Antioxidant Therapies for Neuroprotection—A Review
Abstract
1. Introduction
2. Oxidative Stress Mechanisms
3. Antioxidant Defense Mechanism
4. Antioxidants for Neuroprotection
4.1. Vitamins A, E, and C
4.2. Phenolic Compounds
4.3. Carotenoids
5. Conclusions and Future Perspectives
Author Contributions
Conflicts of Interest
References
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative stress, protein modification and alzheimer disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Varzakova, D.P.; Kazakov, Y.E.; Vidrevich, M.B. Noninvasive electrochemical antioxidant activity estimation: Saliva analysis. Biointerface Res. Appl. Chem. 2018, 8, 3381–3387. [Google Scholar]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 9. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxidative Med. Cell. Longev. 2016, 2016, 15. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 13. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxidative Med. Cell. Longev. 2017, 2017, 11. [Google Scholar] [CrossRef]
- Bjørklund, G.; Chirumbolo, S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (et)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, C.; Tsao, R. Nomenclature and general classification of antioxidant activity/capacity assays. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 1–19. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef]
- Griffiths, H.R. Is the generation of neo-antigenic determinants by free radicals central to the development of autoimmune rheumatoid disease? Autoimmun. Rev. 2008, 7, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Wang, R.; Geller, D.A.; Wink, D.A. Nitric oxide and hepatocellular cancer. Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Seabra, A.B.; Duran, N. Nitric oxide donors for prostate and bladder cancers: Current state and challenges. Eur. J. Pharmacol. 2018, 826, 158–168. [Google Scholar] [CrossRef]
- Paskas, S.; Mazzon, E.; Basile, M.S.; Cavalli, E.; Al-Abed, Y.; He, M.; Rakocevic, S.; Nicoletti, F.; Mijatovic, S.; Maksimovic-Ivanic, D. Lopinavir-no, a nitric oxide-releasing hiv protease inhibitor, suppresses the growth of melanoma cells in vitro and in vivo. Investig. New Drugs 2019, 37, 1014–1028. [Google Scholar] [CrossRef]
- Basile, M.S.; Mazzon, E.; Krajnovic, T.; Draca, D.; Cavalli, E.; Al-Abed, Y.; Bramanti, P.; Nicoletti, F.; Mijatovic, S.; Maksimovic-Ivanic, D. Anticancer and differentiation properties of the nitric oxide derivative of lopinavir in human glioblastoma cells. Molecules 2018, 23, 2463. [Google Scholar] [CrossRef]
- Maksimovic-Ivanic, D.; Mojic, M.; Bulatovic, M.; Radojkovic, M.; Kuzmanovic, M.; Ristic, S.; Stosic-Grujicic, S.; Miljkovic, D.; Cavalli, E.; Libra, M.; et al. The no-modified hiv protease inhibitor as a valuable drug for hematological malignancies: Role of p70s6k. Leuk. Res. 2015, 39, 1088–1095. [Google Scholar] [CrossRef]
- Danilov, A.I.; Jagodic, M.; Wiklund, N.P.; Olsson, T.; Brundin, L. Effects of long term nos inhibition on disease and the immune system in mog induced eae. Nitric Oxide:Biol. Chem. 2005, 13, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Mangano, K.; Quattrocchi, C.; Cavalli, E.; Mammana, S.; Lombardo, G.A.; Pennisi, V.; Zocca, M.B.; He, M.; Al-Abed, Y. Effects of no-hybridization on the immunomodulatory properties of the hiv protease inhibitors lopinavir and ritonavir. Basic Clin. Pharmacol. Toxicol. 2015, 117, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Obulesu, M. Chapter 3-antioxidants in alzheimer’s therapy. In Alzheimer’s Disease Theranostics; Obulesu, M., Ed.; Academic Press: Cambridge/Waltham, MA, USA, 2019; pp. 13–18. [Google Scholar]
- Panahi, Y.; Rajaee, S.M.; Johnston, T.P.; Sahebkar, A. Neuroprotective effects of antioxidants in the management of neurodegenerative disorders: A literature review. J. Cell. Biochem. 2019, 120, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef]
- Lalkovičová, M.; Danielisová, V. Neuroprotection and antioxidants. Neural Regen Res 2016, 11, 865–874. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food antioxidants: Chemical insights at the molecular level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in parkinson’s disease and alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for drug delivery to the central nervous system. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neuronanomedicine: An up-to-date overview. Pharmaceutics 2019, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Cory-Slechta, D.A. Chapter nine-enduring behavioral and brain impacts of prenatal stress and childhood adversity and their potential multigenerational consequences. In Advances in Neurotoxicology; Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge/Waltham, MA, USA, 2018; Volume 2, pp. 265–300. [Google Scholar]
- Sies, H. Chapter 13-oxidative stress: Eustress and distress in redox homeostasis. In Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: Cambridge/Waltham, MA, USA, 2019; pp. 153–163. [Google Scholar]
- Adeyemi, O.S.; Olajide, I.O.; Adeyanju, A.A.; Awakan, O.J.; Otohinoyi, D.A. Modulation of rat plasma kynurenine level by platinum nanoparticles and likely association with oxidative stress. Biointerface Res. Appl. Chem. 2018, 8, 3364–3367. [Google Scholar]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H. Molecular mechanisms of ros production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. Ros and ros-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 18. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016, 100, 81–85. [Google Scholar] [CrossRef]
- Milatovic, D.; Zaja-Milatovic, S.; Gupta, R.C. Chapter 29-oxidative stress and excitotoxicity: Antioxidants from nutraceuticals. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 401–413. [Google Scholar]
- Young, R.; Francis, S. Chapter 23 - form and function of the animal cell. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 459–475. [Google Scholar]
- Vašková, J.; Vaško, L. Introductory chapter: Unregulated mitochondrial production of reactive oxygen species in testing the biological activity of compounds. In Medicinal Chemistry; IntechOpen: London, UK, 2018. [Google Scholar]
- Munro, D.; Treberg, J.R. A radical shift in perspective: Mitochondria as regulators of reactive oxygen species. J. Exp. Biol. 2017, 220, 1170–1180. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ros generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ros and rns sources in physiological and pathological conditions. Oxidative Med. Cell. Longev. 2016, 2016, 44. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, T.A.F.; Navarro, B.C.H.; Pérez, J.A.M. Endogenous antioxidants: A review of their role in oxidative stress. In A Master Regulator of Oxidative Stress-the Transcription Factor nrf2; IntechOpen: London, UK, 2016. [Google Scholar]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Suski, J.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ros formation. In Mitochondrial Bioenergetics: Methods and Protocols; Palmeira, C.M., Moreno, A.J., Eds.; Springer New York: New York, NY, USA, 2018; pp. 357–381. [Google Scholar]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity:Supplementary issue: Brain plasticity and repair. J. Exp. Neurosci. 2016, 10s1, JEN.S39887. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Geuss, E.; Kleikers, P.W.M.; Mencl, S.; Herrmann, A.M.; Buendia, I.; Egea, J.; Meuth, S.G.; Lopez, M.G.; Kleinschnitz, C.; et al. Nox4-dependent neuronal autotoxicity and bbb breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. USA 2017, 114, 12315–12320. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Pula, G. The role of nadph oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef]
- Dao, V.T.; Altenhöfer, S.; Elbatreek, M.H.; Casas, A.I.; Lijnen, P.; Meens, M.J.; Knaus, U.; Schmidt, H.H.H.W. Isoform-specific nadph oxidase inhibition for pharmacological target validation. bioRxiv 2018, 382226. [Google Scholar]
- Park, H.R.; Oh, R.; Wagner, P.; Panganiban, R.; Lu, Q. Chapter two-new insights into cellular stress responses to environmental metal toxicants. In International Review of Cell and Molecular Biology; Galluzzi, L., Ed.; Academic Press: Cambridge/Waltham, MA, USA, 2017; Volume 331, pp. 55–82. [Google Scholar]
- de Araújo, R.F.; Martins, D.B.G.; Borba, M.A.C. Oxidative stress and disease. In A Master Regulator of Oxidative Stress-the Transcription Factor nrf2; IntechOpen: London, UK, 2016. [Google Scholar]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef]
- Qin, Y. 9 - health benefits of bioactive seaweed substances. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge/Waltham, MA, USA, 2018; pp. 179–200. [Google Scholar]
- Verma, H.; Silakari, O. Chapter 10-benzoxazolinone: A scaffold with diverse pharmacological significance. In Key Heterocycle Cores for Designing Multitargeting Molecules; Silakari, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 343–367. [Google Scholar]
- Adwas, A.; Elsayed, A.; Azab, A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar]
- Girija, A.R. 6 - peptide nutraceuticals. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Koutsopoulos, S., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2018; pp. 157–181. [Google Scholar]
- Perchyonok, T.; Reher, V.; Grobler, S. Chapter 9-bioactive-functionalized interpenetrating network hydrogel (biof-ipn). In Engineering of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 287–306. [Google Scholar]
- Horton, W.; Török, M. Chapter 3.27-natural and nature-inspired synthetic small molecule antioxidants in the context of green chemistry. In Green Chemistry; Török, B., Dransfield, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 963–979. [Google Scholar]
- Blanco, A.; Blanco, G. Chapter 10-antioxidants. In Medical Biochemistry; Blanco, A., Blanco, G., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 205–214. [Google Scholar]
- Soleimani, M.; Dehabadi, L.; Wilson, L.D.; Tabil, L.G. Antioxidants classification and applications in lubricants. In Lubrication-Tribology, Lubricants and Additives; Johnson, D.W., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Hermund, D.B. 10-antioxidant properties of seaweed-derived substances. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Boston, MA, USA, 2018; pp. 201–221. [Google Scholar]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Nabi, S. Endogenous antioxidants. In Toxic Effects of Mercury; Springer India: New Delhi, India, 2014; pp. 117–120. [Google Scholar]
- Undeland, I. Chapter 11-oxidative stability of seafood. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Hu, M., Jacobsen, C., Eds.; AOCS Press: Urbana, IL, USA, 2016; pp. 391–460. [Google Scholar]
- Docampo, R.; Moreno, S.N.J. 17-biochemistry of trypanosoma cruzi. In American Trypanosomiasis Chagas Disease, 2nd ed.; Telleria, J., Tibayrenc, M., Eds.; Elsevier: London, UK, 2017; pp. 371–400. [Google Scholar]
- Lumb, A.B. Chapter 24-oxygen toxicity and hyperoxia. In Nunn’s Applied Respiratory Physiology, 8th ed.; Lumb, A.B., Ed.; Elsevier: London, UK, 2017; pp. 341–356.e342. [Google Scholar]
- Asakura, H.; Kitahora, T. Chapter 23-antioxidants and polyphenols in inflammatory bowel disease: Ulcerative colitis and crohn disease. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 279–292. [Google Scholar]
- Homma, T.; Fujii, J. Chapter 5-oxidative stress and dysfunction of the intracellular proteolytic machinery: A pathological hallmark of nonalcoholic fatty liver disease. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 59–70. [Google Scholar]
- Liu, X.; Kokare, C. Chapter 11-microbial enzymes of use in industry. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 267–298. [Google Scholar]
- Velayati, A.A.; Farnia, P.; Saif, S. Chapter 2-identification of nontuberculous mycobacterium: Conventional versus rapid molecular tests. In Nontuberculous Mycobacteria (ntm); Velayati, A.A., Farnia, P., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 11–59. [Google Scholar]
- Thimraj, T.A.; George, L.; Asrafuzzaman, S.; Upadhyay, S.; Ganguly, K. Chapter 7-oxidative signaling in chronic obstructive airway diseases. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 79–98. [Google Scholar]
- Mulgund, A.; Doshi, S.; Agarwal, A. Chapter 25-the role of oxidative stress in endometriosis. In Handbook of Fertility; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 273–281. [Google Scholar]
- Mischley, L.K. Chapter forty-nutrition and nonmotor symptoms of parkinson’s disease. In International Review of Neurobiology; Chaudhuri, K.R., Titova, N., Eds.; Academic Press: San Diego, CA, USA, 2017; Volume 134, pp. 1143–1161. [Google Scholar]
- Gupta, P.K. Chapter 8-biotransformation. In Fundamentals of Toxicology; Gupta, P.K., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 73–85. [Google Scholar]
- Ağardan, N.B.M.; Torchilin, V.P. Chapter 1-engineering of stimuli-sensitive nanopreparations to overcome physiological barriers and cancer multidrug resistance. In Engineering of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 1–28. [Google Scholar]
- Drisko, J.A. Chapter 107-chelation therapy. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: London, UK, 2018; pp. 1004–1015.e1003. [Google Scholar]
- Abel, R. Chapter 85-age-related macular degeneration. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: London, UK, 2018; pp. 838–846.e831. [Google Scholar]
- Simmons, A.D. Chapter 15-parkinson’s disease. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: London, UK, 2018; pp. 143–151.e143. [Google Scholar]
- Paladino, S.; Conte, A.; Caggiano, R.; Pierantoni, G.M.; Faraonio, R. Nrf2 pathway in age-related neurological disorders: Insights into micrornas. Cell. Physiol. Biochem. 2018, 47, 1951–1976. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Hardingham, G.E. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic. Biol. Med. 2016, 100, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ghosh, J.; Sil, P. Drug metabolism and oxidative stress: Cellular mechanism and new therapeutic insights. Biochem. Anal. Biochem. 2016, 5. [Google Scholar] [CrossRef]
- Adamczyk, B.; Adamczyk-Sowa, M. New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxidative Med. Cell. Longev. 2016, 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Benfeito, S.; Fonseca, A.; Oliveira, C.; Garrido, J.; Garrido, E.M.; Borges, F. 15-photodamage and photoprotection: Toward safety and sustainability through nanotechnology solutions. In Food Preservation; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 527–565. [Google Scholar]
- Combs, G.F.; McClung, J.P. Chapter 6-vitamin a. In The Vitamins, 5th ed.; Combs, G.F., McClung, J.P., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 109–159. [Google Scholar]
- van Lith, R.; Ameer, G.A. Chapter ten-antioxidant polymers as biomaterial. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 251–296. [Google Scholar]
- Power, M.L.; Koutsos, L. Chapter 4-marmoset nutrition and dietary husbandry. In The Common Marmoset in Captivity and Biomedical Research; Marini, R., Wachtman, L., Tardif, S., Mansfield, K., Fox, J., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 63–76. [Google Scholar]
- Parian, A.M.; Mullin, G.E.; Langhorst, J.; Brown, A.C. Chapter 50-inflammatory bowel disease. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: London, UK, 2018; pp. 501–516.e508. [Google Scholar]
- Alam, P.; Siddiqi, M.K.; Malik, S.; Chaturvedi, S.K.; Uddin, M.; Khan, R.H. Elucidating the inhibitory potential of vitamin a against fibrillation and amyloid associated cytotoxicity. Int. J. Biol. Macromol. 2019, 129, 333–338. [Google Scholar] [CrossRef] [PubMed]
- González-Fuentes, J.; Selva, J.; Moya, C.; Castro-Vázquez, L.; Lozano, M.V.; Marcos, P.; Plaza-Oliver, M.; Rodríguez-Robledo, V.; Santander-Ortega, M.J.; Villaseca-González, N.; et al. Neuroprotective natural molecules, from food to brain. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Role of vitamin e in the treatment of alzheimer’s disease: Evidence from animal models. Int. J. Mol. Sci. 2017, 18, 2504. [Google Scholar] [CrossRef]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin e: Emerging aspects and new directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The effectiveness of vitamin e treatment in alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, L.; Wang, X. Chapter 24-pathophysiology of ischemia-reperfusion injury and hemorrhagic transformation in the brain. In Primer on Cerebrovascular Diseases, 2nd ed.; Caplan, L.R., Biller, J., Leary, M.C., Lo, E.H., Thomas, A.J., Yenari, M., Zhang, J.H., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 121–124. [Google Scholar]
- Mohamed, W.; Sayeed, S.; Saxena, A.; Oothuman, P. Oxidative stress status and neuroprotection of tocotrienols in chronic cerebral hypoperfusion-induced neurodegeneration rat animal model. Int. J. Nutr. Pharmacol. Neurol. Dis. 2018, 8, 47–52. [Google Scholar]
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of antioxidant supplement use and dementia in the prevention of alzheimer’s disease by vitamin e and selenium trial (preadvise). JAMA Neurol. 2017, 74, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Man Anh, H.; Linh, D.M.; My Dung, V.; Thi Phuong Thao, D. Evaluating dose- and time-dependent effects of vitamin c treatment on a parkinson’s disease fly model. Parkinson’s Dis. 2019, 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Siepelmeyer, A.; Micka, A.; Simm, A.; Bernhardt, J. Chapter 8-nutritional biomarkers of aging. In Molecular Basis of Nutrition and Aging; Malavolta, M., Mocchegiani, E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 109–120. [Google Scholar]
- Pathak, J.; Ahmed, H.; Singh, P.R.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Chapter 7-mechanisms of photoprotection in cyanobacteria. In Cyanobacteria; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 145–171. [Google Scholar]
- Gagné, L.; Maizes, V. Chapter 36-osteoporosis. In Integrative Medicine, 4nd ed.; Rakel, D., Ed.; Elsevier: London, UK, 2018; pp. 370–381.e375. [Google Scholar]
- Figurski, A.C. Chapter 44-cholelithiasis. In Integrative medicine, 4nd ed.; Rakel, D., Ed.; Elsevier: London, UK, 2018; pp. 450–456.e452. [Google Scholar]
- Gromova, O.A.; Torshin, I.Y.; Pronin, A.V.; Kilchevsky, M.A. Synergistic application of zinc and vitamin c to support memory and attention and to decrease the risk of developing nervous system diseases. Neurosci. Behav. Physiol. 2019, 49, 357–364. [Google Scholar] [CrossRef]
- Bonnet, U. The sour side of vitamin c might mediate neuroprotective, anticonvulsive and antidepressant-like effects. Med. Hypotheses 2019, 131, 109320. [Google Scholar] [CrossRef]
- Basambombo, L.L.; Carmichael, P.-H.; Côté, S.; Laurin, D. Use of vitamin e and c supplements for the prevention of cognitive decline. Ann. Pharmacother. 2017, 51, 118–124. [Google Scholar] [CrossRef]
- Sangeetha, A.; Kumaresan, M. Chronic administration of vitamin c increases brain derived neurotrophic factor in chronic stress induced rats. Biomed. (India) 2018, 38, 1–7. [Google Scholar]
- Zhang, X.-Y.; Xu, Z.-P.; Wang, W.; Cao, J.-B.; Fu, Q.; Zhao, W.-X.; Li, Y.; Huo, X.-L.; Zhang, L.-M.; Li, Y.-F.; et al. Vitamin c alleviates lps-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2018, 65, 438–447. [Google Scholar] [CrossRef]
- Khordad, E.; Alipour, F.; Beheshti, F.; Hosseini, M.; Rajabzadeh, A.A.; Asiaei, F.; Seghatoleslam, M. Vitamin c prevents hypothyroidism associated neuronal damage in the hippocampus of neonatal and juvenile rats: A stereological study. J. Chem. Neuroanat. 2018, 93, 48–56. [Google Scholar] [CrossRef]
- Huang, Y.-N.; Yang, L.-Y.; Wang, J.-Y.; Lai, C.-C.; Chiu, C.-T.; Wang, J.-Y. L-ascorbate protects against methamphetamine-induced neurotoxicity of cortical cells via inhibiting oxidative stress, autophagy, and apoptosis. Mol. Neurobiol. 2017, 54, 125–136. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2-phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 33–50. [Google Scholar]
- do Nascimento, K.S.; Gasparotto Sattler, J.A.; Lauer Macedo, L.F.; Serna González, C.V.; Pereira de Melo, I.L.; da Silva Araújo, E.; Granato, D.; Sattler, A.; de Almeida-Muradian, L.B. Phenolic compounds, antioxidant capacity and physicochemical properties of brazilian apis mellifera honeys. LWT 2018, 91, 85–94. [Google Scholar] [CrossRef]
- Souidi, K.; Lkrik, A.; Joly, N.; Martin, P. Effect of polyphenols extracted from (olea europaea. L) solid residues and leaves on the oxidative stability of a commercial olive oil. Biointerface Res. Appl. Chem. 2017, 7, 1963–1968. [Google Scholar]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Chapter 12-phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 253–271. [Google Scholar]
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge, Y.-Y.; Gunaratne, A.; Ge, J.; Corke, H. 9-bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains; Feng, H., Nemzer, B., DeVries, J.W., Eds.; AACC International Press: Saint Paul, MN, USA, 2019; pp. 191–246. [Google Scholar]
- San Miguel-Chávez, R. Phenolic antioxidant capacity: A review of the state of the art. Phenolic Compd. -Biol. Act. 2017. [Google Scholar]
- Saranraj, P.; Behera, S.S.; Ray, R.C. Chapter 7-traditional foods from tropical root and tuber crops: Innovations and challenges. In Innovations in Traditional Foods; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 159–191. [Google Scholar]
- Abbasi, A.M.; Shah, M.H. Assessment of phenolic contents, essential/toxic metals and antioxidant capacity of fruits of viburnum foetens decne. Biointerface Res. Appl. Chem. 2018, 8, 3178–3186. [Google Scholar]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic compounds: Functional properties, impact of processing and bioavailability. Phenolic Compd. —Biol. Act. 2017, 236. [Google Scholar]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the tlr4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Jakaria, M.; Kim, I.-S.; Kim, J.; Haque, M.E.; Choi, D.-K. Regulation of toll-like receptor (tlr) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: Focus on tlr4 signaling. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Oikonomou, E.; Papamikroulis, A.; Tousoulis, D. Chapter 3.2-anti-oxidant treatment. In Coronary Artery Disease; Tousoulis, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 273–300. [Google Scholar]
- Wang, T.-y.; Li, Q.; Bi, K.-s. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Jan, S.; Abbas, N. Chapter 4 - chemistry of himalayan phytochemicals. In Himalayan Phytochemicals; Jan, S., Abbas, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–166. [Google Scholar]
- Šegota, S.; Crnolatac, I.; Čadež, V.; Jembrek, M.J.; Sikirić, M.D. Neuroprotection and neuronal recovery under the oxidative stress achieved by enhanced lipid membrane interaction with flavonoids. In Proceedings of the Third Regional Roundtable: Refractory, Process Industry, Nanotechnologies and Nanomedicine ROSOV PINN 2017, Belgrade, Serbia, 1–2 June 2017. [Google Scholar]
- Frandsen, J.R.; Narayanasamy, P. Neuroprotection through flavonoid: Enhancement of the glyoxalase pathway. Redox Biol. 2018, 14, 465–473. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogacnik, L. Food, polyphenols and neuroprotection. Neural Regen. Res. 2017, 12, 582–583. [Google Scholar]
- Putteeraj, M.; Lim, W.L.; Teoh, S.L.; Yahaya, M.F. Flavonoids and its neuroprotective effects on brain ischemia and neurodegenerative diseases. Curr. Drug Targets 2018, 19, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Neuroprotective effects of flavonoids in epilepsy. In Neuroprotective Natural Products; Wiley: Hoboken, NJ, USA, 2017; pp. 279–291. [Google Scholar]
- Ahmed, S.I.; Hayat, M.Q.; Zahid, S.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R. Isolation and identification of flavonoids from anticancer and neuroprotective extracts of trigonella foenum graecum. Trop. J. Pharm. Res. 2017, 16, 1391–1398. [Google Scholar] [CrossRef]
- Ma, J.; Gao, S.-S.; Yang, H.-J.; Wang, M.; Cheng, B.-F.; Feng, Z.-W.; Wang, L. Neuroprotective effects of proanthocyanidins, natural flavonoids derived from plants, on rotenone-induced oxidative stress and apoptotic cell death in human neuroblastoma sh-sy5y cells. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Shi, C.; Cao, J.; Sun, Y.; Zhao, X.; Guo, Y.; Wang, C.; Lei, H.; Jiang, H.; Ablat, N.; et al. Neuroprotective effects of a standardized flavonoid extract of safflower against neurotoxin-induced cellular and animal models of parkinson’s disease. Sci. Rep. 2016, 6, 22135. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Activation of nrf2 pathway contributes to neuroprotection by the dietary flavonoid tiliroside. Mol. Neurobiol. 2018, 55, 8103–8123. [Google Scholar] [CrossRef] [PubMed]
- Koçancı, F.G.; Aslim, B. Neuroprotective effects of rutin and quercetin flavonoids in glaucium corniculatum methanol and water extracts. Int. J. Second. Metab. 2017, 4, 85–93. [Google Scholar] [CrossRef]
- Clarkson, A.N.; Boothman-Burrell, L.; Dósa, Z.; Nagaraja, R.Y.; Jin, L.; Parker, K.; van Nieuwenhuijzen, P.S.; Neumann, S.; Gowing, E.K.; Gavande, N.; et al. The flavonoid, 2′-methoxy-6-methylflavone, affords neuroprotection following focal cerebral ischaemia. J. Cereb. Blood Flow Metab. 2019, 39, 1266–1282. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Qiao, J.; Guo, Y.; Miao, M. Neuroprotective effect of total flavonoids from ilex pubescens against focal cerebral ischemia/reperfusion injury in rats. Mol. Med. Rep. 2017, 16, 7439–7449. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The neuroprotective effects of phenolic acids: Molecular mechanism of action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma sh-sy5y cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Yang, L.; Guo, R.-Z.; Li, J. Phenolic acid derivatives with neuroprotective effect from the aqueous extract of clerodendranthus spicatus. J. Asian Nat. Prod. Res. 2017, 19, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Biernasiuk, A.; Wozniak, M.; Bogucka-Kocka, A. Determination of free and bounded phenolic acids in the rhizomes and herb of Sanguisorba officinalis L. Curr. Issues Pharm. Med Sci. 2015, 28, 254–256. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Rabiee, N.; Zabihnejad, S.; Roghani, M. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of parkinson’s disease: Possible involvement of erβ/nrf2/ho-1 signaling. Brain Res. 2017, 1662, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández, A.; Rendeiro, C.; Spencer, J.P.E.; del Coso, D.G.; de Llano, M.D.G.; Bartolomé, B.; Moreno-Arribas, M.V. Neuroprotective effects of selected microbial-derived phenolic metabolites and aroma compounds from wine in human sh-sy5y neuroblastoma cells and their putative mechanisms of action. Front. Nutr. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R.; Ferreira, G.C.; Schuck, P.F. Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in sh-sy5y cells: Role for pi3k/akt/nrf2 pathway. Toxicol. Vitr. 2016, 32, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Fonteles, A.A.; de Souza, C.M.; de Sousa Neves, J.C.; Menezes, A.P.F.; Santos do Carmo, M.R.; Fernandes, F.D.P.; de Araújo, P.R.; de Andrade, G.M. Rosmarinic acid prevents against memory deficits in ischemic mice. Behav. Brain Res. 2016, 297, 91–103. [Google Scholar] [CrossRef]
- Linnewiel-Hermoni, K.; Paran, E.; Wolak, T. Chapter 34-carotenoid supplements and consumption: Implications for healthy aging. In Molecular Basis of Nutrition and Aging; Malavolta, M., Mocchegiani, E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 473–489. [Google Scholar]
- Shidfar, F.; Arjomand, G.-N. Chapter 24-glucose intake and utilization in pre-diabetes and diabetes: Tomato and diabetes. In Glucose Intake and Utilization in Pre-Diabetes and Diabetes; Watson, R.R., Dokken, B.B., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 301–313. [Google Scholar]
- Schieber, A.; Weber, F. 5-carotenoids. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2016; pp. 101–123. [Google Scholar]
- Nagarajan, J.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.M.; Krishnamurthy, N.P. Chapter 8-carotenoids. In Nutraceutical and Functional Food Components; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 259–296. [Google Scholar]
- Merhan, O. Biochemistry and antioxidant properties of carotenoids. Carotenoids 2017, 5, 51. [Google Scholar]
- Young, A.J.; Lowe, G.L. Carotenoids-antioxidant properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Fabra, M.J.; Castro-Mayorga, J.L.; Sánchez, G.; Martínez-Sanz, M.; López-Rubio, A. Chapter 2 - nanostructuring biopolymers for improved food quality and safety. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 33–64. [Google Scholar]
- Uthayakumaran, S.; Wrigley, C. Chapter 5-wheat: Grain-quality characteristics and management of quality requirements. In Cereal Grains, 2nd ed.; Wrigley, C., Batey, I., Miskelly, D., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2017; pp. 91–134. [Google Scholar]
- Sharif, M.K.; Shah, F.-u.-H.; Butt, M.S.; Sharif, H.R. 15-role of nanotechnology in enhancing bioavailability and delivery of dietary factors. In Nutrient Delivery; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 587–618. [Google Scholar]
- Hua, Y.; Xu, N.; Ma, T.; Liu, Y.; Xu, H.; Lu, Y. Anti-inflammatory effect of lycopene on experimental spinal cord ischemia injury via cyclooxygenase-2 suppression. NeuroImmunoModulation 2019, 26, 84–92. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, P.; Gao, X.; Shao, B.; Zhao, S.; Li, Y. Lycopene attenuates aluminum-induced hippocampal lesions by inhibiting oxidative stress-mediated inflammation and apoptosis in the rat. J. Inorg. Biochem. 2019, 193, 143–151. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, Y.S.; Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B.; Hong, J.T. Astaxanthin ameliorates lipopolysaccharide-induced neuroinflammation, oxidative stress and memory dysfunction through inactivation of the signal transducer and activator of transcription 3 pathway. Mar. Drugs 2019, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.O.; Panda, B.P.; Parvez, S.; Kaundal, M.; Hussain, S.; Akhtar, M.; Najmi, A.K. Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by aβ peptides in animal model of alzheimer’s disease. Biomed. Pharmacother. 2019, 110, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, X.; Feng, J.; Xie, T.; Si, P.; Wang, W. Neuroprotective effect of astaxanthin on newborn rats exposed to prenatal maternal seizures. Brain Res. Bull. 2019, 148, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (aβ 1-42 ) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, X.; Hu, W.; Li, Z.; Kong, F.; Chen, X.; Wang, D. Investigation of the neuroprotective effects of crocin via antioxidant activities in ht22 cells and in mice with alzheimer’s disease. Int. J. Mol. Med. 2019, 43, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Haeri, P.; Mohammadipour, A.; Heidari, Z.; Ebrahimzadeh-bideskan, A. Neuroprotective effect of crocin on substantia nigra in mptp-induced parkinson’s disease model of mice. Anat. Sci. Int. 2019, 94, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; Hemalatha, P.; Yetish, S.; Muralidhara, M.; Rajini, P.S. Prophylactic neuroprotective propensity of crocin, a carotenoid against rotenone induced neurotoxicity in mice: Behavioural and biochemical evidence. Metab. Brain Dis. 2019. [Google Scholar] [CrossRef]
- Zhou, L.; Ouyang, L.; Lin, S.; Chen, S.; Liu, Y.; Zhou, W.; Wang, X. Protective role of β-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int. Immunopharmacol. 2018, 61, 92–99. [Google Scholar] [CrossRef]
- Winyard, P.G.; Ryan, B.; Eggleton, P.; Nissim, A.; Taylor, E.; Lo Faro, M.L.; Burkholz, T.; Szabo-Taylor, K.E.; Fox, B.; Viner, N.; et al. Measurement and meaning of markers of reactive species of oxygen, nitrogen and sulfur in healthy human subjects and patients with inflammatory joint disease. Biochem. Soc. Trans. 2011, 39, 1226–1232. [Google Scholar] [CrossRef]
- Lazarevic, M.; Mazzon, E.; Momcilovic, M.; Basile, M.S.; Colletti, G.; Petralia, M.C.; Bramanti, P.; Nicoletti, F.; Miljkovic, D. The h(2)s donor gyy4137 stimulates reactive oxygen species generation in bv2 cells while suppressing the secretion of tnf and nitric oxide. Molecules 2018, 23, 2966. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Ding, Y.-P.; Wang, Z.; Kong, Y.; Gao, R.; Chen, G. Hydrogen sulfide therapy in brain diseases: From bench to bedside. Med. Gas. Res. 2017, 7, 113–119. [Google Scholar] [PubMed]
| Class of Compounds | Compound Name | Chemical Structure | ||
|---|---|---|---|---|
| Vitamins | Vitamin A | retinol | 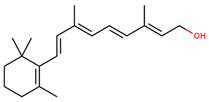 | |
| retinal | 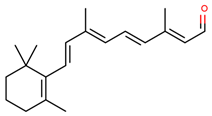 | |||
| retinoic acid | 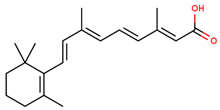 | |||
| Vitamin E (α-tocopherol) | 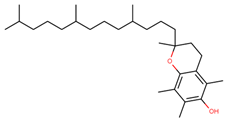 | |||
| Vitamin C (ascorbic acid) | 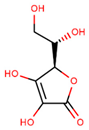 | |||
| Phenolic compounds | Flavonoids | amurensin | 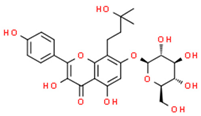 | |
| cosmosiin | 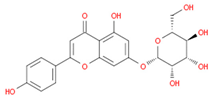 | |||
| tiliroside | 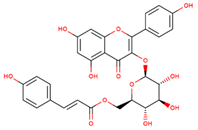 | |||
| rutin | 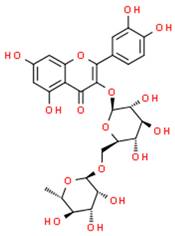 | |||
| quercetin | 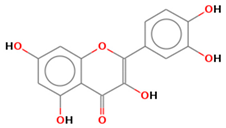 | |||
| 2′-methoxy-6-methylflavone | 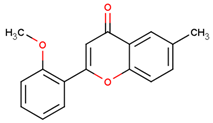 | |||
| Non-flavonoids | 3,4-dihydroxyphenylpropionic acid | 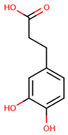 | ||
| 3,4-dihydroxyphenylacetic acid | 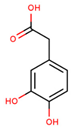 | |||
| gallic acid | 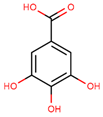 | |||
| ellagic acid | 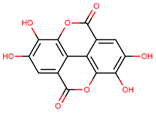 | |||
| 3-hydroxyphenylacetic acid | 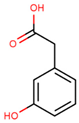 | |||
| salicylic β-d-O-glucuronide | 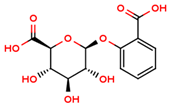 | |||
| carnosic acid | 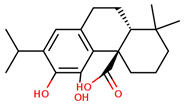 | |||
| rosmarinic acid | 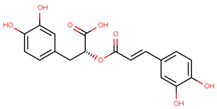 | |||
| Carotenoids | α-carotene | 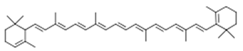 | ||
| β-carotene | 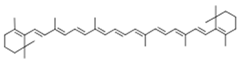 | |||
| lycopene | 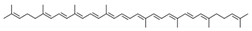 | |||
| lutein | 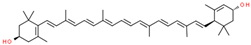 | |||
| cryptoxanthin | 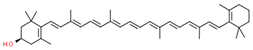 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant Therapies for Neuroprotection—A Review. J. Clin. Med. 2019, 8, 1659. https://doi.org/10.3390/jcm8101659
Teleanu RI, Chircov C, Grumezescu AM, Volceanov A, Teleanu DM. Antioxidant Therapies for Neuroprotection—A Review. Journal of Clinical Medicine. 2019; 8(10):1659. https://doi.org/10.3390/jcm8101659
Chicago/Turabian StyleTeleanu, Raluca Ioana, Cristina Chircov, Alexandru Mihai Grumezescu, Adrian Volceanov, and Daniel Mihai Teleanu. 2019. "Antioxidant Therapies for Neuroprotection—A Review" Journal of Clinical Medicine 8, no. 10: 1659. https://doi.org/10.3390/jcm8101659
APA StyleTeleanu, R. I., Chircov, C., Grumezescu, A. M., Volceanov, A., & Teleanu, D. M. (2019). Antioxidant Therapies for Neuroprotection—A Review. Journal of Clinical Medicine, 8(10), 1659. https://doi.org/10.3390/jcm8101659








