The Role of Physical Fitness in Cognitive-Related Biomarkers in Persons at Genetic Risk of Familial Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
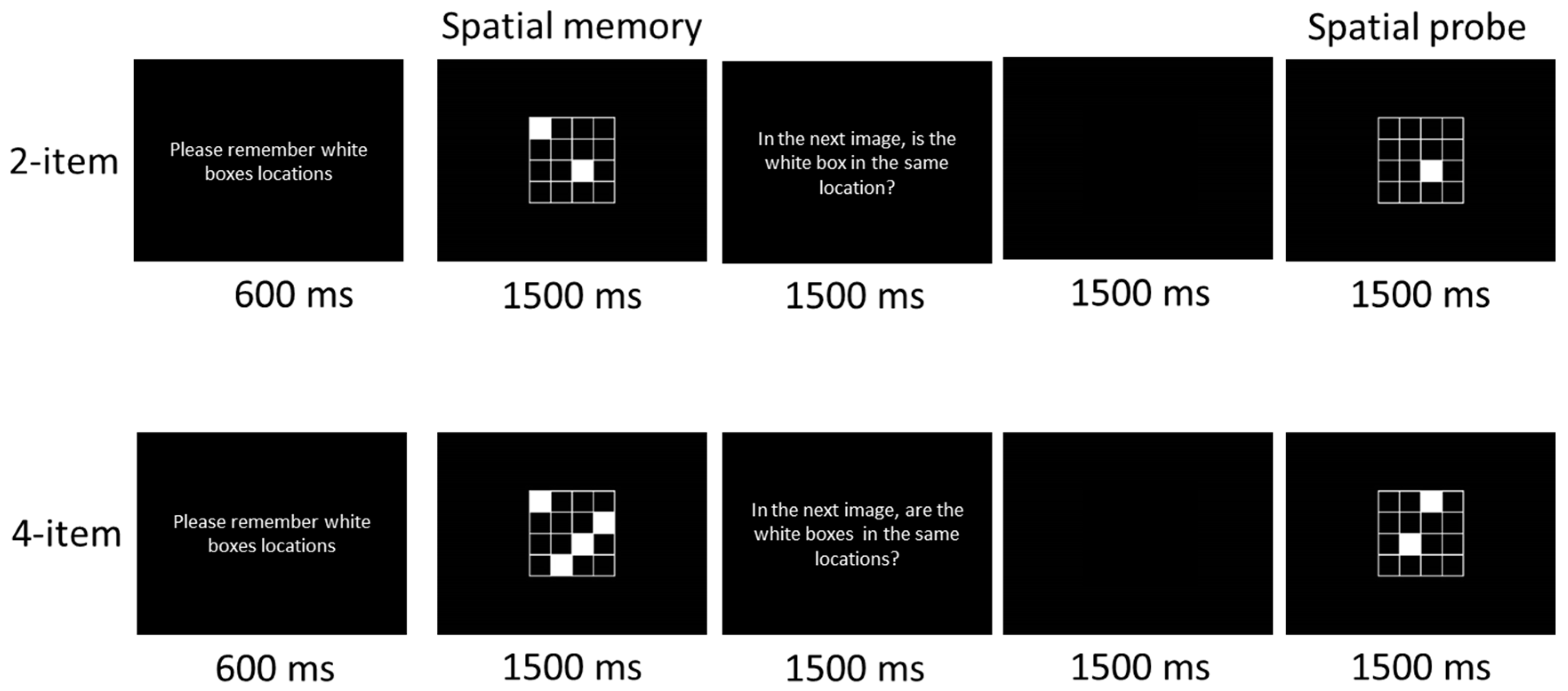
2.3. Cognitive Task
2.4. Blood Sampaling and Analysis
2.5. Time-Frequency Analysis
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Behavioral Performance
3.2.1. Accuracy Rates (ARs)
3.2.2. Reaction Time
3.3. Alpha Power Oscillations
3.4. Molecular Biomarkers
3.5. Correlation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bastiaansen, M.C.; Posthuma, D.; Groot, P.F.; de Geus, E.J. Event-related alpha and theta responses in a visuo-spatial working memory task. Clin. Neurophysiol. 2002, 113, 1882–1893. [Google Scholar] [CrossRef]
- Proskovec, A.L.; Heinrichs-Graham, E.; Wilson, T.W. Load modulates the alpha and beta oscillatory dynamics serving verbal working memory. Neuroimage 2019, 184, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Jost, B.C.; Grossberg, G.T. The natural history of Alzheimer’s disease: A brain bank study. J. Am. Geriatr. Soc. 1995, 43, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Ercoli, L.M.; Silverman, D.H.; Huang, S.C.; Komo, S.; Bookheimer, S.Y.; Lavretsky, H.; Miller, K.; Siddarth, P.; Rasgon, N.L.; et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 6037–6042. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Risch, N.J.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C., Jr.; Rimmler, J.B.; Locke, P.A.; Conneally, P.M.; Schmader, K.E.; et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 1994, 7, 180–184. [Google Scholar] [CrossRef]
- Reiman, E.M.; Chen, K.; Liu, X.; Bandy, D.; Yu, M.; Lee, W.; Ayutyanont, N.; Keppler, J.; Reeder, S.A.; Langbaum, J.B.; et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 6820–6825. [Google Scholar] [CrossRef]
- Chen, Y.; Durakoglugil, M.S.; Xian, X.; Herz, J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc. Natl. Acad. Sci. USA 2010, 107, 12011–12016. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, H.; Basak, J.; Kim, J. Apolipoprotein E in synaptic plasticity and Alzheimer’s disease: Potential cellular and molecular mechanisms. Mol. Cells 2014, 37, 767–776. [Google Scholar] [CrossRef]
- Nwabuisi-Heath, E.; Rebeck, G.W.; Ladu, M.J.; Yu, C. ApoE4 delays dendritic spine formation during neuron development and accelerates loss of mature spines in vitro. ASN Neuro 2014, 6, e00134. [Google Scholar] [CrossRef]
- Dumanis, S.B.; DiBattista, A.M.; Miessau, M.; Moussa, C.E.; Rebeck, G.W. APOE genotype affects the pre-synaptic compartment of glutamatergic nerve terminals. J. Neurochem. 2013, 124, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Bookheimer, S.Y.; Strojwas, M.H.; Cohen, M.S.; Saunders, A.M.; Pericak-Vance, M.A.; Mazziotta, J.C.; Small, G.W. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000, 343, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Reiman, E.M.; Caselli, R.J.; Yun, L.S.; Chen, K.; Bandy, D.; Minoshima, S.; Thibodeau, S.N.; Osborne, D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N. Engl. J. Med. 1996, 334, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Wishart, H.A.; Saykin, A.J.; McAllister, T.W.; Rabin, L.A.; McDonald, B.C.; Flashman, L.A.; Roth, R.M.; Mamourian, A.C.; Tsongalis, G.J.; Rhodes, C.H. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology 2006, 67, 1221–1224. [Google Scholar] [CrossRef]
- Persson, J.; Lind, J.; Larsson, A.; Ingvar, M.; Cruts, M.; Van Broeckhoven, C.; Adolfsson, R.; Nilsson, L.G.; Nyberg, L. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: A risk for AD? Neurology 2006, 66, 1029–1033. [Google Scholar] [CrossRef]
- Feskens, E.J.; Havekes, L.M.; Kalmijn, S.; de Knijff, P.; Launer, L.J.; Kromhout, D. Apolipoprotein e4 allele and cognitive decline in elderly men. Br. Med. J. 1994, 309, 1202–1206. [Google Scholar] [CrossRef]
- Yaffe, K.; Cauley, J.; Sands, L.; Browner, W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch. Neurol. 1997, 54, 1110–1114. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Ivnik, R.J.; Tangalos, E.G.; Schaid, D.J.; Thibodeau, S.N.; Kokmen, E.; Waring, S.C.; Kurland, L.T. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA 1995, 273, 1274–1278. [Google Scholar] [CrossRef]
- Van Duijn, C.M.; Hofman, A. Risk factors for Alzheimer’s disease: The EURODEM collaborative re-analysis of case-control studies. Neuroepidemiology 1992, 11, 106–113. [Google Scholar] [CrossRef]
- Bendlin, B.B.; Ries, M.L.; Canu, E.; Sodhi, A.; Lazar, M.; Alexander, A.L.; Carlsson, C.M.; Sager, M.A.; Asthana, S.; Johnson, S.C. White matter is altered with parental family history of Alzheimer’s disease. Alzheimers Dement. 2010, 6, 394–403. [Google Scholar] [CrossRef]
- La Rue, A.; Matsuyama, S.S.; McPherson, S.; Sherman, J.; Jarvik, L.F. Cognitive performance in relatives of patients with probable Alzheimer disease: An age at onset effect? J. Clin. Exp. Neuropsychol. 1992, 14, 533–538. [Google Scholar] [CrossRef] [PubMed]
- La Rue, A.; O’Hara, R.; Matsuyama, S.S.; Jarvik, L.F. Cognitive changes in young-old adults: Effect offamily histoηr of dementia. J. Clin. Exp. Neuropsychol. 1995, 17, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Caselli, R.J.; Reiman, E.M.; Osborne, D.; Hentz, J.G.; Baxter, L.C.; Hernandez, J.L.; Alexander, G.G. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology 2004, 62, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.M.; Lambert, C.; Sunderland, T.; Parasuraman, R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy middle-aged adults: Results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology 2005, 19, 199–211. [Google Scholar] [CrossRef]
- Levy, J.A.; Bergeson, J.; Putnam, K.; Rosen, V.; Cohen, R.; Lalonde, F.; Mirza, N.; Linker, G.; Sunderland, T. Context-specific memory and apolipoprotein E (APOE) epsilon 4: Cognitive evidence from the NIMH prospective study of risk for Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2004, 10, 362–370. [Google Scholar] [CrossRef]
- Rosen, V.M.; Bergeson, J.L.; Putnam, K.; Harwell, A.; Sunderland, T. Working memory and apolipoprotein E: What’s the connection? Neuropsychologia 2002, 40, 2226–2233. [Google Scholar] [CrossRef]
- Laczó, J.; Andel, R.; Vlček, K.; Macoška, V.; Vyhnálek, M.; Tolar, M.; Bojar, M.; Hort, J. Spatial navigation and APOE in amnestic mild cognitive impairment. Neurodegener. Dis. 2011, 8, 169–177. [Google Scholar] [CrossRef]
- Head, D.; Bugg, J.M.; Goate, A.M.; Fagan, A.M.; Mintun, M.A.; Benzinger, T.; Holtzman, D.M.; Morris, J.C. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch. Neurol. 2012, 69, 636–643. [Google Scholar]
- Etnier, J.L.; Caselli, R.J.; Reiman, E.M.; Alexander, G.E.; Sibley, B.A.; Tessier, D.; McLemore, E.C. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med. Sci. Sports Exerc. 2007, 39, 199–207. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Morris, J.C. Clinical assessment of Alzheimer’s disease. Neurology 1997, 49, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E.; Dickson, D.; Hutton, M. Filling the gaps in the abeta cascade hypothesis of Alzheimer’s disease. Curr. Alzheimer Res. 2006, 3, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef]
- Castellano, J.M.; Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef]
- Mosconi, L.; De Santi, S.; Brys, M.; Tsui, W.H.; Pirraglia, E.; Glodzik-Sobanska, L.; Rich, K.E.; Switalski, R.; Mehta, P.D.; Pratico, D.; et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol. Psychiatry 2008, 63, 609–618. [Google Scholar] [CrossRef]
- Galluzzi, S.; Marizzoni, M.; Babiloni, C.; Albani, D.; Antelmi, L.; Bagnoli, C.; Bartres-Faz, D.; Cordone, S.; Didic, M.; Farotti, L.; et al. Clinical and biomarker profiling of prodromal Alzheimer’s disease in workpackage 5 of the Innovative Medicines Initiative PharmaCog project: A ‘European ADNI study’. J. Intern. Med. 2016, 279, 576–591. [Google Scholar] [CrossRef]
- Diniz, B.S.; Teixeira, A.L.; Ojopi, E.B.; Talib, L.L.; Mendonça, V.A.; Gattaz, W.F.; Forlenza, O.V. Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J. Alzheimers Dis. 2010, 22, 1305–1311. [Google Scholar] [CrossRef]
- McGeer, E.G.; McGeer, P.L. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: A field in its infancy. J. Alzheimers Dis. 2010, 19, 355–361. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Diniz, B.S.; Talib, L.L.; Mendonca, V.A.; Ojopi, E.B.; Gattaz, W.F.; Teixeira, A.L. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009, 28, 507–512. [Google Scholar] [CrossRef]
- Guerreiro, R.J.; Santana, I.; Bras, J.M.; Santiago, B.; Paiva, A.; Oliveira, C. Peripheral inflammatory cytokines as biomarkers in Alzheimer’s disease and mild cognitive impairment. Neurodegener. Dis. 2007, 4, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Gezen-Ak, D.; Dursun, E.; Hanağası, H.; Bilgiç, B.; Lohman, E.; Araz, Ö.S.; Atasoy, I.L.; Alaylıoğlu, M.; Önal, B.; Gürvit, H.; et al. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J. Alzheimers Dis. 2013, 37, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 2005, 93, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Aleixandre, M.; Linares, C.; Masliah, E.; Moessler, H. Apathy and APOE4 are associated with Reduced BDNF Levels in Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 1347–1355. [Google Scholar] [CrossRef]
- Adlard, P.A.; Perreau, V.M.; Pop, V.; Cotman, C.W. Voluntary exercise decreases amyloid load in a Transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 4217–4221. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Nyberg, J.; Åberg, M.A.; Schiöler, L.; Nilsson, M.; Wallin, A.; Torén, K.; Kuhn, H.G. Cardiovascular and cognitive fitness at age 18 and risk of early-onset dementia. Brain 2014, 137, 1514–1523. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pai, M.C.; Ukropec, J.; Ukropcová, B. Distinctive effects of aerobic and resistance exercise modes on neurocognitive and biochemical changes in individuals with mild cognitive impairment. Curr. Alzheimer Res. 2019, 16, 316–332. [Google Scholar] [CrossRef]
- Ward, L.M. Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci. 2003, 7, 553–559. [Google Scholar] [CrossRef]
- Basar, E.; Schurmann, M.; Demiralp, T.; Basar-Eroglu, C.; Ademoglu, A. Event-related oscillations are ‘real brain responses’-Wavelet analysis and new strategies. Int. J. Psychophysiol. 2001, 39, 91–127. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Rispoli, V.; Ragusa, S.; Nisticò, R.; Marra, R.; Russo, E.; Leo, A.; Felicitá, V.; Rotiroti, D. Huperzine a restores cortico-hippocampal functional connectivity after bilateral AMPA lesion of the nucleus basalis of meynert. J. Alzheimers Dis. 2013, 35, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Wianda, E.; Ross, B. The roles of alpha oscillation in working memory retention. Brain Behav. 2019, 9, e01263. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Rikli, R.E.; Jones, C.J. Senior Fitness Test Manual, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Kline, G.M.; Porcari, J.P.; Hintermeister, R.; Freedson, P.S.; Ward, A.; McCarron, R.F.; Ross, J.; Rippe, J.M. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med. Sci. Sports Exerc. 1987, 19, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.A.; Abrahams, S.; Logie, R.H.; Méndez, L.G.; Lopera, F.; Della Sala, S. Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain 2010, 133, 2702–2713. [Google Scholar] [CrossRef]
- McCarthy, G.; Puce, A.; Constable, R.T.; Krystal, J.H.; Gore, J.C.; Goldman-Rakic, P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb. Cortex 1996, 6, 600–611. [Google Scholar] [CrossRef]
- Knott, V.; Millar, A.; Dulude, L.; Bradford, L.; Alwahhabi, F.; Lau, T.; Shea, C.; Wiens, A. Event-related potentials in young and elderly adults during a visual spatial working memory task. Clin. EEG Neurosci. 2004, 35, 185–192. [Google Scholar] [CrossRef]
- Hsu, T.Y.; Tseng, P.; Liang, W.K.; Cheng, S.K.; Juan, C.H. Transcrainal direct current stimulation over right posterior parietal cortex changes prestimulus alpha oscillation in viusal short-term memory task. Neuroimage 2014, 98, 306–313. [Google Scholar] [CrossRef]
- Wang, C.H.; Lo, Y.H.; Pan, C.Y.; Chen, F.C.; Liang, W.K.; Tsai, C.L. Frontal midline theta as a neurophysiological correlate for deficits of attentional orienting in children with developmental coordination disorder. Psychophysiology 2015, 52, 801–812. [Google Scholar] [CrossRef]
- Wang, C.H.; Tseng, Y.T.; Liu, D.; Tsai, C.L. Neural oscillation reveals deficits in visuospatial working memory in children with developmental coordination disorder. Child Dev. 2017, 88, 1716–1726. [Google Scholar] [CrossRef]
- Roach, B.J.; Mathalon, D.H. Event-related EEG time-frequency analysis: An overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr. Bull. 2008, 34, 907–926. [Google Scholar] [CrossRef]
- Chu, C.H.; Yang, K.T.; Song, T.F.; Liu, J.H.; Hung, T.M.; Chang, Y.K. Cardiorespiratory fitness is associated with executive control in late-middle-aged adults: An event-related (De) synchronization (ERD/ERS) study. Front. Psychol. 2016, 7, 1135. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar]
- Cohen, J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Klimesch, W.; Schack, B.; Sauseng, P. The functional significance of theta and upper alpha oscillations. Exp. Psychol. 2005, 52, 99–108. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Schabus, M.; Doppelmayr, M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 2005, 57, 97–103. [Google Scholar] [CrossRef]
- Klimesch, W.; Doppelmayr, M.; Russegger, H.; Pachinger, T.; Schwaiger, J. Induced alpha band power changes in the human EEG and attention. Neurosci. Lett. 1998, 244, 73–76. [Google Scholar] [CrossRef]
- Stipacek, A.; Grabner, R.H.; Neuper, C.; Fink, A.; Neubauer, A.C. Sensitivity of human EEG alpha band desynchronization to different working memory components and increasinglevels of memory load. Neurosci. Lett. 2003, 353, 193–196. [Google Scholar] [CrossRef]
- Green, J.; Levey, A.I. Event-related potential changes in groups at increased risk for Alzheimer disease. Arch. Neurol. 1999, 56, 1398–1403. [Google Scholar] [CrossRef]
- Sun, X.; Chiu, C.C.; Liebson, E.; Crivello, N.A.; Wang, L.; Claunch, J.; Folstein, M.; Rosenberg, I.; Mwamburi, D.M.; Peter, I.; et al. Depression and plasma amyloid beta peptides in the elderly with and without the apolipoprotein E4 allele. Alzheimer Dis. Assoc. Disord. 2009, 23, 238–244. [Google Scholar] [CrossRef]
- Babiloni, C.; Benussi, L.; Binetti, G.; Cassetta, E.; Dal Forno, G.; Del Percio, C.; Ferreri, F.; Ferri, R.; Frisoni, G.; Ghidoni, R.; et al. Apolipoprotein E and alpha brain rhythms in mild cognitive impairment: A multicentric electroencephalogram study. Ann. Neurol. 2006, 59, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A.D. Exploring the central executive. Q. J. Exp. Psychol. 1996, 49, 5–28. [Google Scholar] [CrossRef]
- Cantero, J.L.; Atienza, M.; Cruz-Vadell, A.; Suarez-Gonzalez, A.; Gil-Neciga, E. Increased synchronization and decreased neural complexity underlie thalamocortical oscillatory dynamics in mild cognitive impairment. Neuroimage 2009, 46, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Bookheimer, S.; Burggren, A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer’s and cognitive aging. Annu. Rev. Clin. Psychol. 2009, 5, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Price, J.L.; Morris, J.C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 1999, 45, 358–368. [Google Scholar] [CrossRef]
- Niebauer, J.; Maxwell, A.J.; Lin, P.S.; Tsao, P.S.; Kosek, J.; Bernstein, D.; Cooke, J.P. Impaired aerobic capacity in hypercholesterolemic mice: Partial reversal by exercise training. Am. J. Physiol. 1999, 276, H1346–H1354. [Google Scholar] [CrossRef]
- Themanson, J.R.; Hillman, C.H.; Curtin, J.J. Age and physical activity influences on action monitoring during task switching. Neurobiol. Aging 2006, 27, 1335–1345. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pan, C.Y.; Chen, F.C.; Tseng, Y.T. Open- and closed-skill exercise interventions produce different neurocognitive effects on executive functions in the elderly: A 6-month randomized, controlled trial. Front. Aging Neurosci. 2017, 9, 294. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pai, M.C.; Ukropec, J.; Ukropcová, B. The role of physical fitness in the neurocognitive performance of task switching in older persons with mild cognitive impairment. J. Alzheimers Dis. 2016, 53, 143–159. [Google Scholar] [CrossRef]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef]
- Ten Brinke, L.F.; Bolandzadeh, N.; Nagamatsu, L.S.; Hsu, C.L.; Davis, J.C.; Miran-Khan, K.; Liu-Ambrose, T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Sports Med. 2015, 49, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Ukropec, J.; Ukropcová, B.; Pai, M.C. An acute bout of aerobic or strength exercise specifically modifies circulating exerkine levels and neurocognitive functions in elderly individuals with mild cognitive impairment. NeuroImage Clin. 2018, 17, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Metti, A.L.; Cauley, J.A.; Ayonayon, H.N.; Harris, T.B.; Rosano, C.; Williamson, J.D.; Yaffe, K. The demographic and medical correlates of plasma aβ40 and aβ42. Alzheimer Dis. Assoc. Disord. 2013, 27, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, J.M.; Ferrell, R.E.; Katzel, L.I.; Dengel, D.R.; Sorkin, J.D.; Goldberg, A.P. Apolipoprotein E genotype and exercise training induced increases in high-density lipoprotein (HDL)- and HDL2-cholesterol levels in overweight men. Metabolism 1999, 48, 943–945. [Google Scholar] [CrossRef]
- Leon, A.S.; Togashi, K.; Rankinen, T.; Després, J.P.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Association of apolipoprotein E polymorphism with blood lipids and maximal oxygen uptake in the sedentary state and after exercise training in the HERITAGE family study. Metabolism 2004, 53, 108–116. [Google Scholar] [CrossRef]
- Meckes, C.L.; Moytna, N.A.; Tsongalis, G.; Miles, M. The increase in maximal oxygen uptake with exercise training is reduced in subjects homozygous for the apolipoprotein E3 allele. Circulation 2001, 104, 343. [Google Scholar]
- Thompson, P.D.; Tsongalis, G.J.; Seip, R.L.; Bilbie, C.; Miles, M.; Zoeller, R.; Visich, P.; Gordon, P.; Angelopoulos, T.J.; Pescatello, L.; et al. Apolipoprotein E genotype and changes in serum lipids and maximal oxygen uptake with exercise training. Metabolism 2004, 53, 193–202. [Google Scholar] [CrossRef]
- Lue, L.F.; Sabbagh, M.N.; Chiu, M.J.; Jing, N.; Snyder, N.L.; Schmitz, C.; Guerra, A.; Belden, C.M.; Chen, T.F.; Yang, C.C.; et al. Plasma levels of Aβ42 and tau identified probable Alzheimer’s dementia: Findings in two cohorts. Front. Aging Neurosci. 2017, 9, 226. [Google Scholar] [CrossRef]
- Tzen, K.Y.; Yang, S.Y.; Chen, T.F.; Cheng, T.W.; Horng, H.E.; Wen, H.P.; Huang, Y.Y.; Shiue, C.Y.; Chiu, M.J. Plasma Aβ but not tau is related to brain PiB retention in early Alzheimer’s disease. ACS Chem. Neurosci. 2014, 5, 830–836. [Google Scholar] [CrossRef]
- Yang, S.Y.; Chiu, M.J.; Chen, T.F.; Lin, C.H.; Jeng, J.S.; Tang, S.C.; Lee, Y.F.; Yang, C.C.; Liu, B.H.; Chen, H.H.; et al. Analytical performance of reagent for assaying tau protein in human plasma and feasibility study screening neurodegenerative diseases. Sci. Rep. 2017, 7, 9304. [Google Scholar] [CrossRef]
- Mehta, P.D.; Pirttilä, T.; Mehta, S.P.; Sersen, E.A.; Aisen, P.S.; Wisniewski, H.M. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch. Neurol. 2000, 57, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Vanderstichele, H.; Van Kerschaver, E.; Hesse, C.; Davidsson, P.; Buyse, M.A.; Andreasen, N.; Minthon, L.; Wallin, A.; Blennow, K.; Vanmechelen, E. Standardization of measurement of beta-amyloid(1-42) in cerebrospinal fluid and plasma. Amyloid 2000, 7, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Sager, M.A.; Hermann, B.; La Rue, A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J. Geriatr. Psychiatry Neurol. 2005, 18, 245–249. [Google Scholar] [CrossRef] [PubMed]

| ApoE-4 | Non-ApoE-4 | p | |
|---|---|---|---|
| Age (years) | 53.19 ± 8.57 | 53.94 ± 7.09 | 0.789 |
| Gender (male/female) | 7/9 | 5/11 | 0.465 |
| Education (years) | 15.06 ± 3.17 | 14.19 ± 2.43 | 0.388 |
| Systolic pressure (mmHg) | 118.06 ± 16.43 | 126.13 ± 19.12 | 0.211 |
| Diastolic pressure (mmHg) | 78.63 ± 12.65 | 81.56 ± 13.20 | 0.525 |
| Height (cm) | 163.41 ± 5.76 | 159.76 ± 6.95 | 0.117 |
| Weight (kg) | 63.99 ± 12.22 | 59.05 ± 8.43 | 0.193 |
| Body Mass Index (kg/m2) | 23.81 ± 3.17 | 23.12 ± 2.83 | 0.525 |
| Social participation | 9.88 ± 2.85 | 10.31 ± 2.98 | 0.674 |
| MMSE | 29.75 ± 0.45 | 29.50 ± 0.63 | 0.207 |
| MoCA | 28.75 ± 1.29 | 29.06 ± 0.93 | 0.438 |
| ACE-III | 95.50 ± 4.87 | 96.19 ± 2.64 | 0.623 |
| BDI-II | 3.75 ± 3.11 | 1.81 ± 2.54 | 0.063 |
| Grip (kg) | 31.68 ± 9.28 | 29.82 ± 7.15 | 0.531 |
| Arm Curl (number) | 27.25 ± 10.46 | 24.56 ± 7.54 | 0.411 |
| Chair Stand (sec) | 17.16 ± 4.84 | 16.38 ± 4.39 | 0.882 |
| 8-Foot Up-and-Go (sec) | 5.14 ± 1.17 | 4.91 ± 0.57 | 0.496 |
| Back Scratch (cm) | 2.86 ± 5.75 | 5.54 ± 7.71 | 0.274 |
| Chair Sit-and-Reach (cm) | 1.56 ± 16.00 | 9.18 ± 10.84 | 0.128 |
| VO2max (mL/kg/min) | 28.00 ± 10.23 | 31.02 ± 10.12 | 0.408 |
| ApoE-4 | Non-ApoE-4 | t | p | |
|---|---|---|---|---|
| n = 16 | n = 16 | |||
| IL-1β (pg/mL) | 0.05 ± 0.06 | 0.06 ± 0.10 | −0.59 | 0.561 |
| IL-6 (pg/mL) | 0.72 ± 1.13 | 0.22 ± 0.25 | 1.74 | 0.100 |
| IL-8 (pg/mL) | 3.21 ± 1.89 | 2.50 ± 1.78 | 1.09 | 0.284 |
| BDNF (ng/mL) | 8.47 ± 6.43 | 10.57 ± 5.00 | −1.03 | 0.311 |
| Aβ1-40 (pg/mL) | 57.54 ± 35.40 | 46.23 ± 32.19 | 0.95 | 0.352 |
| Aβ1-42 (pg/dL) | 27.01 ± 21.20 | 25.76 ± 23.66 | 0.16 | 0.876 |
| Group | Grip | Arm Curl | Chair Stand | 8-Foot Up-and-Go | Back Scratch | Chair Sit-and-Reach | VO2max | |
|---|---|---|---|---|---|---|---|---|
| ApoE-4 | ||||||||
| 2-item AR | r = 0.48, p = 0.059 | r = 0.38, p = 0.142 | r = 0.25, p = 0.351 | r = 0.29, p = 0.273 | r = −0.34, p = 0.902 | r = 0.15, p = 0.590 | r = 0.59, p = 0.017 | |
| 4-item AR | r = 0.46, p = 0.071 | r = 0.41, p = 0.119 | r = 0.24, p = 0.366 | r = 0.43, p = 0.100 | r = −0.45, p = 0.077 | r = −0.28, p = 0.295 | r = 0.63, p = 0.009 | |
| 2-item RT | r = −0.41, p = 0.120 | r = −0.18, p = 0.518 | r = −0.08, p = 0.779 | r = −0.40, p = 0.129 | r = 0.35, p = 0.179 | r = 0.07, p = 0.805 | r = −0.52, p = 0.039 | |
| 4-item RT | r = −0.30, p = 0.254 | r = −0.06, p = 0.826 | r = 0.07, p = 0.792 | r = −0.34, p = 0.204 | r = 0.56, p = 0.023 | r = 0.26, p = 0.328 | r = −0.55, p = 0.027 | |
| non-ApoE-4 | ||||||||
| 2-item AR | r = 0.32, p = 0.230 | r = 0.40, p = 0.121 | r = 0.46, p = 0.074 | r = −0.39, p = 0.131 | r = −0.12, p = 0.672 | r = 0.51, p = 0.042 | r = 0.56, p = 0.024 | |
| 4-item AR | r = 0.44, p = 0.090 | r = 0.22, p = 0.410 | r = 0.19, p = 0.488 | r = −0.34, p = 0.202 | r = −0.10, p = 0.726 | r = 0.38, p = 0.149 | r = 0.46, p = 0.072 | |
| 2-item RT | r = −0.24, p = 0.377 | r = −0.37, p = 0.160 | r = −0.10, p = 0.707 | r = 0.17, p = 0.538 | r = −0.23, p = 0.399 | r = −0.49, p = 0.056 | r = −0.31, p = 0.238 | |
| 4-item RT | r = −0.11, p = 0.687 | r = −0.19, p = 0.478 | r = −0.46, p = 0.865 | r = 0.25, p = 0.352 | r = −0.19, p = 0.489 | r = −0.29, p = 0.269 | r = −0.24, p = 0.366 | |
| All participants | ||||||||
| 2-item AR | r = 0.37, p = 0.038 | r = 0.37, p = 0.035 | r = 0.32, p = 0.078 | r = −0.00, p = 0.994 | r = −0.12, p = 0.527 | r = 0.25, p = 0.160 | r = 0.52, p = 0.003 | |
| 4-item AR | r = 0.37, p = 0.035 | r = 0.26, p = 0.147 | r = 0.20, p = 0.273 | r = 0.18, p = 0.323 | r = −0.17, p = 0.349 | r = 0.03, p = 0.876 | r = 0.56, p = 0.001 | |
| 2-item RT | r = −0.30, p = 0.092 | r = −0.25, p = 0.174 | r = −0.08, p = 0.660 | r = −0.15, p = 0.399 | r = −0.02, p = 0.903 | r = −0.19, p = 0.306 | r = −0.40, p = 0.022 | |
| 4-item RT | r = −0.19, p = 0.310 | r = −0.07, p = 0.690 | r = 0.03, p = 0.859 | r = −0.11, p = 0.556 | r = 0.09, p = 0.638 | r = −0.03, p = 0.893 | r = −0.42, p = 0.017 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-L.; Sun, H.-S.; Kuo, Y.-M.; Pai, M.-C. The Role of Physical Fitness in Cognitive-Related Biomarkers in Persons at Genetic Risk of Familial Alzheimer’s Disease. J. Clin. Med. 2019, 8, 1639. https://doi.org/10.3390/jcm8101639
Tsai C-L, Sun H-S, Kuo Y-M, Pai M-C. The Role of Physical Fitness in Cognitive-Related Biomarkers in Persons at Genetic Risk of Familial Alzheimer’s Disease. Journal of Clinical Medicine. 2019; 8(10):1639. https://doi.org/10.3390/jcm8101639
Chicago/Turabian StyleTsai, Chia-Liang, H.-Sunny Sun, Yu-Min Kuo, and Ming-Chyi Pai. 2019. "The Role of Physical Fitness in Cognitive-Related Biomarkers in Persons at Genetic Risk of Familial Alzheimer’s Disease" Journal of Clinical Medicine 8, no. 10: 1639. https://doi.org/10.3390/jcm8101639
APA StyleTsai, C.-L., Sun, H.-S., Kuo, Y.-M., & Pai, M.-C. (2019). The Role of Physical Fitness in Cognitive-Related Biomarkers in Persons at Genetic Risk of Familial Alzheimer’s Disease. Journal of Clinical Medicine, 8(10), 1639. https://doi.org/10.3390/jcm8101639





