Risk of Recurrent Peptic Ulcer Disease in Patients Receiving Cumulative Defined Daily Dose of Nonsteroidal Anti-Inflammatory Drugs
Abstract
1. Introduction
2. Methods
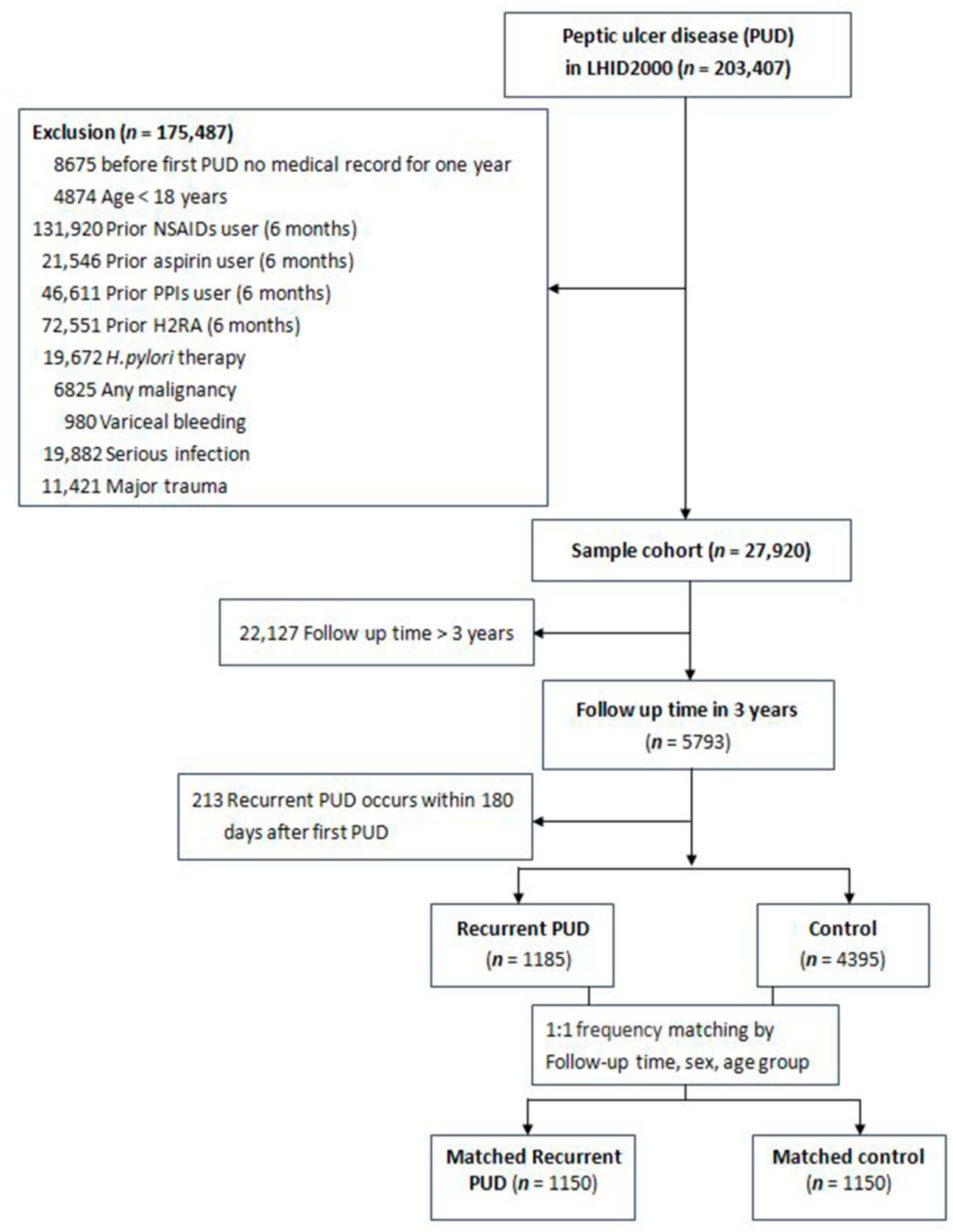
2.1. Study Population
2.2. NSAIDs Exposure
2.3. Other Ulcerogenic Agents and Potential Confounders
2.4. Helicobacter Pylori Therapy and Eradication
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sostres, C.; Gargallo, C.J.; Lanas, A. Interaction between Helicobacter pylori infection, nonsteroidal anti-inflammatory drugs and/or low-dose aspirin use: Old question new insights. World J. Gastroenterol. 2014, 20, 9439–9450. [Google Scholar] [PubMed]
- Nagata, N.; Niikura, R.; Sekine, K.; Sakurai, T.; Shimbo, T.; Kishida, Y.; Tanaka, S.; Aoki, T.; Okubo, H.; Watanabe, K.; et al. Risk of peptic ulcer bleeding associated with Helicobacter pylori infection, nonsteroidal anti-inflammatory drugs, low-dose aspirin, and antihypertensive drugs: A case-control study. J. Gastroenterol. Hepatol. 2015, 30, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.L.; Ye, F.; Liu, W.; Pan, X.L.; Qian, J.; Zhang, G.X. Eradication of Helicobacter pylori infection reduces the incidence of peptic ulcer disease in patients using nonsteroidal anti-inflammatory drugs: A meta-analysis. Helicobacter 2012, 17, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Vergara, M.; Catalán, M.; Gisbert, J.P.; Calvet, X. Meta-analysis: Role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment. Pharmacol. Ther. 2005, 21, 1411–1418. [Google Scholar] [CrossRef]
- Fock, K.M.; Katelaris, P.; Sugano, K.; Ang, T.L.; Hunt, R.; Talley, N.J.; Lam, S.K.; Xiao, S.D.; Tan, H.J.; Wu, C.Y.; et al. Second AsiaPacific Consensus Guidelines for Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2009, 24, 1587–1600. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Chan, F.K.; Chung, S.C.; Suen, B.Y.; Lee, Y.T.; Leung, W.K.; Leung, V.K.; Wu, J.C.; Lau, J.Y.; Hui, Y.; Lai, M.S.; et al. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N. Engl. J. Med. 2001, 344, 967–973. [Google Scholar] [CrossRef]
- Lai, K.C.; Lau, C.S.; Ip, W.Y.; Wong, B.C.; Hui, W.M.; Hu, W.H.; Wong, R.W.; Lam, S.K. Effect of treatment of Helicobacter pylori on the prevention of gastroduodenal ulcers in patients receiving long-term NSAIDs: A double-blind, placebo-controlled trial. Aliment. Pharmacol. Ther. 2003, 17, 799–805. [Google Scholar] [CrossRef]
- De Leest, H.T.; Steen, K.S.; Lems, W.F.; Bijlsma, J.W.; Van de Laar, M.A.; Huisman, A.M.; Vonkeman, H.E.; Houben, H.H.; Kadir, S.W.; Kostense, P.J.; et al. Eradication of Helicobacter pylori does not reduce the incidence of gastroduodenal ulcers in patients on long-term NSAID treatment: Double-blind, randomized, placebo-controlled trial. Helicobacter 2007, 12, 477–485. [Google Scholar] [CrossRef]
- Barber, C.; Lacaille, D.; Fortin, P.R. Systematic review of validation studies of the use of administrative data to identify serious infections. Arthritis Care Res. 2013, 65, 1343–1357. [Google Scholar] [CrossRef]
- Osler, T.; Rutledge, R.; Deis, J.; Bedrick, E. ICISS: An international classification of disease-9 based injury severity score. J. Trauma 1996, 41, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Wiese, A.D.; Griffin, M.R.; Stein, C.M.; Schaffner, W.; Greevy, R.A.; Mitchel, E.F., Jr.; Grijalva, C.G. Validation of discharge diagnosis codes to identify serious infections among middle age and older adults. BMJ Open. 2018, 8, e020857. [Google Scholar] [CrossRef] [PubMed]
- Abajas-Bustillo, R.; Amo-Setién, F.J.; Leal-Costa, C.; Ortego-Mate, M.d.C.; Seguí-Gómez, M.; Durá-Ros, M.J. Comparison of injury severity scores (ISS) obtained by manual coding versus “Two-step conversion” from ICD-9-CM. PLoS ONE 2019, 14, e0216206. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Center for Drugs Statistics Methodology: ATC/DDD Index 2013. Available online: http://www.whocc.no/atc_ddd_index/ (accessed on 1 January 2013).
- Kieszak, S.M.; Flanders, W.D.; Kosinski, A.S.; Shipp, C.C.; Karp, H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J. Clin. Epidemiol. 1999, 52, 137–142. [Google Scholar] [CrossRef]
- Wu, C.Y.; Kuo, K.N.; Wu, M.S.; Chen, Y.J.; Wang, C.B.; Lin, J.T. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology 2009, 137, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Tseng, K.L.; Hsu, C.N.; Liang, C.M.; Tai, W.C.; Ku, M.K.; Hung, T.H.; Yuan, L.T.; Nguang, S.H.; Yang, S.C.; et al. Association between Helicobacter pylori eradication and the risk of coronary heart diseases. PLoS ONE 2018, 13, e0190219. [Google Scholar] [CrossRef]
- Liang, C.M.; Hsu, C.N.; Tai, W.C.; Yang, S.C.; Wu, C.K.; Shih, C.W.; Ku, M.K.; Yuan, L.T.; Wang, J.W.; Tseng, K.L.; et al. Taiwan Acid-Related Disease (TARD) Study Group. Risk factors influencing the outcome of peptic ulcer bleeding in chronic kidney disease after initial endoscopic hemostasis: A nationwide cohort study. Medicine 2016, 95, e4795. [Google Scholar] [CrossRef]
- Laine, L.; Jensen, D.M. Management of patients with ulcer bleeding. Am. J. Gastroenterol. 2012, 107, 345–360. [Google Scholar] [CrossRef]
- Chan, F.K.; Wong, V.W.; Suen, B.Y.; Wu, J.C.; Ching, J.Y.; Hung, L.C.; Hui, A.J.; Leung, V.K.; Lee, V.W.; Lai, L.H.; et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: A double-blind, randomised trial. Lancet 2007, 369, 1621–1626. [Google Scholar] [CrossRef]
- Yuan, J.Q.; Tsoi, K.K.; Yang, M.; Wang, J.Y.; Threapleton, D.E.; Yang, Z.Y. Systematic review with network meta-analysis: Comparative effectiveness and safety of strategies for preventing NSAID-associated gastrointestinal toxicity. Aliment. Pharmacol. Ther. 2016, 43, 1262–1275. [Google Scholar] [CrossRef]
- Rostom, A.; Dube, C.; Wells, G.; Tugwell, P.; Welch, V.; Jolicoeur, E.; McGowan, J. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst. Rev. 2002, 4, CD002296. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Chang, F.Y.; Chen, T.S.; Chen, C.Y.; Jiun, K.L.; Lee, S.D. Helicobacter pylori colonization does not influence the symptomatic response to prokinetic agents in patients with functional dyspepsia. J. Gastroenterol. Hepatol. 1998, 13, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Garcia Rodriguez, L.A.; Hernandez-Diaz, S. Relative risk of upper gastrointestinal complications among users of acetaminophen and nonsteroidal anti-inflammatory drugs. Epidemiology 2001, 57, 570–576. [Google Scholar] [CrossRef]
- Garcia Rodriguez, L.A.; Barreales Tolosa, L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology 2007, 132, 498–506. [Google Scholar] [CrossRef]
- Lanas, A.; Garcia-Rodriguez, L.A.; Arroyo, M.T.; Gomollon, F.; Feu, F.; Gonzalez-Perez, A. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut 2006, 55, 1731–1738. [Google Scholar] [CrossRef]
- Massó González, E.L.; Patrignani, P.; Tacconelli, S.; García Rodríguez, L.A. Variability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleeding. Arthritis Rheumatol. 2010, 62, 1592–1601. [Google Scholar] [CrossRef]
- Dinçer, D.; Ulukal Karancı, E.; Akın, M.; Adanır, H. NSAID, antiaggregant, and/or anticoagulant-related upper gastrointestinal bleeding: Is there any change in prophylaxis rate after a 10-year period? Turk. J. Gastroenterol. 2019, 30, 505–510. [Google Scholar]
- Bytzer, P.; Howell, S.; Leemon, M.; Young, L.J.; Jones, M.P.; Talley, N.J. Low socioeconomic class is a risk factor for upper and lower gastrointestinal symptoms: A population-based study in 15,000 Australian adults. Gut 2001, 49, 66–72. [Google Scholar] [CrossRef]
| Variables | Primary Case-Control Group | Matched Case-Control Group | ||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 5580) | Recurrent PUD (n = 1185) | Control (n = 4395) | p Value | All (n = 2300) | Recurrent PUD (n = 1150) | Control (n = 1150)) | p Value | |
| Follow up period (months) | 18.33 ± 10.66 | 20.47 ± 8.79 | 17.75 ± 11.04 | <0.0001 | 20.65 ± 8.77 | 20.65 ± 8.77 | 20.65 ± 8.77 | 1.0000 |
| NSAID use | <0.0001 | 0.0028 | ||||||
| DDD = 0 | 1910 | 298(25.15) | 1612(36.68) | 632 | 284(24.70) | 348(30.26) | ||
| DDD > 0 | 3670 | 887(74.85) | 2783(63.32) | 1668 | 866(75.30) | 802(69.74) | ||
| NSAID cDDD group | <0.0001 | 0.0156 | ||||||
| DDD = 0 | 1910 | 298(25.15) | 1612(36.68) | 632 | 284(24.70) | 348(30.26) | ||
| 0 < DDD ≤ 28 | 2736 | 632(53.33) | 2104(47.87) | 1203 | 614(53.39) | 589(51.22) | ||
| 28 < DDD ≤ 84 | 667 | 183(15.44) | 484(11.01) | 335 | 180(15.65) | 155(13.48) | ||
| 84 < DDD | 267 | 72(6.08) | 195(4.44) | 130 | 72(6.26) | 58(5.04) | ||
| HP eradication therapy | 263 | 102(8.61) | 161(3.66) | <0.0001 | 139 | 99(8.61) | 40(3.48) | <0.0001 |
| Gender | <0.0001 | 1.0000 | ||||||
| Female | 2623 | 459(38.73) | 2164(49.24) | 912 | 456(39.65) | 456(39.65) | ||
| Male | 2957 | 726(61.27) | 2231(50.76) | 1388 | 694(60.35) | 694(60.35) | ||
| Age group (years) | <0.0001 | 1.0000 | ||||||
| 18–30 | 655 | 118(9.96) | 537(12.22) | 224 | 112(9.74) | 112(9.74) | ||
| 31–40 | 900 | 193(16.29) | 707(16.09) | 384 | 192(16.70) | 192(16.70) | ||
| 41–50 | 1123 | 288(24.30) | 835(19.00) | 566 | 283(24.61) | 283(24.61) | ||
| 51–60 | 1086 | 254(21.43) | 832(18.93) | 480 | 240(20.87) | 240(20.87) | ||
| 61–70 | 724 | 164(13.84) | 560(12.74) | 310 | 155(13.48) | 155(13.48) | ||
| >70 | 1092 | 168(14.18) | 924(21.02) | 336 | 168(14.61) | 168(14.61) | ||
| Comorbidity | ||||||||
| Acute myocardial infarction | 30 | 3(0.25) | 27(0.61) | 0.1313 | 8 | 3(0.26) | 5(0.43) | 0.4787 |
| Congestive heart failure | 111 | 5(0.42) | 106(2.41) | <0.0001 | 25 | 5(0.43) | 20(1.74) | 0.0026 |
| Peripheral vascular disease | 26 | 0(0.00) | 26(0.59) | 0.0080 | 7 | 0(0.00) | 7(0.61) | 0.0081 |
| Cerebral vascular accident | 283 | 41(3.46) | 242(5.51) | 0.0044 | 86 | 40(3.48) | 46(4.00) | 0.5096 |
| Dementia | 69 | 8(0.68) | 61(1.39) | 0.0488 | 16 | 8(0.70) | 8(0.70) | 1.0000 |
| Pulmonary disease | 470 | 93(7.85) | 377(8.58) | 0.4221 | 184 | 93(8.09) | 91(7.91) | 0.8778 |
| Connective tissue disorder | 29 | 7(0.59) | 22(0.50) | 0.7017 | 9 | 7(0.61) | 2(0.17) | 0.0949 |
| Liver disease | 514 | 137(11.56) | 377(8.58) | 0.0016 | 216 | 127(11.04) | 89(7.74) | 0.0066 |
| Diabetes | 485 | 78(6.58) | 407(9.26) | 0.0037 | 169 | 77(6.70) | 92(8.00) | 0.2306 |
| Diabetes complications | 119 | 18(1.52) | 101(2.30) | 0.0995 | 35 | 17(1.48) | 18(1.57) | 0.8647 |
| Paraplegia | 18 | 2(0.17) | 16(0.36) | 0.2928 | 5 | 2(0.17) | 3(0.26) | 0.6544 |
| Renal disease | 188 | 35(2.95) | 153(3.48) | 0.3716 | 66 | 34(2.96) | 32(2.78) | 0.8027 |
| Severe liver disease | 25 | 2(0.17) | 23(0.52) | 0.1048 | 4 | 2(0.17) | 2(0.17) | 1.0000 |
| HIV | 4 | 0(0.00) | 4(0.09) | 0.2989 | 2 | 0(0.00) | 2(0.17) | 0.1571 |
| Hypertension | 1073 | 205(17.30) | 868(19.75) | 0.0575 | 393 | 197(17.13) | 196(17.04) | 0.9558 |
| Hyperlipidemia | 455 | 80(6.75) | 375(8.53) | 0.0467 | 177 | 79(6.87) | 98(8.52) | 0.1372 |
| Medication use in the baseline period | ||||||||
| Antiplatelet | 236 | 44(3.71) | 192(4.37) | 0.3197 | 85 | 44(3.83) | 41(3.57) | 0.7402 |
| Anti-coagulants | 19 | 2(0.17) | 17(0.39) | 0.2529 | 4 | 2(0.17) | 2(0.17) | 1.0000 |
| ACEI/ARB | 519 | 105(8.86) | 414(9.42) | 0.5565 | 208 | 100(8.70) | 108(9.39) | 0.5608 |
| SSRIs | 18 | 2(0.17) | 16(0.36) | 0.2928 | 5 | 2(0.17) | 3(0.26) | 0.6544 |
| Concomitant medications during follow-up | ||||||||
| PPIs | 1036 | 266(22.45) | 770(17.52) | 0.0001 | 456 | 258(22.43) | 198(17.22) | 0.0017 |
| H2RA | 1017 | 317(26.75) | 700(15.93) | <0.0001 | 511 | 309(26.87) | 202(17.57) | <0.0001 |
| Antiplatelet | 560 | 129(10.89) | 431(9.81) | 0.2724 | 258 | 126(10.96) | 132(11.48) | 0.6918 |
| Anti-coagulants | 44 | 14(1.18) | 30(0.68) | 0.0849 | 20 | 14(1.22) | 6(0.52) | 0.0724 |
| Cerenin® | 40 | 9(0.76) | 31(0.71) | 0.8445 | 18 | 9(0.78) | 9(0.78) | 1.0000 |
| Aspirin | 269 | 67(5.65) | 202(4.60) | 0.1314 | 133 | 64(5.57) | 69(6.00) | 0.6551 |
| SSRIs | 116 | 22(1.86) | 94(2.14) | 0.5456 | 50 | 21(1.83) | 29(2.52) | 0.2527 |
| Variables | Matched Case-Control Group | ||
|---|---|---|---|
| aOR | 95% CI | p-Value | |
| NSAID cDDD group (ref: cDDD = 0) | |||
| cDDD ≤ 28 | 1.24 | (1.01–1.52) | 0.0430 |
| 28 < cDDD ≤ 84 | 1.52 | (1.14–2.02) | 0.0043 |
| 84 < cDDD | 1.67 | (1.09–2.56) | 0.0179 |
| HP eradication therapy | 2.73 | (1.80–4.15) | <0.0001 |
| Kinds of NSAIDs | |||
| diclofenac | 1.33 | (1.09–1.61) | 0.0042 |
| mefenamic acid | 1.05 | (0.86–1.28) | 0.6379 |
| ibuprofen | 0.98 | (0.78–1.22) | 0.8409 |
| ketorolac | 0.95 | (0.73–1.23) | 0.7039 |
| ketoprofen | 1.27 | (0.90–1.79) | 0.1696 |
| naproxen | 1.38 | (0.98–1.93) | 0.0630 |
| acemetacin | 0.86 | (0.61–1.21) | 0.3839 |
| meloxicam | 1.15 | (0.76–1.76) | 0.5045 |
| celecoxib | 0.80 | (0.50–1.28) | 0.3477 |
| piroxicam | 1.59 | (1.03–2.44) | 0.0346 |
| indometacin | 1.04 | (0.65–1.68) | 0.8585 |
| flubiprofen | 1.15 | (0.75–1.76) | 0.5224 |
| sulindac | 1.59 | (1.03–2.45) | 0.0367 |
| Comorbidity | |||
| Acute myocardial infarction | 0.54 | (0.11–2.71) | 0.4517 |
| Congestive heart failure | 0.20 | (0.07–0.61) | 0.0048 |
| Cerebral vascular accident | 0.85 | (0.52–1.40) | 0.5339 |
| Dementia | 1.41 | (0.47–4.22) | 0.5425 |
| Pulmonary disease | 1.09 | (0.79–1.51) | 0.6021 |
| Connective tissue disorder | 3.56 | (0.69–18.29) | 0.1276 |
| Liver disease | 1.47 | (1.09–2.00) | 0.0128 |
| Diabetes | 0.75 | (0.52–1.10) | 0.1407 |
| Diabetes complications | 0.89 | (0.41–1.95) | 0.7742 |
| Paraplegia | 1.59 | (0.24–10.53) | 0.6336 |
| Renal disease | 0.97 | (0.55–1.71) | 0.9150 |
| Severe liver disease | 0.45 | (0.06–3.45) | 0.4414 |
| Hypertension | 1.16 | (0.84–1.61) | 0.3554 |
| Hyperlipidemia | 0.78 | (0.55–1.11) | 0.1668 |
| Medications use in the baseline period | |||
| Antiplatelet | 1.35 | (0.78–2.35) | 0.2802 |
| Anti-coagulants | 2.22 | (0.14–35.03) | 0.5703 |
| ACEI/ARB | 0.89 | (0.60–1.34) | 0.5849 |
| SSRIs | 0.87 | (0.13–5.73) | 0.8878 |
| Concomitant medications during follow up | |||
| PPIs | 1.13 | (0.89–1.44) | 0.3022 |
| H2RA | 1.85 | (1.47–2.32) | <0.0001 |
| Antiplatelet | 0.65 | (0.41–1.03) | 0.0688 |
| Anti-coagulants | 4.21 | (1.21–14.68) | 0.0242 |
| Cerenin® | 1.08 | (0.40–2.91) | 0.8794 |
| Aspirin | 1.30 | (0.76–2.23) | 0.3382 |
| SSRIs | 0.63 | (0.35–1.15) | 0.1320 |
| H pylori Therapy | Without H pylori Therapy | |||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| NSAID use | ||||||
| cDDD > 0 (ref:DDD = 0) | 1.09 | (0.43–2.77) | 0.8490 | 1.31 | (1.08–1.59) | 0.0068 |
| NSAID cDDD group (ref: cDDD = 0) | ||||||
| cDDD ≤ 28 | - | 1.24 | (1.01–1.52) | 0.0424 | ||
| 28 < cDDD ≤ 84 | - | 1.47 | (1.11–1.94) | 0.0074 | ||
| 84 < cDDD | - | 1.64 | (1.10–2.45) | 0.0152 | ||
| Comorbidity | ||||||
| Acute myocardial infarction | - | 0.48 | (0.10–2.32) | 0.3591 | ||
| Congestive heart failure | - | 0.23 | (0.08–0.71) | 0.0104 | ||
| Cerebral vascular accident | 0.52 | (0.06–4.75) | 0.5597 | 0.83 | (0.50–1.39) | 0.4737 |
| Dementia | - | 1.26 | (0.42–3.74) | 0.6772 | ||
| Pulmonary disease | 2.57 | (0.28–23.39) | 0.4030 | 0.99 | (0.72–1.36) | 0.9463 |
| Connective tissue disorder | - | 3.31 | (0.67–16.41) | 0.1420 | ||
| Liver disease | 3.84 | (0.43–34.17) | 0.2283 | 1.50 | (1.11–2.02) | 0.0083 |
| Diabetes | 0.72 | (0.13–4.06) | 0.7049 | 0.80 | (0.55–1.17) | 0.2567 |
| Diabetes complications | - | 0.85 | (0.39–1.88) | 0.6929 | ||
| Paraplegia | - | 1.12 | (0.17–7.36) | 0.9042 | ||
| Renal disease | - | 0.86 | (0.49–1.51) | 0.6063 | ||
| Severe liver disease | - | 0.55 | (0.07–4.24) | 0.5704 | ||
| Hypertension | 0.67 | (0.15–3.01) | 0.6036 | 1.17 | (0.86–1.59) | 0.3299 |
| Hyperlipidemia | 1.51 | (0.26–8.88) | 0.6470 | 0.74 | (0.53–1.05) | 0.0886 |
| Medications use in the baseline period | ||||||
| Antiplatelet | 0.79 | (0.11–5.62) | 0.8136 | 1.40 | (0.80–2.45) | 0.2390 |
| Anti-coagulants | - | 0.64 | (0.05–9.04) | 0.7416 | ||
| ACEI/ARB | 1.57 | (0.23–10.80) | 0.6474 | 0.87 | (0.59–1.30) | 0.5029 |
| SSRIs | - | 0.87 | (0.14–5.45) | 0.8850 | ||
| Concomitant medications during follow up | ||||||
| PPIs | 0.38 | (0.17–0.86) | 0.0199 | 1.24 | (0.98–1.58) | 0.0735 |
| H2RA | 1.89 | (0.70–5.09) | 0.2066 | 1.74 | (1.39–2.18) | <0.0001 |
| Anti-platelet agents | 2.98 | (0.55–16.11) | 0.2041 | 0.65 | (0.41–1.02) | 0.0598 |
| Anti-coagulants | - | 6.34 | (1.59–25.32) | 0.0089 | ||
| Cerenin® | - | 0.91 | (0.33–2.50) | 0.8529 | ||
| Aspirin | - | 1.17 | (0.68–2.03) | 0.5663 | ||
| SSRIs | - | 0.59 | (0.32–1.08) | 0.0882 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.-M.; Yang, S.-C.; Wu, C.-K.; Li, Y.-C.; Yeh, W.-S.; Tai, W.-C.; Lee, C.-H.; Yang, Y.-H.; Tsai, T.-H.; Hsu, C.-N.; et al. Risk of Recurrent Peptic Ulcer Disease in Patients Receiving Cumulative Defined Daily Dose of Nonsteroidal Anti-Inflammatory Drugs. J. Clin. Med. 2019, 8, 1722. https://doi.org/10.3390/jcm8101722
Liang C-M, Yang S-C, Wu C-K, Li Y-C, Yeh W-S, Tai W-C, Lee C-H, Yang Y-H, Tsai T-H, Hsu C-N, et al. Risk of Recurrent Peptic Ulcer Disease in Patients Receiving Cumulative Defined Daily Dose of Nonsteroidal Anti-Inflammatory Drugs. Journal of Clinical Medicine. 2019; 8(10):1722. https://doi.org/10.3390/jcm8101722
Chicago/Turabian StyleLiang, Chih-Ming, Shih-Cheng Yang, Cheng-Kun Wu, Yu-Chi Li, Wen-Shuo Yeh, Wei-Chen Tai, Chen-Hsiang Lee, Yao-Hsu Yang, Tzu-Hsien Tsai, Chien-Ning Hsu, and et al. 2019. "Risk of Recurrent Peptic Ulcer Disease in Patients Receiving Cumulative Defined Daily Dose of Nonsteroidal Anti-Inflammatory Drugs" Journal of Clinical Medicine 8, no. 10: 1722. https://doi.org/10.3390/jcm8101722
APA StyleLiang, C.-M., Yang, S.-C., Wu, C.-K., Li, Y.-C., Yeh, W.-S., Tai, W.-C., Lee, C.-H., Yang, Y.-H., Tsai, T.-H., Hsu, C.-N., & Chuah, S.-K. (2019). Risk of Recurrent Peptic Ulcer Disease in Patients Receiving Cumulative Defined Daily Dose of Nonsteroidal Anti-Inflammatory Drugs. Journal of Clinical Medicine, 8(10), 1722. https://doi.org/10.3390/jcm8101722






