Prognostic Significance of Sarcopenia in Patients with Unresectable Advanced Esophageal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment and Treatment Outcome
2.3. Measurement of Body Composition
2.4. Patient Data
2.5. Nutritional Screening
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment-Related Factors
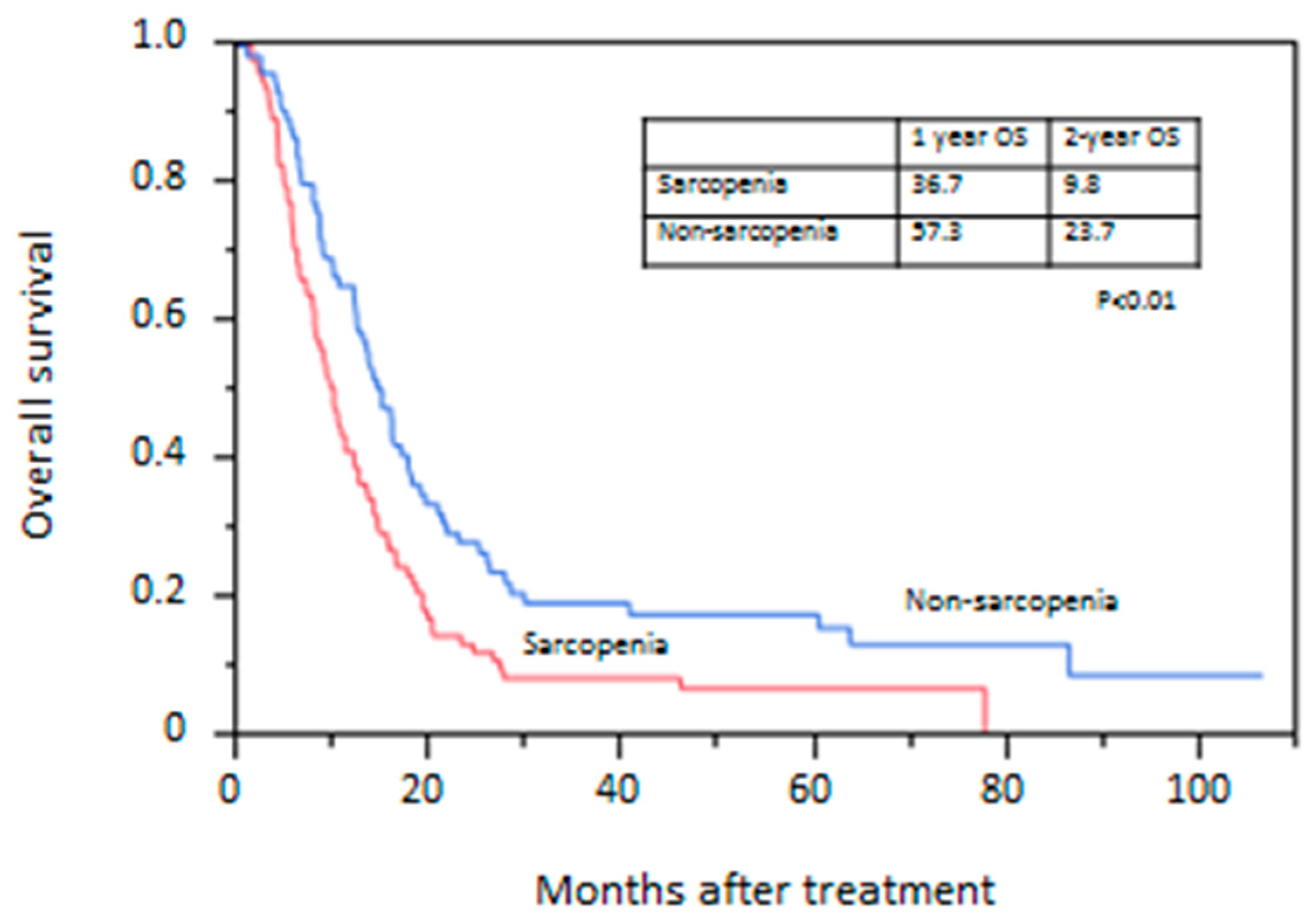
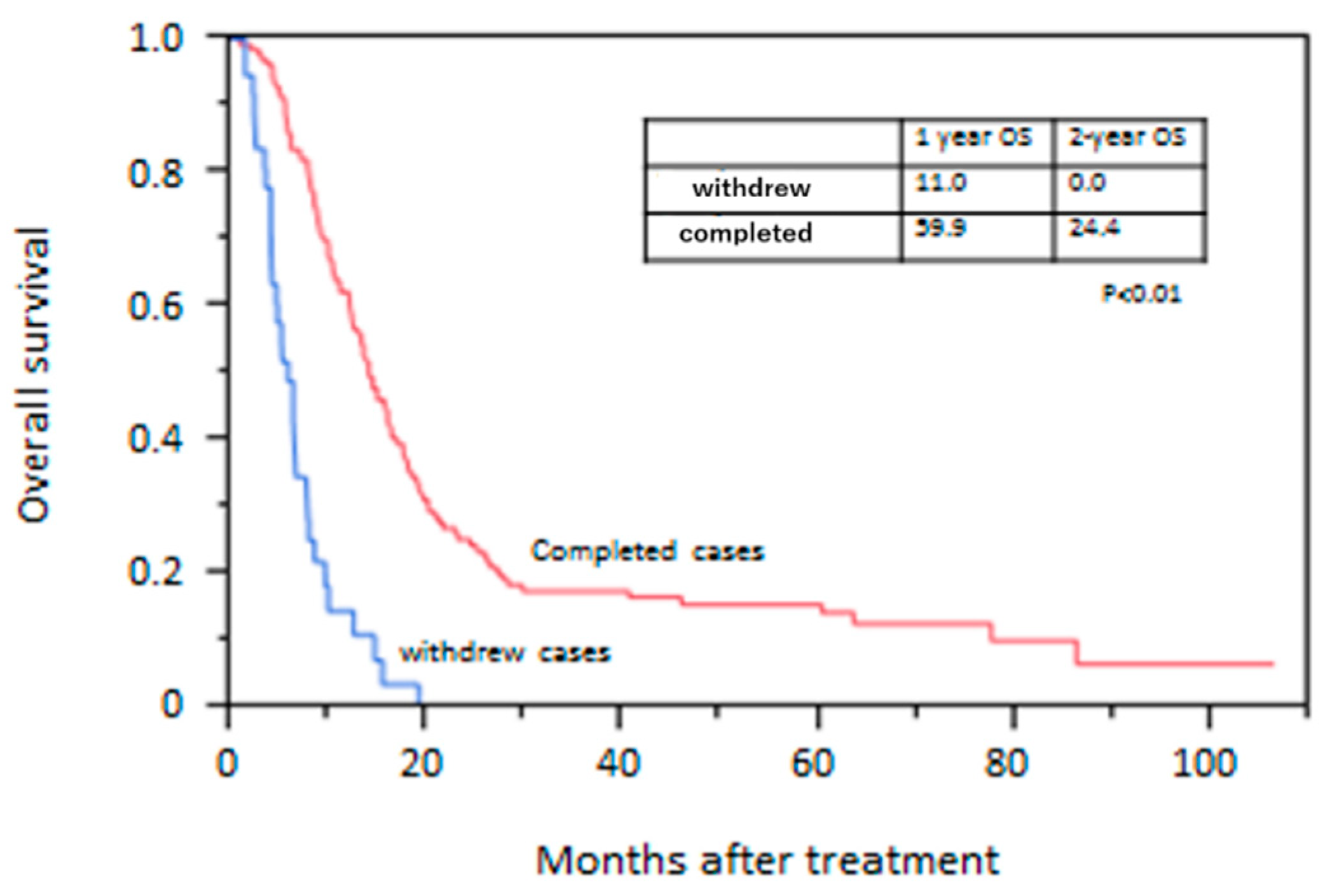
3.3. Overall Survival Rate
3.4. Prognostic Factors for OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Enzinger, P.C.; Mayer, R.J. Esophageal cancer. N. Engl. J. Med. 2003, 349, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Jafri, S.H.; Previgliano, C.; Khandelwal, K.; Shi, R. Cachexia index in advanced non-small-cell lung cancer patients. Clin. Med. Insights Oncol. 2015, 9, 87–93. [Google Scholar] [CrossRef]
- Yabusaki, N.; Fujii, T.; Yamada, S.; Suzuki, K.; Sugimoto, H.; Kanda, M.; Nakayama, G.; Koike, M.; Fujiwara, M.; Kodera, Y. Adverse impact of low skeletal muscle index on the prognosis of hepatocellular carcinoma after hepatic resection. Int. J. Surg. 2016, 30, 136–142. [Google Scholar] [CrossRef]
- Fukushima, H.; Nakanishi, Y.; Kataoka, M.; Tobisu, K.; Koga, F. Prognostic significance of sarcopenia in patients with metastatic renal cell carcinoma. J. Urol. 2016, 195, 26–32. [Google Scholar] [CrossRef]
- Park, I.; Choi, S.J.; Kim, Y.S.; Ahn, H.K.; Hong, J.; Sym, S.J.; Park, J.; Cho, E.K.; Lee, J.H.; Shin, Y.J.; et al. Prognostic factors for risk stratification of patients with recurrent or metastatic pancreatic adenocarcinoma who were treated with gemcitabine-based chemotherapy. Cancer Res. Treat. 2016, 48, 1264–1273. [Google Scholar] [CrossRef]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.; Jenkins, J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef]
- Sabel, M.S.; Lee, J.; Cai, S.; Englesbe, M.J.; Holcombe, S.; Wang, S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann. Surg. Oncol. 2011, 18, 3579–3585. [Google Scholar] [CrossRef] [PubMed]
- Villasenor, A.; Ballard-Barbash, R.; Baumgartner, K.; Baumgartner, R.; Bernstein, L.; McTiernan, A.; Neuhouser, M.L. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: The HEAL Study. J. Cancer Surviv. 2012, 6, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Doyle, S.L.; Murphy, C.F.; King, S.; Guinan, E.M.; Beddy, P.; Ravi, N.; Reynolds, J.V. Sarcopenia: Prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann. Surg. 2017, 266, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Paireder, M.; Asari, R.; Kristo, I.; Rieder, E.; Tamandl, D.; Ba-Ssalamah, A.; Schoppmann, S.F. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur. J. Surg. Oncol. 2017, 43, 478–484. [Google Scholar] [CrossRef]
- Yip, C.; Goh, V.; Davies, A.; Gossage, J.; Mitchell-Hay, R.; Hynes, O.; Maisey, N.; Ross, P.; Gaya, A.; Landau, D.B.; et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur. Radiol. 2014, 24, 998–1005. [Google Scholar] [CrossRef]
- Mayanagi, S.; Tsubosa, Y.; Omae, K.; Niihara, M.; Uchida, T.; Tsushima, T.; Yokota, T.; Sato, H.; Naito, T.; Yasui, H. Negative impact of skeletal muscle wasting after neoadjuvant chemotherapy followed by surgery on survival for patients with thoracic esophageal cancer. Ann. Surg. Oncol. 2017, 24, 3741–3747. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Motoyama, S.; Sato, Y.; Wakita, A.; Kawakita, Y.; Saito, H.; Minamiya, Y. Decreased skeletal muscle mass after neoadjuvant therapy correlates with poor prognosis in patients with esophageal cancer. Anticancer Res. 2016, 36, 6677–6685. [Google Scholar] [CrossRef]
- Grotenhuis, B.A.; Shapiro, J.; van Adrichem, S.; de Vries, M.; Koek, M.; Wijnhoven, B.P.; van Lanschot, J.J. Sarcopenia/muscle mass is not a prognostic factor for short- and long-term outcome after esophagectomy for cancer. World J. Surg. 2016, 40, 2698–2704. [Google Scholar] [CrossRef]
- Boshier, P.R.; Heneghan, R.; Markar, S.R.; Baracos, V.E.; Low, D.E. Assessment of body composition and sarcopenia in patients with esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2018, 31. [Google Scholar] [CrossRef]
- Deng, H.Y.; Zha, P.; Peng, L.; Hou, L.; Huang, K.L.; Li, X.Y. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: A comprehensive systematic review and meta-analysis. Dis. Esophagus 2019, 32. [Google Scholar] [CrossRef]
- Makiura, D.; Ono, R.; Inoue, J.; Kashiwa, M.; Oshikiri, T.; Nakamura, T.; Kakeji, Y.; Sakai, Y.; Miura, Y. Preoperative sarcopenia is a predictor of postoperative pulmonary complications in esophageal cancer following esophagectomy: A retrospective cohort study. J. Geriatr. Oncol. 2016, 7, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Iritani, S.; Imai, K.; Takai, K.; Hanai, T.; Ideta, T.; Miyazaki, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M.; Moriwaki, H. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol. 2015, 50, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Watanabe, S.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Sarcopenia impairs prognosis of patients with hepatocellular carcinoma: The role of liver functional reserve and tumor-related factors in loss of skeletal muscle volume. Nutrients 2017, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Kuwano, H.; Nishimura, Y.; Oyama, T.; Kato, H.; Kitagawa, Y.; Kusano, M.; Shimada, H.; Takiuchi, H.; Toh, Y.; Doki, Y.; et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 2015, 12, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.P.; Detsky, A.S.; Wesson, D.E.; Wolman, S.L.; Stewart, S.; Whitewell, J.; Langer, B.; Jeejeebhoy, K.N. Nutritional assessment: A comparison of clinical judgement and objective measurements. N. Engl. J. Med. 1982, 306, 969–972. [Google Scholar] [CrossRef]
- Wakahara, T.; Shiraki, M.; Murase, K.; Fukushima, H.; Matsuura, K.; Fukao, A.; Kinoshita, S.; Kaifuku, N.; Arakawa, N.; Tamura, T.; et al. Nutritional screening with Subjective Global Assessment predicts hospital stay in patients with digestive diseases. Nutrition 2007, 23, 634–639. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Pamoukdjian, F.; Bouillet, T.; Levy, V.; Soussan, M.; Zelek, L.; Paillaud, E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin. Nutr. 2018, 37, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kunisaki, C.; Suematsu, H.; Tanaka, Y.; Miyamoto, H.; Kosaka, T.; Yukawa, N.; Tanaka, K.; Sato, K.; Akiyama, H.; et al. Impact of Sarcopenia in Patients with Unresectable Locally Advanced Esophageal Cancer Receiving Chemoradiotherapy. In Vivo 2018, 32, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H. Presbyphagia and sarcopenic dysphagia: Association between aging, sarcopenia, and deglutition disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Akagi, J. Treatment of sarcopenic dysphagia with rehabilitation and nutritional support: A comprehensive approach. J. Acad. Nutr. Diet. 2016, 116, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, A.; Sakuma, K. Vitamin D signaling in myogenesis: Potential for treatment of sarcopenia. Biomed. Res. Int. 2014, 2014, 121254. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Cramer, J.T.; Cruz-Jentoft, A.J.; Landi, F.; Hickson, M.; Zamboni, M.; Pereira, S.L.; Hustead, D.S.; Mustad, V.A. Impacts of high-protein oral nutritional supplements among malnourished men and women with sarcopenia: A multicenter, randomized, double-blinded, controlled trial. J. Am. Med. Dir. Assoc. 2016, 17, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Cao, Y. Angiogenesis modulates adipogenesis and obesity. J. Clin. Investig. 2007, 117, 2362–2368. [Google Scholar] [CrossRef]
- Van Kruijsdijk, R.C.; van derWall, E.; Visseren, F.L. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Prev. Biomark. 2009, 18, 2569–2578. [Google Scholar] [CrossRef]
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta 2013, 1831, 1533–1541. [Google Scholar] [CrossRef]
- Freese, K.E.; Kokai, L.; Edwards, R.P.; Philips, B.J.; Sheikh, M.A.; Kelley, J.; Comerci, J.; Marra, K.; Rubin, J.P.; Linkov, F. Adipose-derived stems cells and their role in human cancer development, growth, progression, and metastasis: A systematic review. Cancer Res. 2015, 75, 1161–1168. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Doyle, S.L.; Reynolds, J.V. Visceral adiposity, insulin resistance and cancer risk. Diabetol. Metab. Syndr. 2011, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.L.; Donohoe, C.L.; Lysaght, J.; Reynolds, J.V. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc. Nutr. Soc. 2012, 71, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Okamura, A.; Watanabe, M.; Yamashita, K.; Yuda, M.; Hayami, M.; Imamura, Y.; Mine, S. Implication of visceral obesity in patients with esophageal squamous cell carcinoma. Langenbeck Arch. Surg. 2018, 403, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Baba, Y.; Ishimoto, T.; Kosumi, K.; Tokunaga, R.; Izumi, D.; Ida, S.; Imamura, Y.; Iwagami, S.; Miyamoto, Y.; et al. Low Visceral Fat Content is Associated with Poor Prognosis in a Database of 507 Upper Gastrointestinal Cancers. Ann. Surg. Oncol. 2015, 22, 3946–3953. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, T.; Okabe, H.; Tanaka, E.; Tsunoda, S.; Hisamori, S.; Sakai, Y. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J. Surg. Oncol. 2016, 113, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Anandavadivelan, P.; Lagergren, P. Cachexia in patients with oesophageal cancer. Nat. Rev. Clin. Oncol. 2016, 13, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
| Variables | Sarcopenia | Non-Sarcopenia | p-Value |
|---|---|---|---|
| (n = 101) | (n = 75) | ||
| Age (years) | 65.1 ± 6.1 | 65.3 ± 6.2 | 0.84 |
| Sex (male/female) | 96/5 | 54/21 | <0.01 |
| PS (0 or 1/2 or 3) | 91/10 | 73/2 | 0.08 |
| Brinkman index | 952 ± 723 | 624 ± 482 | <0.01 |
| Alcohol drinking, n (%) | 93 (92.1) | 67 (89.3) | 0.6 |
| cStage (IVa/IVb) | 34/67 | 40/35 | 0.01 |
| Primary tumor location (Ce/Ut/Mt/Lt) | 7/21/41/32 | 2/10/45/18 | 0.06 |
| Tumor length (mm) | 60.0 ± 31.8 | 57.2 ± 28.9 | 0.54 |
| Body mass index (kg/m2) | 19.2 ± 2.8 | 22.3 ± 3.0 | <0.01 |
| Visceral fat mass (cm2) | 54.2 ± 44.9 | 89.7 ± 60.7 | <0.01 |
| Subcutaneous fat mass (cm2) | 49.1 ± 65.8 | 84.7 ± 44.8 | <0.01 |
| Albumin (g/dL) | 3.85 ± 0.46 | 4.08 ± 0.37 | <0.01 |
| SGA (A/B or C) | 49/52 | 63/12 | <0.01 |
| Body weight loss rate (%) | 6.7 ± 7.7 | 4.2 ± 5.8 | 0.01 |
| Variables | Sarcopenia | Non-Sarcopenia | |
|---|---|---|---|
| (n = 101) | (n = 75) | p-Value | |
| Treatment (CT/CRT/RT/BSC) | 28/69/2/2 | 19/54/1/1 | 0.94 |
| Chemotherapy regimen (FP/DCF/5FU + NDP) | 89/6/2 | 65/4/4 | 0.48 |
| Disease control rate *, n (%) | 64 (65.3) | 51 (68.9) | 0.67 |
| Withdrawn cases, n (%) | 30 (31.3) | 8 (10.9) | <0.01 |
| Aspiration pneumonia | 20 | 1 | |
| General condition deterioration | 9 | 4 | |
| Deterioration of other disease | 1 | 0 | |
| Others | 0 | 3 | |
| Adverse events (≥grade 3), n (%) | 32 (31.7%) | 19 (25.3%) | 0.4 |
| Hematological | |||
| Neutropenia | 26 | 18 | 0.86 |
| Increased creatinine | 1 | 2 | 0.57 |
| Nonhematological | |||
| Febrile neutropenia | 14 | 5 | 0.19 |
| Anorexia | 6 | 1 | 0.09 |
| Variables | Withdrawn | Completed | |
|---|---|---|---|
| (n = 30) | (n = 69) | p-Value | |
| Age (years) | 65.2 ± 6.8 | 65.1 ± 5.93 | 0.94 |
| Gender (male/female) | 29/1 | 65/4 | 1 |
| PS (0 or 1/2 or 3) | 2/28 | 7/62 | 1 |
| Brinkman index | 990 ± 679 | 932 ± 754 | 0.72 |
| Alcohol drinking, n (%) | 27 (90.0) | 64 (92.8) | 0.64 |
| Body mass index (kg/m2) | 18.6 ± 3.10 | 19.5 ± 2.63 | 0.11 |
| Visceral fat mass (cm2) | 47.8 ± 42.0 | 57.0 ± 42.1 | 0.38 |
| Subcutaneous fat mass (cm2) | 40.7 ± 51.3 | 53.2 ± 71.5 | 0.42 |
| Albumin (g/dL) | 3.63 ± 0.39 | 3.94 ± 0.47 | <0.01 |
| SGA (A/B or C) | 6/24 | 42/24 | <0.01 |
| Body weight loss rate (%) | 8.8 ± 8.4 | 5.8 ± 7.4 | 0.07 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| n | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age | <70 | 131 | 1 | |||
| ≥70 | 45 | 1.29 (0.88–1.85) | 0.18 | |||
| Gender | Female | 26 | 1 | |||
| Male | 150 | 1.48 (0.95–2.43) | 0.07 | |||
| PS | 0–1 | 164 | 1 | 1 | ||
| 2–3 | 12 | 2.53 (1.19–4.71) | 0.01 | 1.94 (0.89–3.70) | 0.08 | |
| Brinkman index | >620 | 107 | 1 | |||
| ≤620 | 69 | 1.01 (0.73–1.42) | 0.91 | |||
| Alcohol drinking | No | 16 | 1 | |||
| Yes | 160 | 1.45 (0.84–2.78) | 0.19 | |||
| Body mass index (kg/m2) | >23 | 43 | 1 | |||
| ≤23 | 133 | 1.42 (0.97–2.11) | 0.06 | |||
| cStage | IVa | 74 | 1 | 1 | ||
| IVb | 102 | 1.28 (0.91–1.81) | 0.02 | 1.28 (0.91–1.82) | 0.15 | |
| Sarcopenia | Absent | 75 | 1 | 1 | ||
| Present | 101 | 1.63 (1.17–2.29) | <0.01 | 1.48 (1.04–2.10) | 0.03 | |
| Visceral fat mass | >55 | 95 | 1 | |||
| ≤55 | 81 | 1.33 (0.94–1.89) | 0.09 | |||
| Body weight loss rate (%) | <10 | 136 | 1 | |||
| ≥10 | 40 | 1.29 (0.86–1.88) | 0.21 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onishi, S.; Tajika, M.; Tanaka, T.; Hirayama, Y.; Hara, K.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Inaba, Y.; Kodaira, T.; et al. Prognostic Significance of Sarcopenia in Patients with Unresectable Advanced Esophageal Cancer. J. Clin. Med. 2019, 8, 1647. https://doi.org/10.3390/jcm8101647
Onishi S, Tajika M, Tanaka T, Hirayama Y, Hara K, Mizuno N, Kuwahara T, Okuno N, Inaba Y, Kodaira T, et al. Prognostic Significance of Sarcopenia in Patients with Unresectable Advanced Esophageal Cancer. Journal of Clinical Medicine. 2019; 8(10):1647. https://doi.org/10.3390/jcm8101647
Chicago/Turabian StyleOnishi, Sachiyo, Masahiro Tajika, Tsutomu Tanaka, Yutaka Hirayama, Kazuo Hara, Nobumasa Mizuno, Takamichi Kuwahara, Nozomi Okuno, Yoshitaka Inaba, Takeshi Kodaira, and et al. 2019. "Prognostic Significance of Sarcopenia in Patients with Unresectable Advanced Esophageal Cancer" Journal of Clinical Medicine 8, no. 10: 1647. https://doi.org/10.3390/jcm8101647
APA StyleOnishi, S., Tajika, M., Tanaka, T., Hirayama, Y., Hara, K., Mizuno, N., Kuwahara, T., Okuno, N., Inaba, Y., Kodaira, T., Abe, T., Muro, K., Shimizu, M., & Niwa, Y. (2019). Prognostic Significance of Sarcopenia in Patients with Unresectable Advanced Esophageal Cancer. Journal of Clinical Medicine, 8(10), 1647. https://doi.org/10.3390/jcm8101647






