Comparison of Placental Three-Dimensional Power Doppler Vascular Indices and Placental Volume in Pregnancies with Small for Gestational Age Neonates
Abstract
1. Introduction
2. Methods
2.1. Study Population
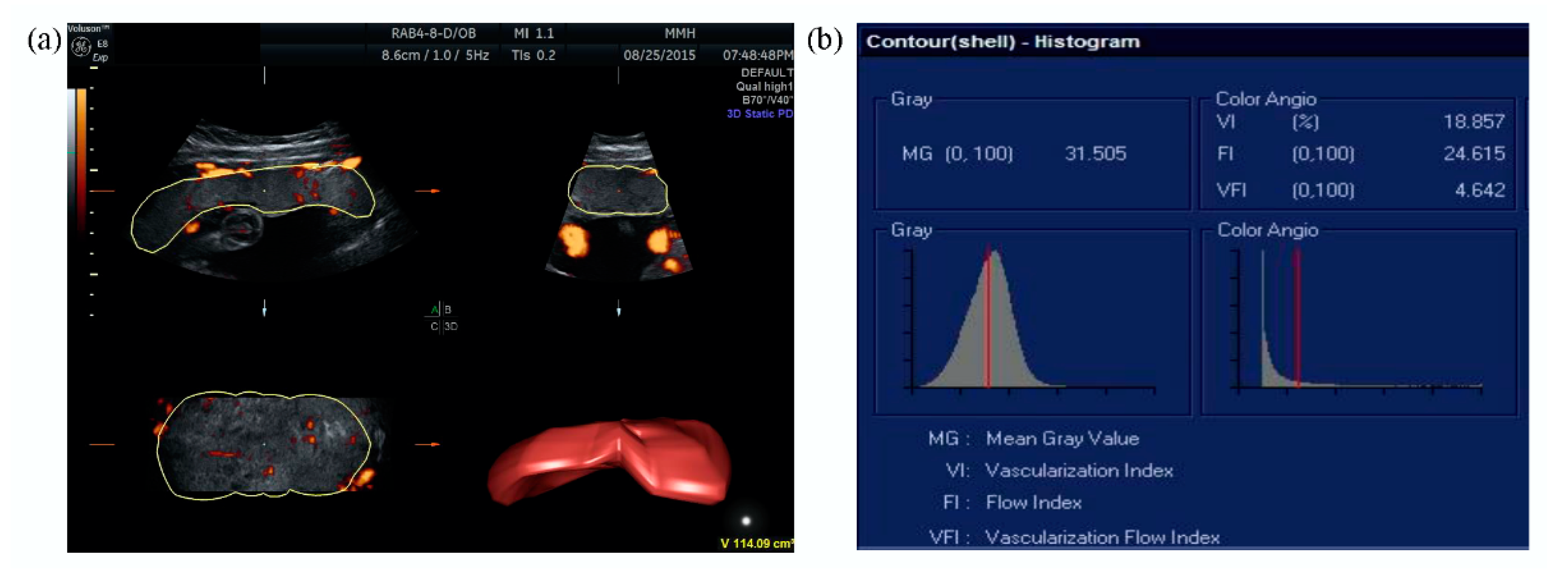
2.2. Ultrasound Examination
2.3. Statistical Analysis
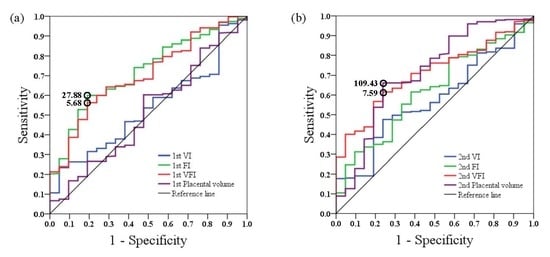
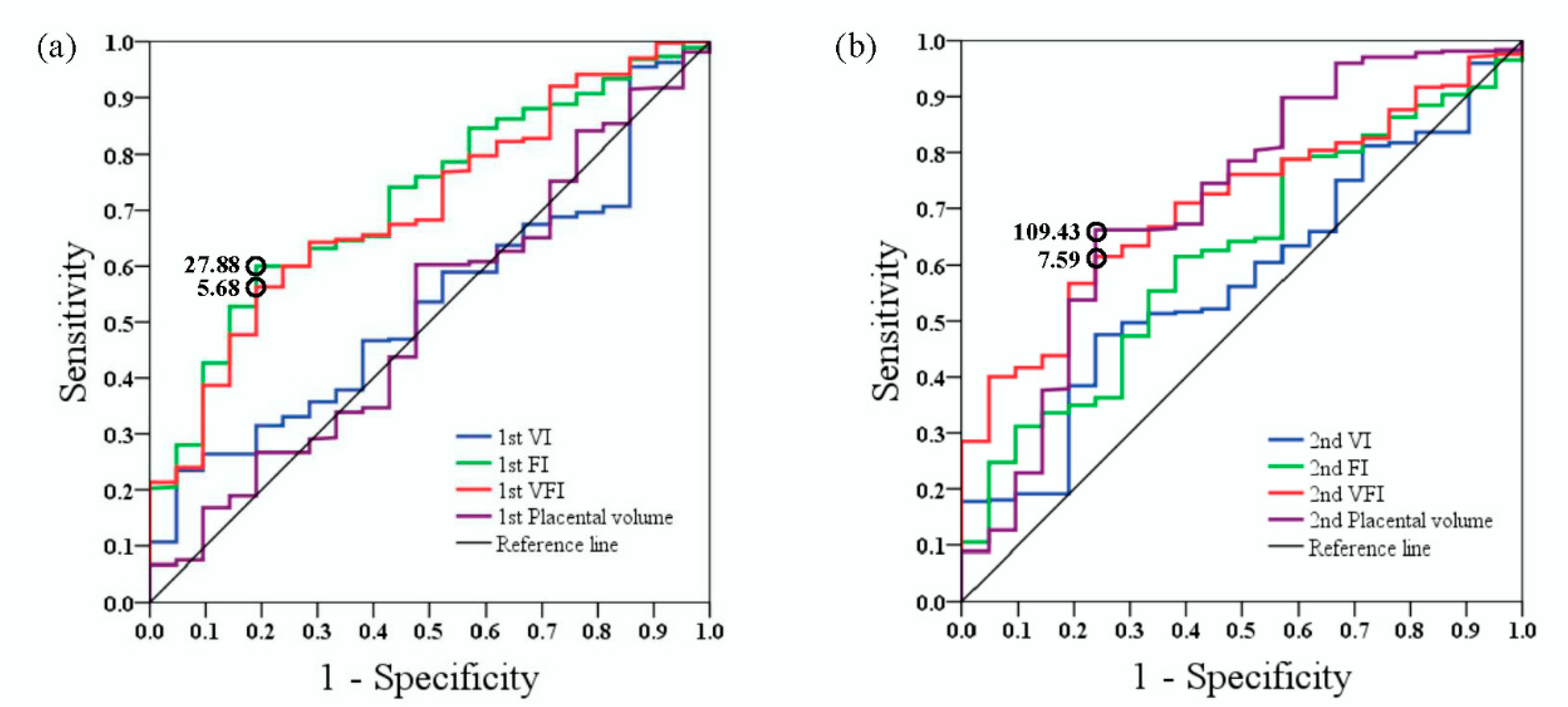
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The American College of Obstetricians and Gynecologists Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 2019, 133, e97–e109.
- Baschat, A.A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet. Gynecol. 2011, 37, 501–514. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, A.H.; Thompson, S.C.; Gecz, J. Cerebral palsy: Causes, pathways, and the role of genetic variants. Am. J. Obstet. Gynecol. 2015, 213, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Rainey, A.; Mayhew, T. Volumes and Numbers of Intervillous Pores and Villous Domains in Placentas Associated with Intrauterine Growth Restriction and/or Pre-eclampsia. Placenta 2010, 31, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [PubMed]
- Audette, M.C.; Kingdom, J.C. Screening for fetal growth restriction and placental insufficiency. Semin. Fetal Neonatal Med. 2018, 23, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.Y.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; Matallana, C.D.P.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Molina, F.S.; et al. Prediction and prevention of small-for-gestational-age neonates: Evidence from SPREE and ASPRE. Ultrasound Obstet. Gynecol. 2018, 52, 52–59. [Google Scholar] [CrossRef]
- Crovetto, F.; Triunfo, S.; Crispi, F.; Rodriguez-Sureda, V.; Domínguez, C.; Figueras, F.; Gratacós, E. Differential performance of first-trimester screening in predicting small-for-gestational-age neonate or fetal growth restriction. Ultrasound Obstet. Gynecol. 2017, 49, 349–356. [Google Scholar] [CrossRef]
- Pairleitner, H.; Steiner, H.; Hasenoehrl, G.; Staudach, A. Three-dimensional power Doppler sonography: Imaging and quantifying blood flow and vascularization. Ultrasound Obstet. Gynecol. 1999, 14, 139–143. [Google Scholar] [CrossRef]
- Farina, A. Placental vascular indices (VI, FI and VFI) in intrauterine growth retardation (IUGR). A pooled analysis of the literature. Prenat. Diagn. 2015, 35, 1065–1072. [Google Scholar] [CrossRef]
- González-González, N.L.; González-Dávila, E.; Marrero, L.G.; Padrón, E.; Castro-Conde, J.R.; Plasencia, W.; Conde, J.R.C. Value of placental volume and vascular flow indices as predictors of intrauterine growth retardation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Tanaka, H.; Noguchi, J.; Hata, K. Three-dimensional ultrasound evaluation of the placenta. Placenta 2011, 32, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Farina, A. Systematic review on first trimester three-dimensional placental volumetry predicting small for gestational age infants. Prenat. Diagn. 2016, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Chrelias, C.; Siristatidis, C.; Kappou, D.; Eleftheriades, M.; Kassanos, D. Placental volume at 11 to 14 gestational weeks in pregnancies complicated with fetal growth restriction and preeclampsia. Prenat. Diagn. 2018, 38, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Hoopmann, M.; Schermuly, S.; Abele, H.; Zubke, W.; Kagan, K.O. First trimester pregnancy volumes and subsequent small for gestational age fetuses. Arch. Gynecol. Obstet. 2014, 290, 41–46. [Google Scholar] [CrossRef]
- Hsieh, W.-S.; Wu, H.-C.; Jeng, S.-F.; Liao, H.-F.; Su, Y.-N.; Lin, S.-J.; Hsieh, C.-J.; Chen, P.-C. Nationwide singleton birth weight percentiles by gestational age in Taiwan, 1998–2002. Acta Paediatr. Taiwanica 2006, 47, 25–33. [Google Scholar]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Guiot, C.; Gaglioti, P.; Oberto, M.; Piccoli, E.; Rosato, R.; Todros, T. Is three-dimensional power Doppler ultrasound useful in the assessment of placental perfusion in normal and growth-restricted pregnancies? Ultrasound Obstet. Gynecol. 2008, 31, 171–176. [Google Scholar] [CrossRef]
- Guimarães Filho, H.A.; Mattar, R.; Araujo Júnior, E.; da Costa, L.L.; de Mello Junior, C.F.; Nardozza, L.M.; Nowak, P.M.; Moron, A.F. Reproducibility of three-dimensional power Doppler placental vascular indices in pregnancies between 26 and 35 weeks. Arch. Gynecol. Obstet. 2011, 283, 213–217. [Google Scholar] [CrossRef]
- Collins, S.L.; Birks, J.S.; Stevenson, G.; Papageorghiou, A.T.; Noble, A.; Impey, L. Measurement of spiral artery jets: General principles and differences observed in small-for-gestational-age pregnancies. Ultrasound Obstet. Gynecol. 2012, 40, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Kingdom, J.; Burton, G.J.; Cindrova-Davies, T. Placental Stem Villus Arterial Remodeling Associated with Reduced Hydrogen Sulfide Synthesis Contributes to Human Fetal Growth Restriction. Am. J. Pathol. 2017, 187, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, M.; Zimmer, M.; Florjanski, J.; Michniewicz, J.; Wiatrowski, A.; Fuchs, T.; Milnerowicz-Nabzdyk, E. Comparative analysis of placental vasculature and placental volume in normal and IUGR pregnancies with the use of three-dimensional Power Doppler. Arch. Gynecol. Obstet. 2012, 285, 331–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moran, M.C.; Mulcahy, C.; Zombori, G.; Ryan, J.; Downey, P.; McAuliffe, F.M. Placental volume, vasculature and calcification in pregnancies complicated by pre-eclampsia and intra-uterine growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 12–17. [Google Scholar] [CrossRef] [PubMed]
- De Paula, C.F.S.; Ruano, R.; Campos, J.A.D.B.; Zugaib, M. Placental volumes measured by 3-dimensional ultrasonography in normal pregnancies from 12 to 40 weeks’ gestation. J. Ultrasound Med. 2008, 27, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.W.; Ou, C.Y.; Tsai, C.C.; Fu, H.C.; Cheng, H.H.; Cheng, B.H.; Chang, M.S.; Hsu, T.Y. Second-trimester placental volume and vascular indices in the prediction of small-for-gestational-age neonates. Fetal. Diagn. Ther. 2015, 37, 123–128. [Google Scholar] [CrossRef]
- Quant, H.S.; Sammel, M.D.; Parry, S.; Schwartz, N. Second-Trimester 3-Dimensional Placental Sonography as a Predictor of Small-for-Gestational-Age Birth Weight. J. Ultrasound Med. 2016, 35, 1693–1702. [Google Scholar] [CrossRef]
- Cignini, P.; Maggio Savasta, L.; Gulino, F.A.; Vitale, S.G.; Mangiafico, L.; Mesoraca, A.; Giorlandino, C. Predictive value of pregnancy-associated plasma protein-A (PAPP-A) and free beta-hCG on fetal growth restriction: Results of a prospective study. Arch. Gynecol. Obstet. 2016, 293, 1227–1233. [Google Scholar] [CrossRef]
- Morris, R.K.; Bilagi, A.; Devani, P.; Kilby, M.D. Association of serum PAPP-A levels in first trimester with small for gestational age and adverse pregnancy outcomes: Systematic review and meta-analysis. Prenat. Diagn. 2017, 37, 253–265. [Google Scholar] [CrossRef]
- Velauthar, L.; Plana, M.N.; Kalidindi, M.; Zamora, J.; Thilaganathan, B.; Illanes, S.E.; Khan, K.S.; Aquilina, J.; Thangaratinam, S. First-trimester uterine artery Doppler and adverse pregnancy outcome: A meta-analysis involving 55 974 women. Ultrasound Obstet. Gynecol. 2014, 43, 500–507. [Google Scholar] [CrossRef]
- Pedroso, M.A.; Palmer, K.R.; Hodges, R.J.; Costa, F.D.S.; Rolnik, D.L. Uterine Artery Doppler in Screening for Preeclampsia and Fetal Growth Restriction. Rev. Bras. Ginecol. Obs. 2018, 40, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.W.; Raine-Fenning, N.J.; Bradley, E.; Bugg, G. Placental 3-D Power Doppler Angiography—Regional Variation and Reliability of Two Ultrasonic Sphere Biopsy Techniques. Ultrasound Med. Biol. 2011, 37, 364–375. [Google Scholar] [CrossRef] [PubMed]


| SGA (n = 21) | Non-SGA (n = 375) | p | |
|---|---|---|---|
| Mother | |||
| Age (years) | 31.67 ± 3.75 | 31.96 ± 3.66 | 0.724 |
| BMI (kg/m2) | 21.65 ± 3.72 | 21.80 ± 3.62 | 0.849 |
| Gravida | 2.19 ± 1.33 | 2.02 ± 1.09 | 0.502 |
| Para | 0.62 ± 0.59 | 0.57 ± 0.65 | 0.740 |
| Cesarean delivery | 11 (52.40) | 93 (24.80) | 0.005 * |
| Chronic hypertension | 4 (19.05) | 9 (2.40) | 0.003 * |
| Gestational hypertension | 0 | 3 (0.80) | >0.999 |
| Preeclampsia | 5 (23.81) | 2 (0.53) | <0.001 * |
| Type I DM | 0 | 2 (0.53) | >0.999 |
| Type II DM | 0 | 5 (1.33) | >0.999 |
| GDM | 1 (4.76) | 30 (8.00) | >0.999 |
| Neonate | |||
| Delivery age (weeks) | 36.67 ± 2.37 | 38.69 ± 1.56 | 0.001 * |
| Birth weight (g) | 2175.00 ± 426.91 | 3139.18 ± 406.73 | <0.001 * |
| Apgar score | |||
| 1 minute | 8.57 ± 1.33 | 9.21 ± 0.97 | 0.005 * |
| 5 minutes | 9.48 ± 0.87 | 9.69 ± 0.63 | 0.277 |
| SGA (n = 21) | Non-SGA (n = 375) | p | |
|---|---|---|---|
| First trimester | |||
| GA at examination (weeks) | 12.90 ± 0.51 | 12.95 ± 0.44 | 0.600 |
| PAPP-A (IU/L) | 3.63 (5.14) | 5.39 (4.73) | 0.226 |
| Free β-hCG (IU/L) | 43.36 (28.37) | 45.50 (38.90) | 0.260 |
| CRL (mm) | 66.39 ± 6.88 | 67.66 ± 5.99 | 0.348 |
| VI | 19.32 ± 8.25 | 20.99 ± 9.63 | 0.436 |
| FI | 25.10 ± 7.51 | 33.10 ± 10.97 | <0.001 * |
| VFI | 4.59 ± 1.95 | 6.28 ± 2.35 | 0.001 * |
| Placental volume (cm3) | 49.46 ± 8.22 | 50.34 ± 9.30 | 0.672 |
| Uterine artery PI | 1.69 ± 0.57 | 1.68 ± 0.44 | 0.939 |
| Second trimester | |||
| GA at examination (weeks) | 22.24 ± 0.45 | 22.42 ± 0.49 | 0.089 |
| BPD (cm) | 5.31 ± 0.31 | 5.49 ± 0.23 | 0.001 * |
| AC (cm) | 17.04 ± 0.94 | 17.68 ± 0.97 | 0.004 * |
| FL (cm) | 3.70 ± 0.20 | 3.81 ± 0.19 | 0.007 * |
| VI | 26.57 ± 9.54 | 29.94 ± 11.68 | 0.195 |
| FI | 27.08 ± 7.97 | 31.54 ± 11.01 | 0.022 * |
| VFI | 6.68 ± 1.71 | 8.68 ± 3.09 | <0.001 * |
| Placental volume (cm3) | 104.80 ± 24.23 | 122.67 ± 26.35 | 0.003 * |
| Uterine artery PI | 1.02 ± 0.42 | 0.93 ± 0.22 | 0.330 |
| ICC | 95% CI | p | |
|---|---|---|---|
| VI | 0.983 | 0.958–0.993 | <0.001 * |
| FI | 0.929 | 0.821–0.972 | <0.001 * |
| VFI | 0.954 | 0.885–0.982 | <0.001 * |
| Placental volume | 0.961 | 0.903–0.985 | <0.001 * |
| Variables | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR | 95% CI | p | Adjusted OR | 95% CI | p | |
| First trimester | ||||||
| VFI | 0.70 | 0.56–0.88 | 0.002 * | 0.76 | 0.60–0.96 | 0.024 * |
| Placental volume | 0.99 | 0.94–1.04 | 0.671 | 0.99 | 0.94–1.05 | 0.872 |
| Second trimester | ||||||
| VFI | 0.74 | 0.61–0.90 | 0.003 * | 0.78 | 0.63–0.96 | 0.021 * |
| Placental volume | 0.97 | 0.94–0.99 | 0.003 * | 0.97 | 0.95–0.99 | 0.007 * |
| First trimester (per 1 SD) | ||||||
| VFI | 0.43 | 0.25–0.73 | 0.002 * | 0.43 | 0.25–0.73 | 0.002 * |
| Placental volume | 0.91 | 0.59–1.41 | 0.671 | 0.99 | 0.65–1.52 | 0.969 |
| Second trimester (per 1 SD) | ||||||
| VFI | 0.41 | 0.23–0.74 | 0.003 * | 0.39 | 0.21–0.74 | 0.004 * |
| Placental volume | 0.39 | 0.22–0.73 | 0.003 * | 0.43 | 0.25–0.77 | 0.004 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-J.; Chen, C.-P.; Sun, F.-J.; Chen, C.-Y. Comparison of Placental Three-Dimensional Power Doppler Vascular Indices and Placental Volume in Pregnancies with Small for Gestational Age Neonates. J. Clin. Med. 2019, 8, 1651. https://doi.org/10.3390/jcm8101651
Chen S-J, Chen C-P, Sun F-J, Chen C-Y. Comparison of Placental Three-Dimensional Power Doppler Vascular Indices and Placental Volume in Pregnancies with Small for Gestational Age Neonates. Journal of Clinical Medicine. 2019; 8(10):1651. https://doi.org/10.3390/jcm8101651
Chicago/Turabian StyleChen, Sue-Jar, Chie-Pein Chen, Fang-Ju Sun, and Chen-Yu Chen. 2019. "Comparison of Placental Three-Dimensional Power Doppler Vascular Indices and Placental Volume in Pregnancies with Small for Gestational Age Neonates" Journal of Clinical Medicine 8, no. 10: 1651. https://doi.org/10.3390/jcm8101651
APA StyleChen, S.-J., Chen, C.-P., Sun, F.-J., & Chen, C.-Y. (2019). Comparison of Placental Three-Dimensional Power Doppler Vascular Indices and Placental Volume in Pregnancies with Small for Gestational Age Neonates. Journal of Clinical Medicine, 8(10), 1651. https://doi.org/10.3390/jcm8101651