramaglia, L.; Cordasco, G.; Lucchese, A.; Fiorillo, L.; Matarese, G. The effect of a functional appliance in the management of temporomandibular joint disorders in patients with juvenile idiopathic arthritis. Minerva Stomatol. 2017, 66, 1–8. [Google Scholar]
- Piancino, M.G.; Isola, G.; Cannavale, R.; Cutroneo, G.; Vermiglio, G.; Bracco, P.; Anastasi, G.P. From periodontal mechanoreceptors to chewing motor control: A systematic review. Arch. Oral Biol. 2017, 78, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Piancino, M.G.; Isola, G.; Merlo, A.; Dalessandri, D.; Debernardi, C.; Bracco, P. Chewing pattern and muscular activation in open bite patients. J. Electromyogr. Kinesiol. 2012, 22, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Piancino, M.G.; Cordero-Ricardo, M.; Cannavale, R.; Vallelonga, T.; Garagiola, U.; Merlo, A. Improvement of masticatory kinematic parameters after correction of unilateral posterior crossbite: Reasons for functional retention. Angle Orthod. 2017, 87, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Schlieve, T.; Funderburk, J.; Flick, W.; Miloro, M.; Kolokythas, A. How do general dentists and orthodontists determine where to refer patients requiring oral and maxillofacial surgical procedures? J. Oral Maxillofac. Surg. 2015, 73, 509–513. [Google Scholar] [CrossRef]
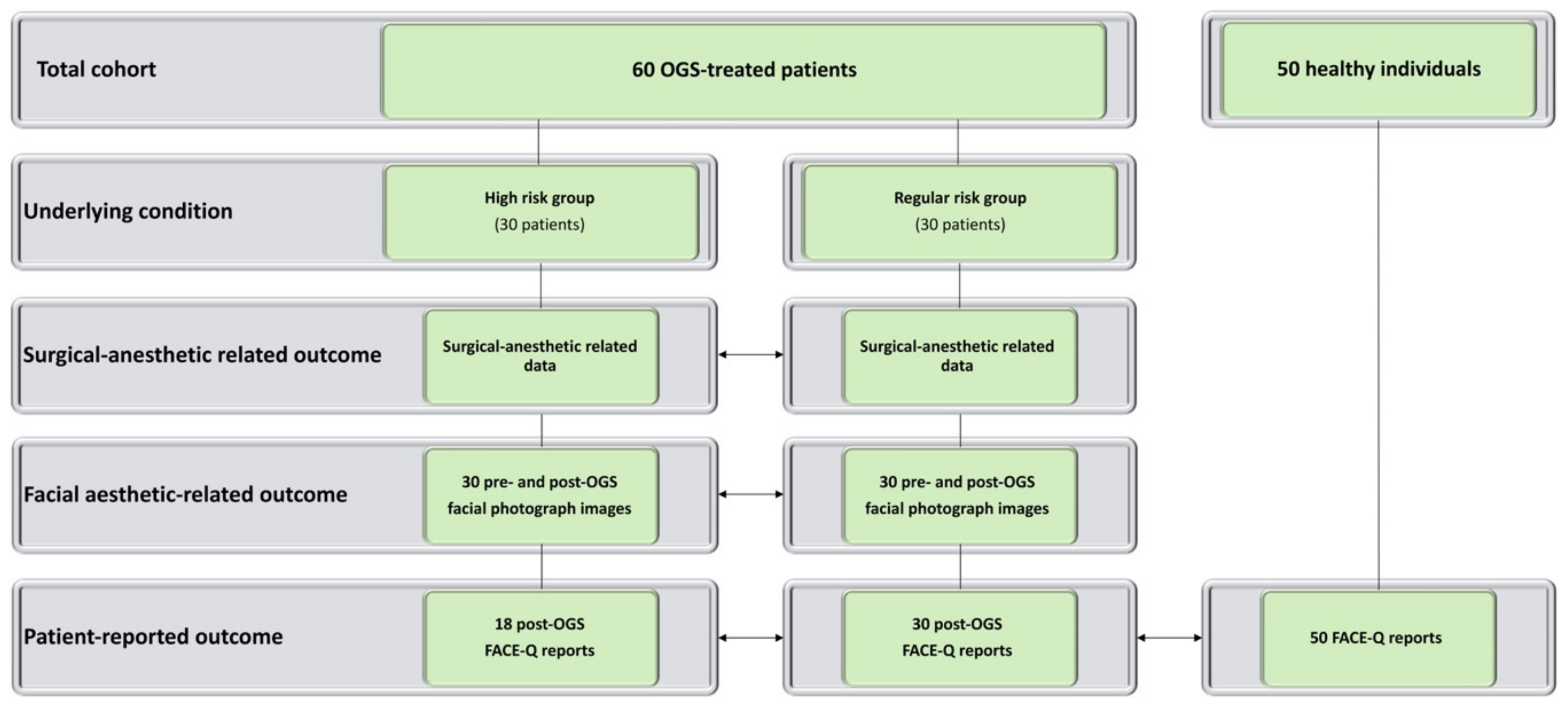


| Parameters | Orthognathic Surgery-Treated Patients | p | |
|---|---|---|---|
| High Risk Group (n = 30) | Regular Risk Group (n = 30) | ||
| Gender n (%) | |||
| Male/Female | 10 (33.3)/20 (66.7) | 12 (40)/18 (60) | >0.05 |
| Age (years, m ± sd) | 25.7 ± 7.4 | 26.3 ± 4.2 | >0.05 |
| Type of underlying disease n (%) | |||
| Genetic | 8 (26.6) | ||
| Autoimmune | 8 (26.6) | ||
| Endocrine | 5 (16.7) | ||
| Neuro-cutaneous | 3 (10) | ||
| Infection | 2 (6.7) | ||
| Congenital cardiac | 2 (6.7) | ||
| Psychiatric | 2 (6.7) | ||
| ASA n (%) | |||
| I/II/III | 3 (10)/23 (76.7)/4 (13.3) | 30 (100)/0 (0)/0 (0) | <0.001 |
| Charlson comorbidity score m ± sd | 1.5 ± 1.5 (1–6) | 0 ± 0 | |
| Number of Charlson comorbidities n (%) | 11 (36.6) | 0 (0) | |
| Charlson score = 1 n (%) | 9 (30) | ||
| Charlson score = 2 n (%) | 1 (3.3) | ||
| Charlson score = 6 n (%) | 1 (3.3) | ||
| Elixhauser comorbidity score m ± sd | 2.9 ± 4.7 (−3–11) | 0 ± 0 | |
| Number of Elixhauser comorbidities n (%) | 20 (66.7) | 0 (0) | |
| Elixhauser score = −1 to −7 n (%) | 4 (13.3) | ||
| Elixhauser score = 0 n (%) | 5 (16.8) | ||
| Elixhauser score = 1 to 5 n (%) | 7 (23.3) | ||
| Elixhauser score = 6+ (%) | 4 (13.3) | ||
| Surgical anesthetic outcomes (m ± sd) | |||
| Blood loss (mL, m ± sd) | 974.3 ± 592.7 | 657.6 ± 355.0 | <0.001 |
| Operation time (min, m ± sd) | 344.5 ± 106.2 | 347 ± 83.9 | >0.05 |
| Hospital length of stay (days, m ± sd) | 4.0 ± 0.8 | 3.7 ± 0.8 | >0.05 |
| Facial aesthetic outcomes (m ± sd) | |||
| Aesthetic (pre/post) | 2.2 ± 1.1/5.1 ± 1.5 | 2.5 ± 1.0/5.3 ± 1.5 | >0.05 |
| Attractive (pre/post) | 2.2 ± 0.9/4.6 ± 1.2 | 2.4 ± 0.9/4.9 ± 1.3 | >0.05 |
| Pleasant (pre/post) | 2.0 ± 1.0/4.5 ± 1.6 | 2.2 ± 1.1/4.8 ± 1.5 | >0.05 |
| Post-OGS FACE-Q outcomes (m ± sd) | |||
| Social function | 71.5 ± 16.6 | 69.8 ± 22.1 | >0.05 |
| Psychological well being | 72.3 ± 23.2 | 70.47 ± 22.3 | >0.05 |
| Satisfaction with decision | 77.3 ± 14.6 | 74.7 ± 23.4 | >0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, P.-Y.; Denadai, R.; Chen, C.; Pai, B.C.-J.; Hsu, K.-H.; Chang, C.-T.; Pascasio, D.; Lin, J.A.-J.; Chen, Y.-R.; Lo, L.-J. Comparison of Orthognathic Surgery Outcomes Between Patients With and Without Underlying High-Risk Conditions: A Multidisciplinary Team-Based Approach and Practical Guidelines. J. Clin. Med. 2019, 8, 1760. https://doi.org/10.3390/jcm8111760
Chou P-Y, Denadai R, Chen C, Pai BC-J, Hsu K-H, Chang C-T, Pascasio D, Lin JA-J, Chen Y-R, Lo L-J. Comparison of Orthognathic Surgery Outcomes Between Patients With and Without Underlying High-Risk Conditions: A Multidisciplinary Team-Based Approach and Practical Guidelines. Journal of Clinical Medicine. 2019; 8(11):1760. https://doi.org/10.3390/jcm8111760
Chicago/Turabian StyleChou, Pang-Yun, Rafael Denadai, Chit Chen, Betty Chien-Jung Pai, Kai-Hsiang Hsu, Che-Tzu Chang, Dax Pascasio, Jennifer Ann-Jou Lin, Yu-Ray Chen, and Lun-Jou Lo. 2019. "Comparison of Orthognathic Surgery Outcomes Between Patients With and Without Underlying High-Risk Conditions: A Multidisciplinary Team-Based Approach and Practical Guidelines" Journal of Clinical Medicine 8, no. 11: 1760. https://doi.org/10.3390/jcm8111760
APA StyleChou, P.-Y., Denadai, R., Chen, C., Pai, B. C.-J., Hsu, K.-H., Chang, C.-T., Pascasio, D., Lin, J. A.-J., Chen, Y.-R., & Lo, L.-J. (2019). Comparison of Orthognathic Surgery Outcomes Between Patients With and Without Underlying High-Risk Conditions: A Multidisciplinary Team-Based Approach and Practical Guidelines. Journal of Clinical Medicine, 8(11), 1760. https://doi.org/10.3390/jcm8111760


_周(Chou).jpg)





