RETRACTED: Nurse’s A-Phase–Silicocarnotite Ceramic–Bone Tissue Interaction in a Rabbit Tibia Defect Model
Abstract
1. Introduction
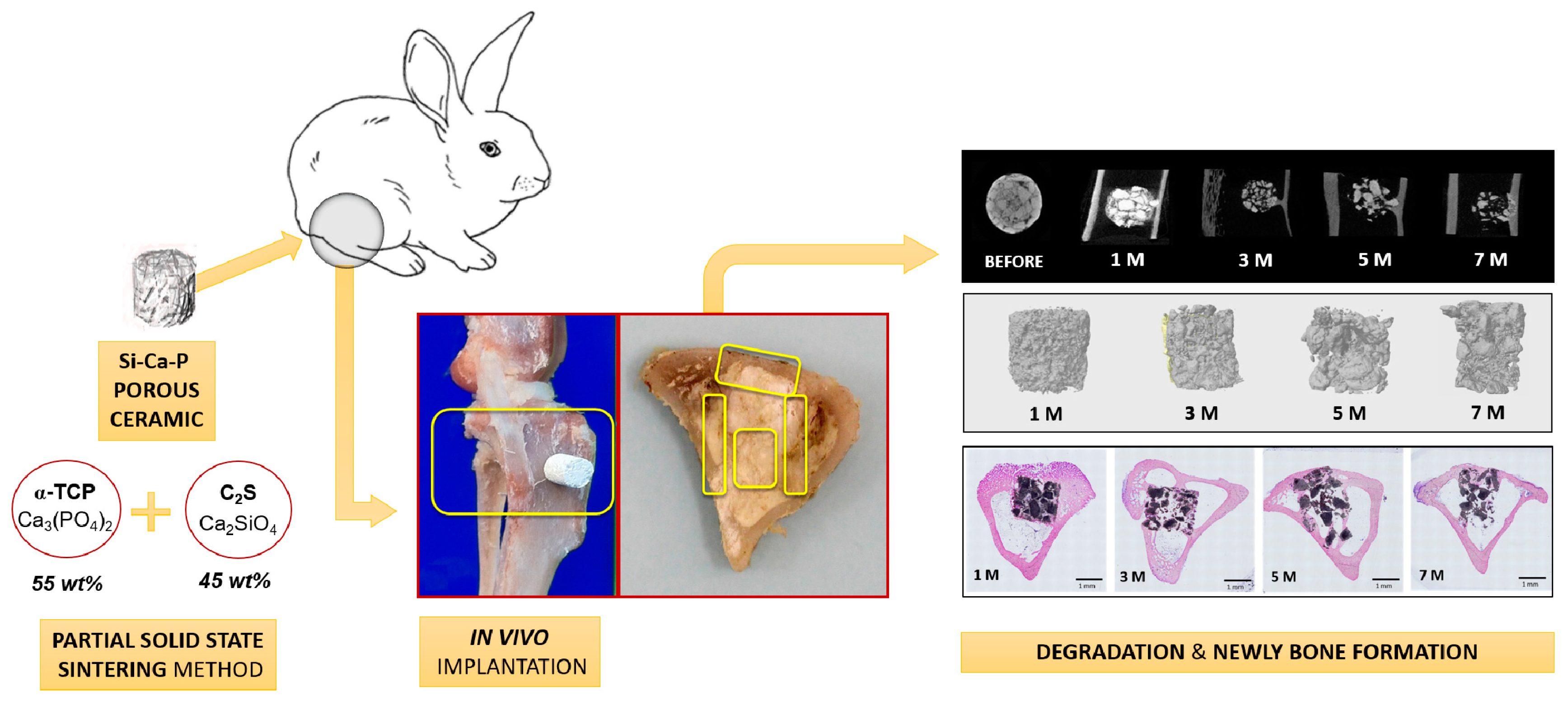
2. Experimental Section
2.1. Preparation of Scaffolds
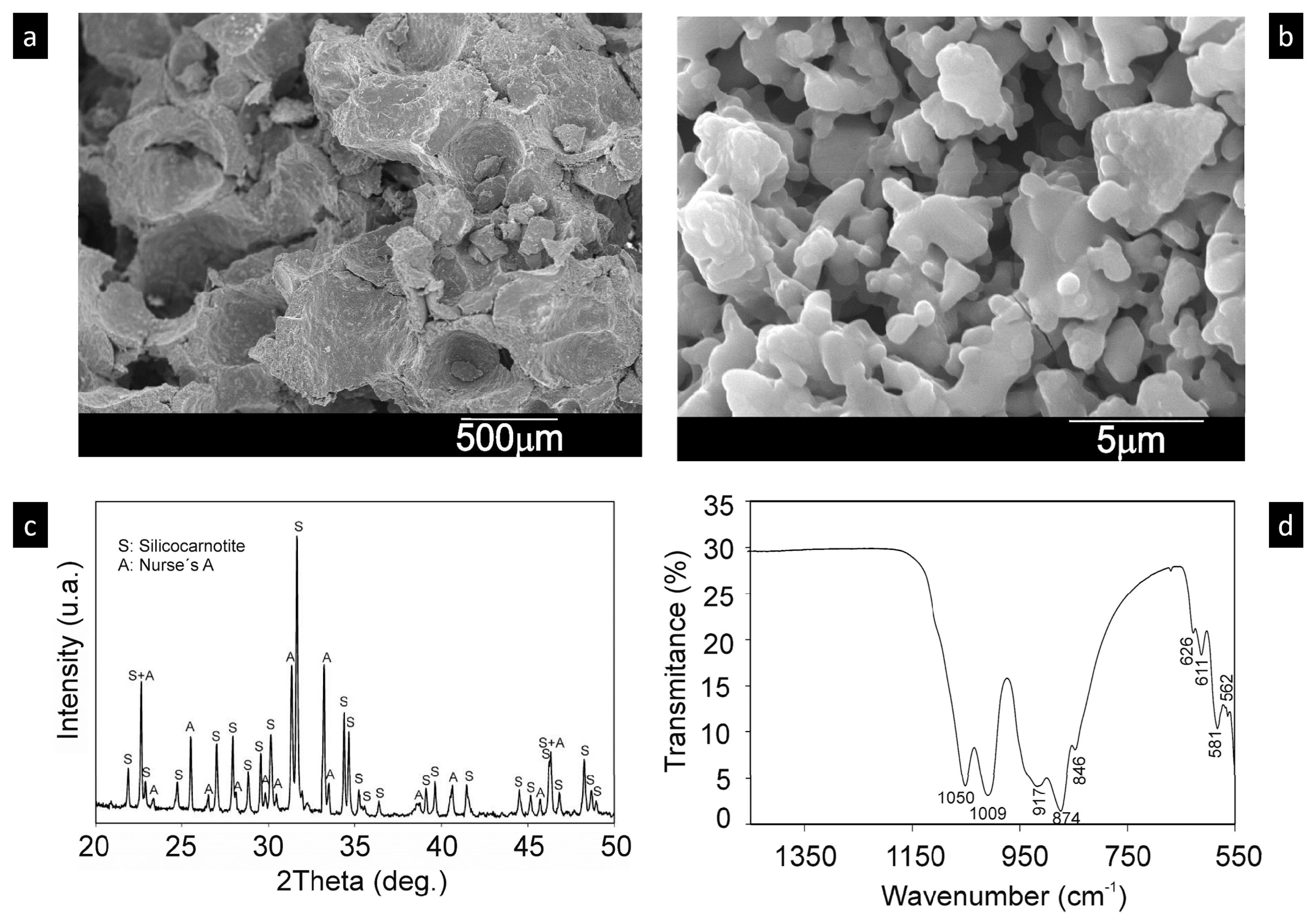
2.2. Characterization of Scaffolds
2.3. Surgical Procedure and Implantation
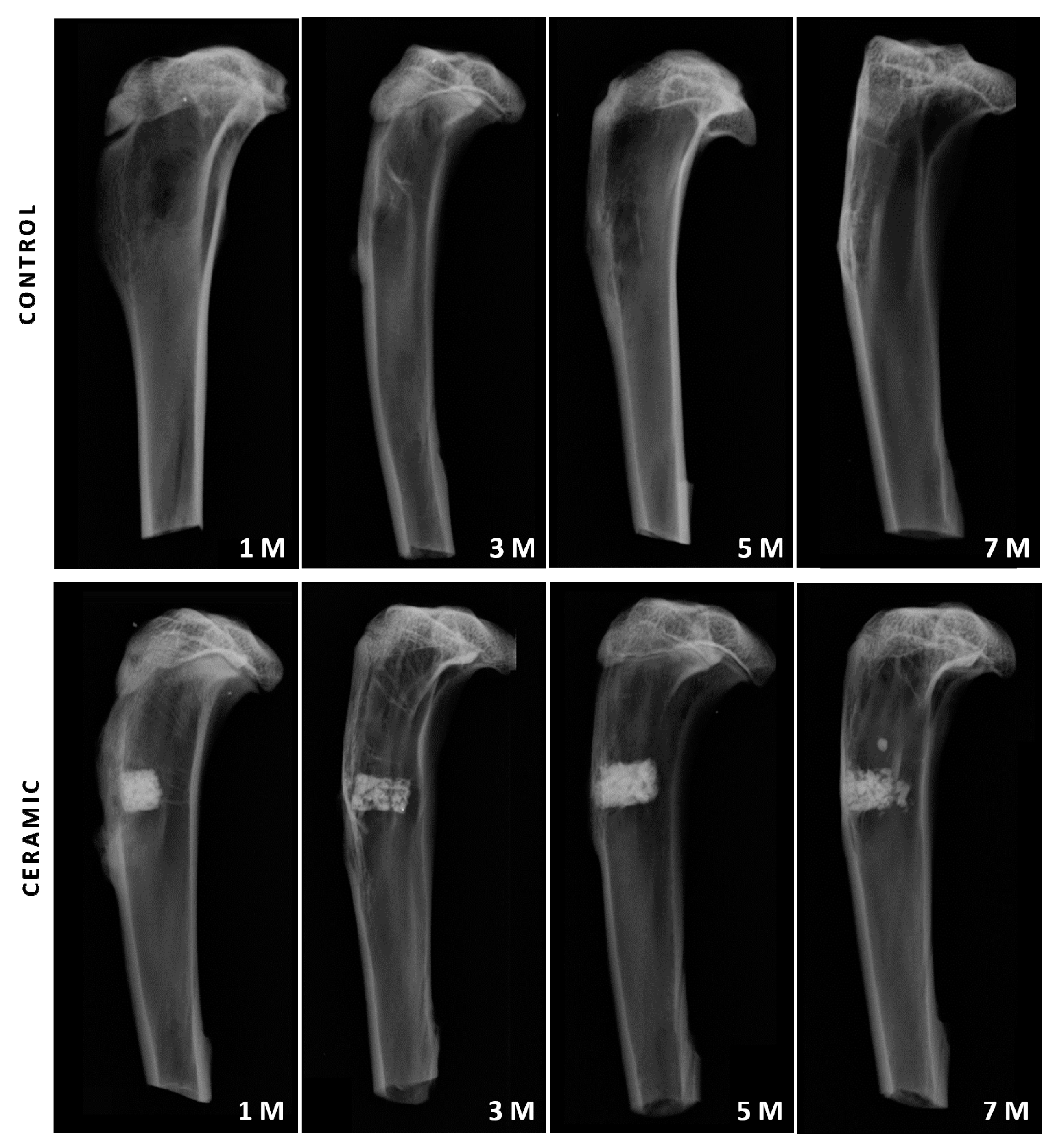
2.4. Conventional Radiological Study
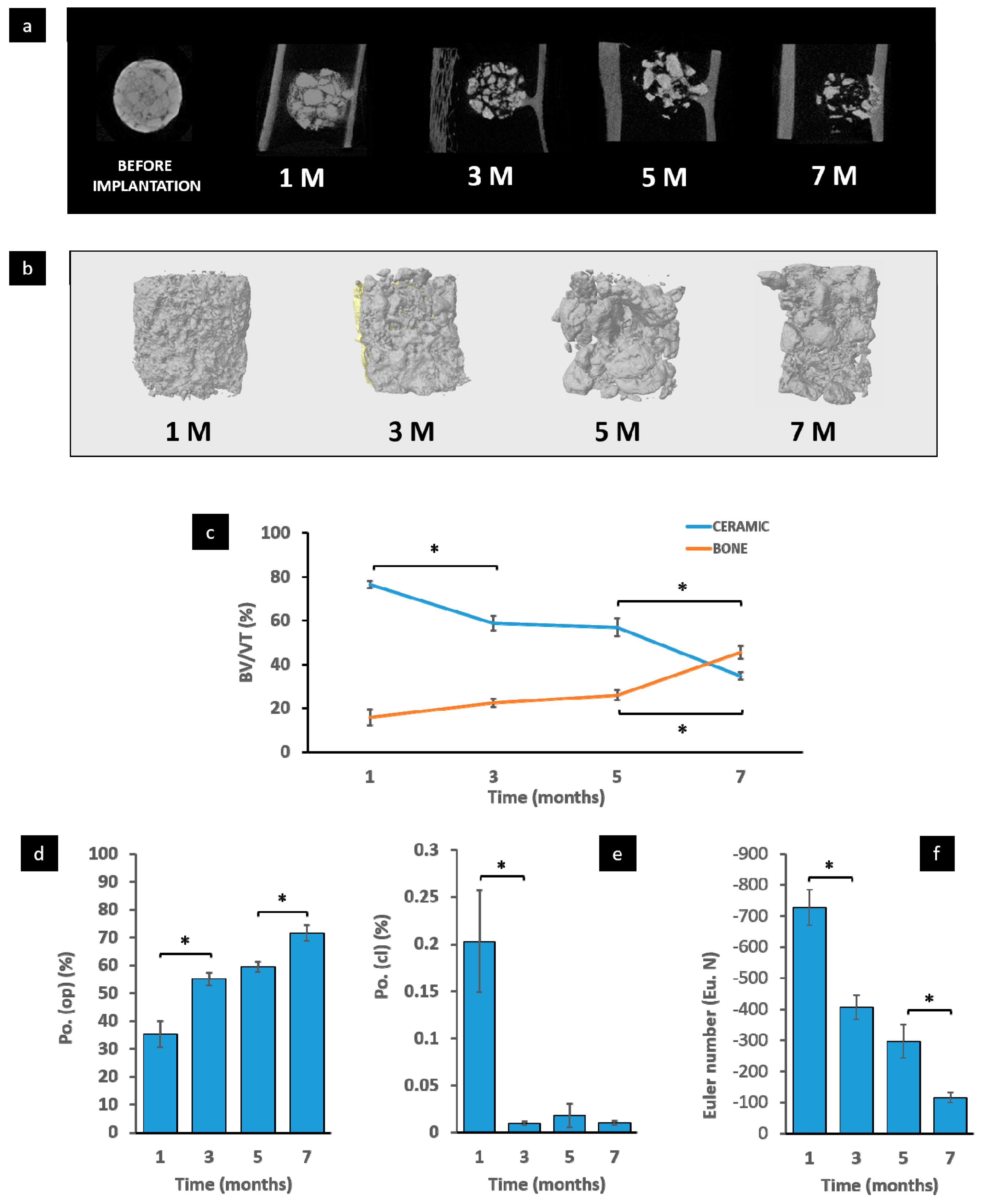
2.5. Micro-CT Evaluation
2.6. Histological and Histomorphometric Study
2.7. Statistical Analysis
3. Results
3.1. Scaffold Characterization
3.2. Conventional Radiological Study
3.3. Micro-CT Evaluation
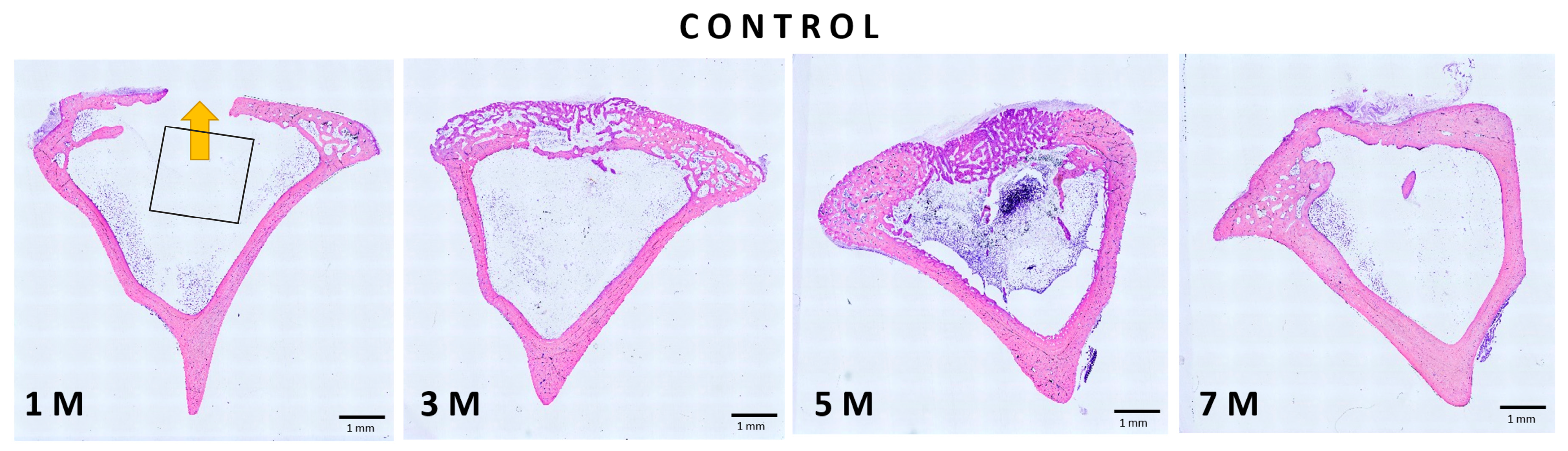
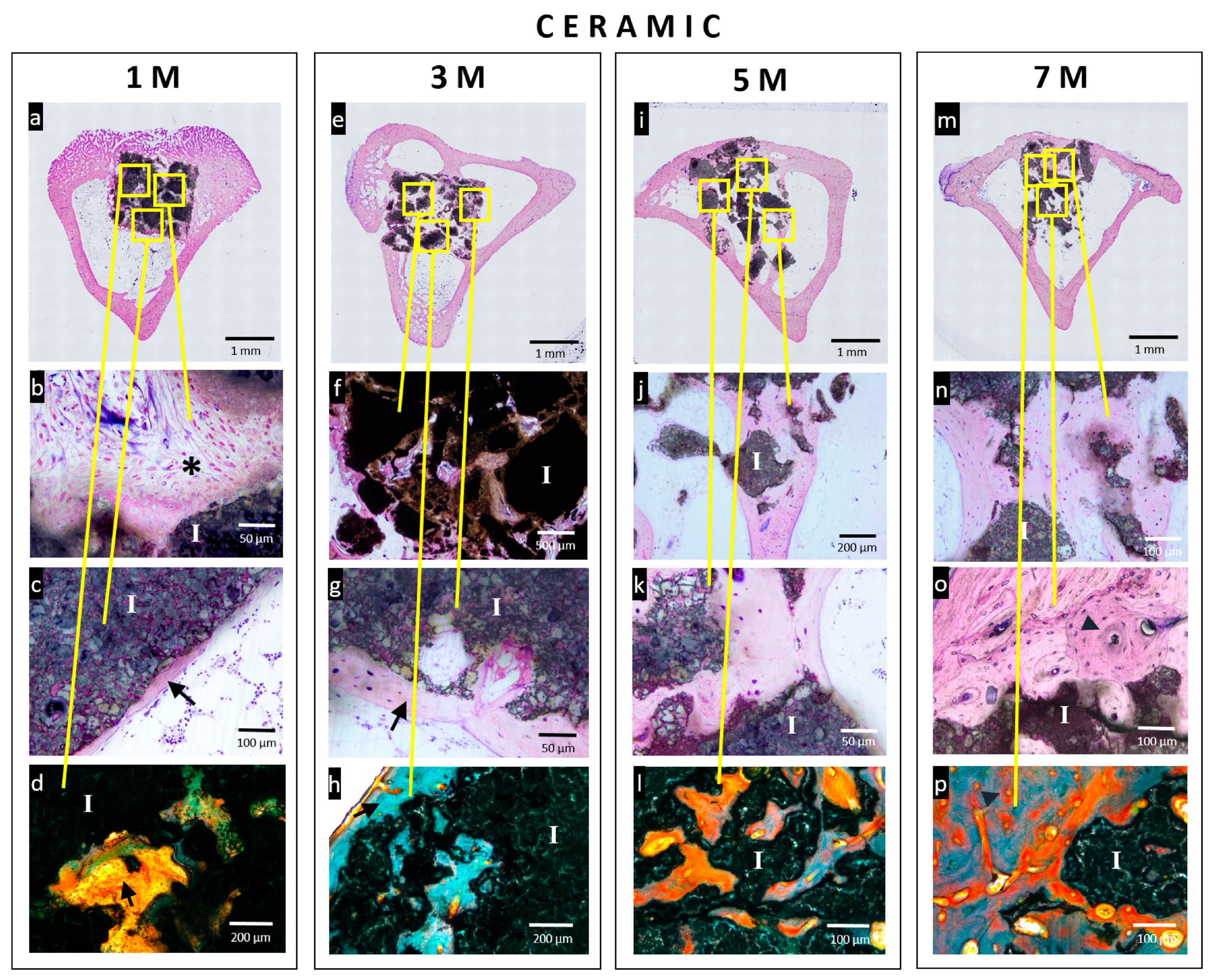
3.4. Histological Analysis
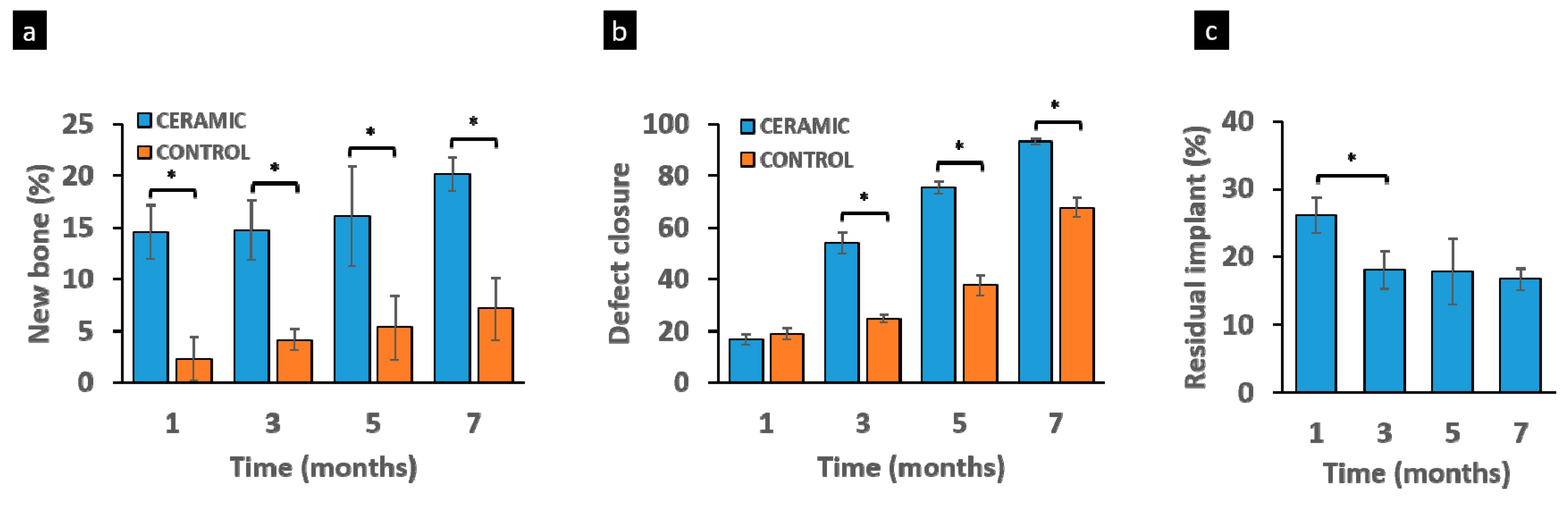
3.5. Histomorphometric Study
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Citron, P.; Nerem, R.M. Bioengineering: 25 years of progress—But still only a beginning. Technol. Soc. 2004, 26, 415–431. [Google Scholar] [CrossRef]
- Bhat, S.; Kumar, A. Biomaterials and bioengineering tomorrow’s healthcare. Biomatter 2013, 3, e24717. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013, 1, 216. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Roy, S.; Mukherjee, P.; Kundu, B.; De, D.K.; Basu, D. Orthopaedic applications of bone graft & graft substitutes: A review. Indian J. Med. Res. 2010, 132, 15–30. [Google Scholar]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Rubio, V.; de la Casa-Lillo, M.A.; De Aza, S.; De Aza, P.N. The System Ca3 (PO4)2–Ca2SiO4: The Sub-System Ca2SiO4–7 CaOP2O52SiO2. J. Am. Ceram. Soc. 2011, 94, 4459–4462. [Google Scholar] [CrossRef]
- Martínez, I.M.; Velásquez, P.; De Aza, P.N. The Sub-System α-TCP ss-Silicocarnotite Within the Binary System Ca3 (PO4)2–Ca2SiO4. J. Am. Ceram. Soc. 2012, 95, 1112–1117. [Google Scholar]
- Ros-Tárraga, P.; Mazón, P.; Meseguer-Olmo, L.; De Aza, P.N. Revising the Subsystem Nurse’s A-Phase–Silicocarnotite within the System Ca3(PO4)2-Ca2SiO4. Materials 2016, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F.; Piras, A.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Hild, N.; Schneider, O.D.; Mohn, D.; Luechinger, N.A.; Koehler, F.M.; Hofmann, S.; Vetsch, J.R.; Thimm, B.W.; Müller, R.; Stark, W.J. Two-layer membranes of calcium phosphate/collagen/PLGA nanofibres: In vitro biomineralisation and osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2011, 3, 401–409. [Google Scholar] [CrossRef] [PubMed]
- De Aza, A.H.; Velasquez, P.; Alemany, M.I.; Pena, P.; De Aza, P.N. In situ bone-like apatite formation from a Bioeutectic® ceramic in SBF dynamic flow. J. Am. Ceram. Soc. 2007, 90, 1200–1207. [Google Scholar] [CrossRef]
- Martínez, I.M.; Velásquez, P.; Meseguer-Olmo, L.; De Aza, P.N. Production and study of in vitro behaviour of monolithic α-Tricalcium Phosphate based ceramics in the system Ca3 (PO4)2–Ca2SiO4. Ceram. Int. 2011, 37, 2527–2535. [Google Scholar] [CrossRef]
- De Val, J.E.M.S.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Ramirez-Fernandez, M.P.; Martinez, I.M.; Granero-Marin, J.M.; Negri, B.; Chiva-Garcia, F.; Martinez-Gonzalez, J.M.; De Aza, P.N. New block graft of α-TCP with silicon in critical size defects in rabbits: Chemical characterization, histological, histomorphometric and micro-CT study. Ceram. Int. 2012, 38, 1563–1570. [Google Scholar] [CrossRef]
- De Aza, P.N.; Luklinska, Z.; Anseau, M.; Guitian, F.; De Aza, S. Electron microscopy of interfaces in a wollastonite—Tricalcium phosphate bioeutectic®. J. Microsc. 1998, 189, 145–153. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- Rabadan-Ros, R.; Revilla-Nuin, B.; Mazón, P.; Aznar-Cervantes, S.; Ros-Tarraga, P.; De Aza, P.N.; Meseguer-Olmo, L. Impact of a Porous Si-Ca-P Monophasic Ceramic on Variation of Osteogenesis-Related Gene Expression of Adult Human Mesenchymal Stem Cells. Appl. Sci. 2018, 8, 46. [Google Scholar]
- Rabadan-Ros, R.; Aznar-Cervantes, S.; Mazón, P.; Ros-Tarraga, P.; De Aza, P.N.; Meseguer-Olmo, L. Nurse’s a-phase material enhance adhesion, growth and differentiation of human bone marrow-derived stromal mesenchymal stem cells. Materials 2017, 10, 347. [Google Scholar] [CrossRef]
- Serena, S.; Caballero, A.; De Aza, P.N.; Sainz, M.A. New evaluation of the in vitro response of silicocarnotite monophasic material. Ceram. Int. 2015, 41, 9411–9419. [Google Scholar] [CrossRef]
- Lugo, G.J.; Mazón, P.; De Aza, P.N. Phase transitions in single phase Si–Ca–P-based ceramic under thermal treatment. J. Eur. Ceram. Soc. 2015, 35, 3693–3700. [Google Scholar] [CrossRef]
- Lugo, G.J.; Mazón, P.; De Aza, P.N. Material processing of a new calcium silicophosphate ceramic. Ceram. Int. 2016, 42, 673–680. [Google Scholar] [CrossRef]
- Parrilla-Almansa, A.; González-Bermúdez, C.A.; Sánchez Sánchez, S.; Meseguer-Olmo, L.; Martínez-Caceres, C.M.; Martinez-Martinez, F.; Calvo-Guirado, J.L.; Piñero de Armas, J.J.; Aragoneses, J.M.; García-Carrillo, N.; et al. Intraosteal behavior of porous scaffolds: The mCT raw-data analysis as a tool for its better understanding. Symmetry 2019, 11, 532. [Google Scholar] [CrossRef]
- Ros-Tárraga, P.; Rabadan-Ros, R.; Murciano, A.; Meseguer-Olmo, L.; De Aza, P. Assessment of Effects of Si-Ca-P Biphasic Ceramic on the Osteogenic Differentiation of a Population of Multipotent Adult Human Stem Cells. Materials 2016, 9, 969. [Google Scholar] [CrossRef]
- Parrilla-Almansa, A.; García-Carrillo, N.; Ros-Tárraga, P.; Martínez, C.M.; Martinez-Martinez, F.; Meseguer-Olmo, L.; De Aza, P.N. Demineralized Bone Matrix Coating Si-Ca-P Ceramic Does Not Improve the Osseointegration of the Scaffold. Materials 2018, 11, 1580. [Google Scholar] [CrossRef]
- Rabadan-Ros, R.; Mazón, P.; Serena, S.; Sainz, M.A.; Meseguer-Olmo, L.; De Aza, P.N. In vitro behaviour of Nurse’s Ass-phase: A new calcium silicophosphate ceramic. J. Eur. Ceram. Soc. 2017, 37, 2943–2952. [Google Scholar] [CrossRef]
- Ros-Tárraga, P.; Mazón, P.; Rodríguez, M.; Meseguer-Olmo, L.; De Aza, P.N. Novel resorbable and osteoconductive calcium silicophosphate scaffold induced bone formation. Materials 2016, 9, 785. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Hildebrand, T.; Laib, A.; Müller, R.; Dequeker, J.; Rüegsegger, P. Direct three-dimensional morphometric analysis of human cancellous bone: Microstructural data from spine, femur, iliac crest and calcaneous. J. Bone Miner. Res. 1999, 14, 1167–1174. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Lugo, G.J.; Mazón, P.; Baudin, C.; De Aza, P.N. Nurse’ s A-Phase: Synthesis and Characterization in the Binary System Ca2SiO4–Ca3 (PO 4) 2. J. Am. Ceram. Soc. 2015, 98, 3042–3046. [Google Scholar] [CrossRef]
- Rubio, V.; Mazón, P.; De la Casa-Lillo, M.A.; De Aza, P.N. Preparation, characterization and in vitro behavior of a new eutectoid bioceramic. J. Eur. Ceram. Soc. 2015, 35, 317–328. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. BoneKey Rep. 2013, 2, 447. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Hing, K.A.; Annaz, B.; Saeed, S.; Revell, P.A.; Buckland, T. Microporosity enhances bioactivity of synthetic bone graft substitutes. J. Mater. Sci. 2005, 16, 467–475. [Google Scholar] [CrossRef]
- Sanzana, E.S.; Navarro, M.; Ginebra, M.P.; Planell, J.A.; Ojeda, A.C.; Montecinos, H.A. Role of porosity and pore architecture in the in vivo bone regeneration capacity of biodegradable glass scaffolds. J. Biomed. Mater. Res. Part A 2014, 102, 1767–1773. [Google Scholar] [CrossRef]
- Klawitter, J.J.; Hulbert, S.F. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. J. Biomed. Mater. Res. 1971, 5, 161–229. [Google Scholar] [CrossRef]
- Schepers, E.; Clercq, M.D.; Ducheyne, P.; Kempeneers, R. Bioactive glass particulate material as a filler for bone lesions. J. Oral Rehabilit. 1991, 18, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Eggli, P.S.; Müller, W.; Schenk, R.K. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin. Orthop. Relat. Res. 1988, 232, 127–138. [Google Scholar] [CrossRef]
- Uchida, A.; Nade, S.M.; McCartney, E.R.; Ching, W. The use of ceramics for bone replacement. A comparative study of three different porous ceramics. J. Bone Jt. Surg. Br. Vol. 1984, 66, 269–275. [Google Scholar] [CrossRef]
- Shimazaki, K.; Mooney, V. Comparative study of porous hydroxyapatite and tricalcium phosphate as bone substitute. J. Orthop. Res. 1985, 3, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Knabe, C.; Berger, G.; Gildenhaar, R.; Meyer, J.; Howlett, C.R.; Markovic, B.; Zreiqat, H. Effect of rapidly resorbable calcium phosphates and a calcium phosphate bone cement on the expression of bone-related genes and proteins in vitro. J. Biomed. Mater. Res. Part A 2004, 69, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kihara, H.; Shiota, M.; Yamashita, Y.; Kasugai, S. Biodegradation process of α-TCP particles and new bone formation in a rabbit cranial defect model. J. Biomed. Mater. Res. Part B 2006, 79, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Merten, H.A.; Wiltfang, J.; Hönig, J.F.; Funke, M.; Luhr, H.G. Intra-individual comparison of alpha-and beta-TCP ceramics in an animal experiment. Mund Kiefer Gesichtschir. 2000, 4, S509–S515. [Google Scholar] [CrossRef]
- Okuda, T.; Ioku, K.; Yonezawa, I.; Minagi, H.; Kawachi, G.; Gonda, Y.; Murayama, H.; Shibata, Y.; Minami, S.; Kamihira, S. The effect of the microstructure of β-tricalcium phosphate on the metabolism of subsequently formed bone tissue. Biomaterials 2007, 28, 2612–2621. [Google Scholar] [CrossRef]
- Velasquez, P.; Luklinska, Z.B.; Meseguer-Olmo, L.; Mate-Sanchez de Val, J.E.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Ramirez-Fernandez, M.P.; De Aza, P.N. αTCP ceramic doped with dicalcium silicate for bone regeneration applications prepared by powder metallurgy method: In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2013, 101, 1943–1954. [Google Scholar] [CrossRef]
- Burchardt, H. The biology of bone graft repair. Clin. Orthop. Relat. Res. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Cypher, T.J.; Grossman, J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- De Aza, P.N.; García-Bernal, D.; Cragnolini, F.; Velasquez, P.; Meseguer-Olmo, L. The effects of Ca2SiO4–Ca3(PO4)2 ceramics on adult human mesenchymal stem cell viability, adhesion, proliferation, differentiation and function. Mater. Sci. Eng. 2013, 33, 4009–4020. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ñíguez Sevilla, B.; Rabadan-Ros, R.; Alcaraz-Baños, M.; Martínez Díaz, F.; Mate Sánchez de Val, J.E.; López-Gónzalez, I.; Calvo-Guirado, J.L.; De Aza, P.N.; Meseguer-Olmo, L. RETRACTED: Nurse’s A-Phase–Silicocarnotite Ceramic–Bone Tissue Interaction in a Rabbit Tibia Defect Model. J. Clin. Med. 2019, 8, 1714. https://doi.org/10.3390/jcm8101714
Ñíguez Sevilla B, Rabadan-Ros R, Alcaraz-Baños M, Martínez Díaz F, Mate Sánchez de Val JE, López-Gónzalez I, Calvo-Guirado JL, De Aza PN, Meseguer-Olmo L. RETRACTED: Nurse’s A-Phase–Silicocarnotite Ceramic–Bone Tissue Interaction in a Rabbit Tibia Defect Model. Journal of Clinical Medicine. 2019; 8(10):1714. https://doi.org/10.3390/jcm8101714
Chicago/Turabian StyleÑíguez Sevilla, Belén, Ruben Rabadan-Ros, Miguel Alcaraz-Baños, Francisco Martínez Díaz, José E. Mate Sánchez de Val, Iván López-Gónzalez, Jose Luis Calvo-Guirado, Piedad N. De Aza, and Luis Meseguer-Olmo. 2019. "RETRACTED: Nurse’s A-Phase–Silicocarnotite Ceramic–Bone Tissue Interaction in a Rabbit Tibia Defect Model" Journal of Clinical Medicine 8, no. 10: 1714. https://doi.org/10.3390/jcm8101714
APA StyleÑíguez Sevilla, B., Rabadan-Ros, R., Alcaraz-Baños, M., Martínez Díaz, F., Mate Sánchez de Val, J. E., López-Gónzalez, I., Calvo-Guirado, J. L., De Aza, P. N., & Meseguer-Olmo, L. (2019). RETRACTED: Nurse’s A-Phase–Silicocarnotite Ceramic–Bone Tissue Interaction in a Rabbit Tibia Defect Model. Journal of Clinical Medicine, 8(10), 1714. https://doi.org/10.3390/jcm8101714







