Divergent Mononuclear Cell Participation and Cytokine Release Profiles Define Hip and Knee Osteoarthritis
Abstract
1. Background
2. Methods
2.1. Study Population
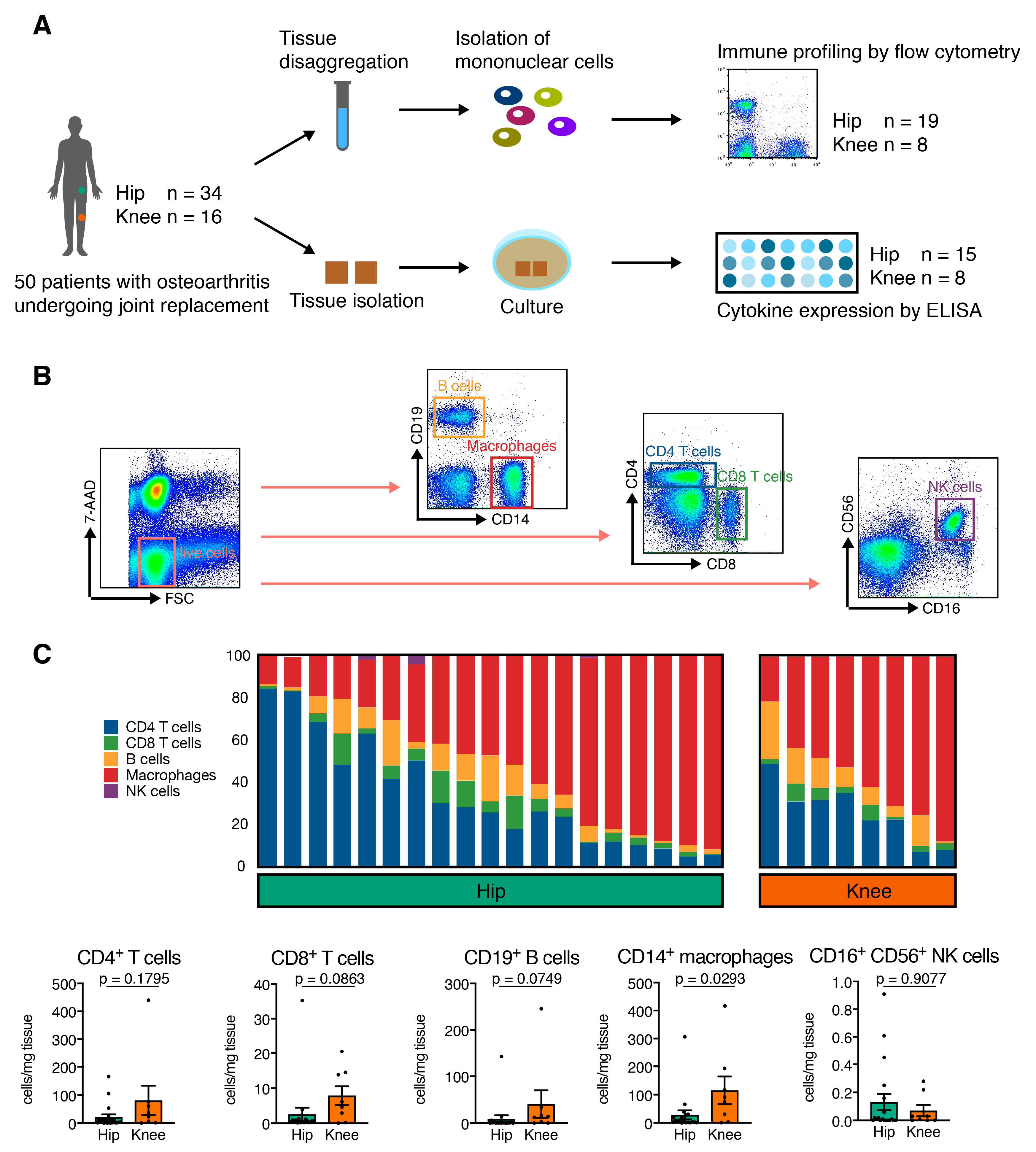
2.2. Sample Preparation for Flow Cytometry Analysis
2.3. Multiplex Cytokine Analysis from Synovial Membrane Supernatants
2.4. Cytokine Expression Analysis
2.5. Statistical Analysis
3. Results
3.1. Description of the Study Population
3.2. Hip OA Synovial Membrane Displays Higher Amounts of Mononuclear Cell Infiltration
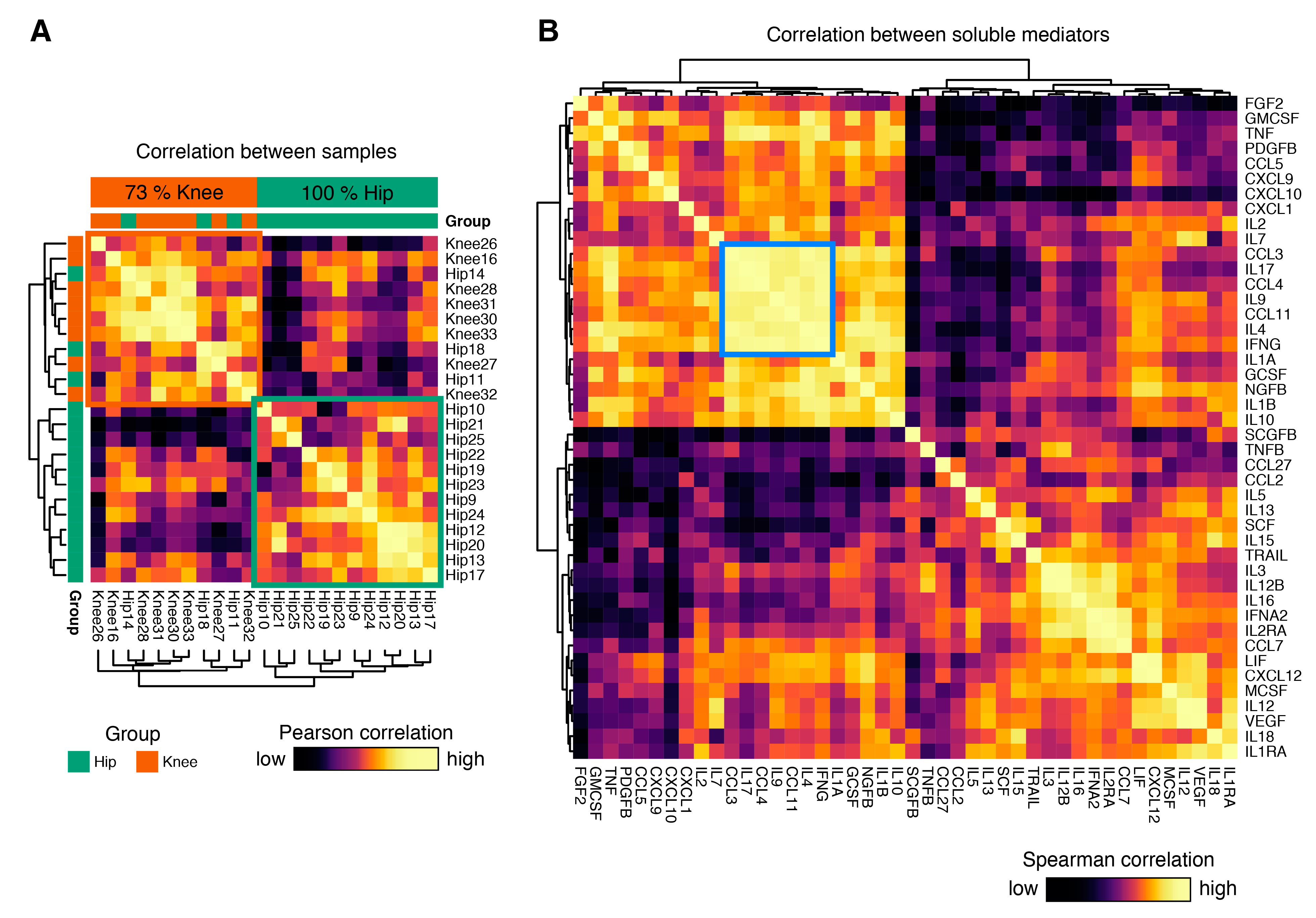
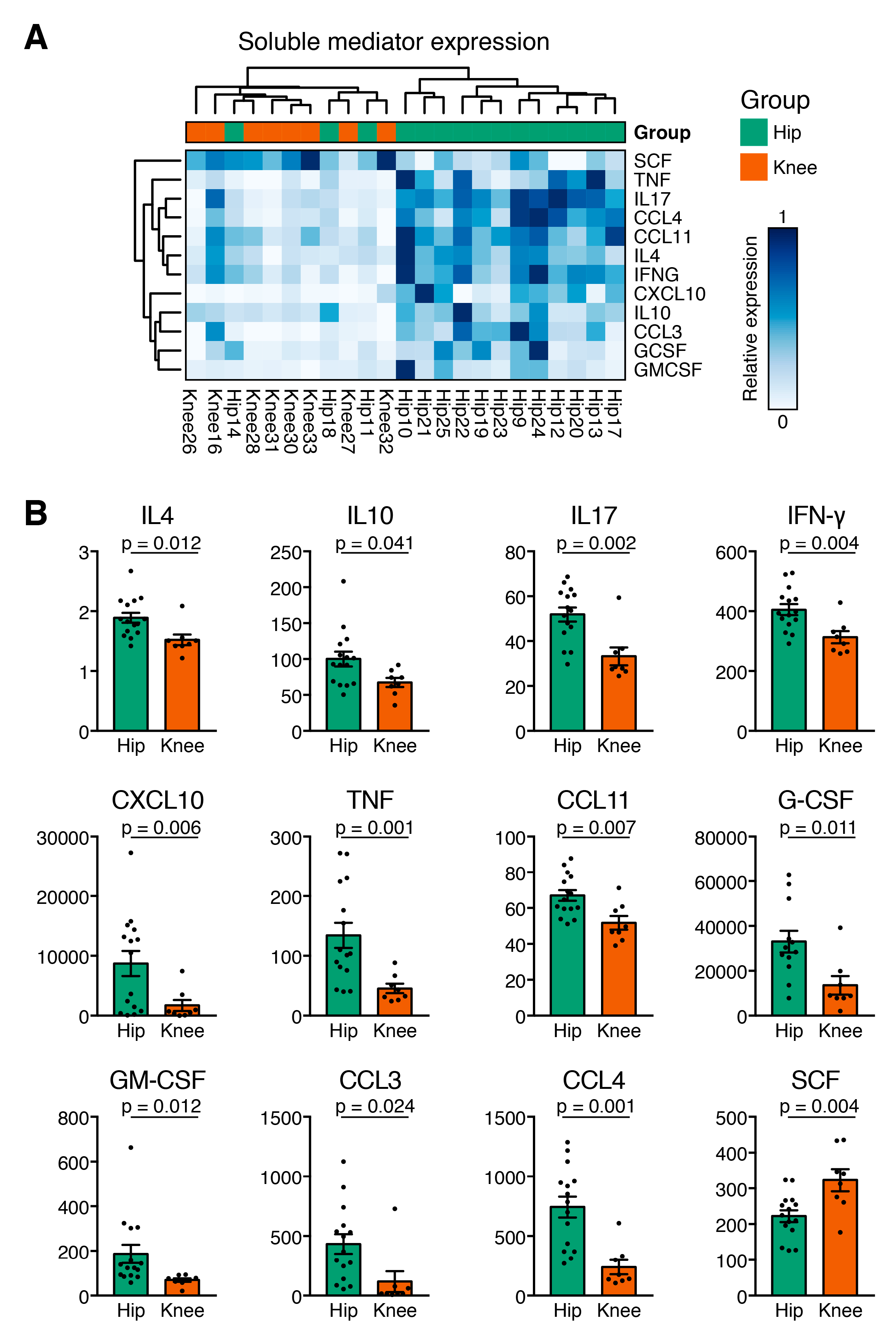
3.3. Cytokine Expression Profiles Distinguish Hip and Knee Osteoarthritis
3.4. Hip OA Presents a Higher Inflammatory Cytokine Expression Profile
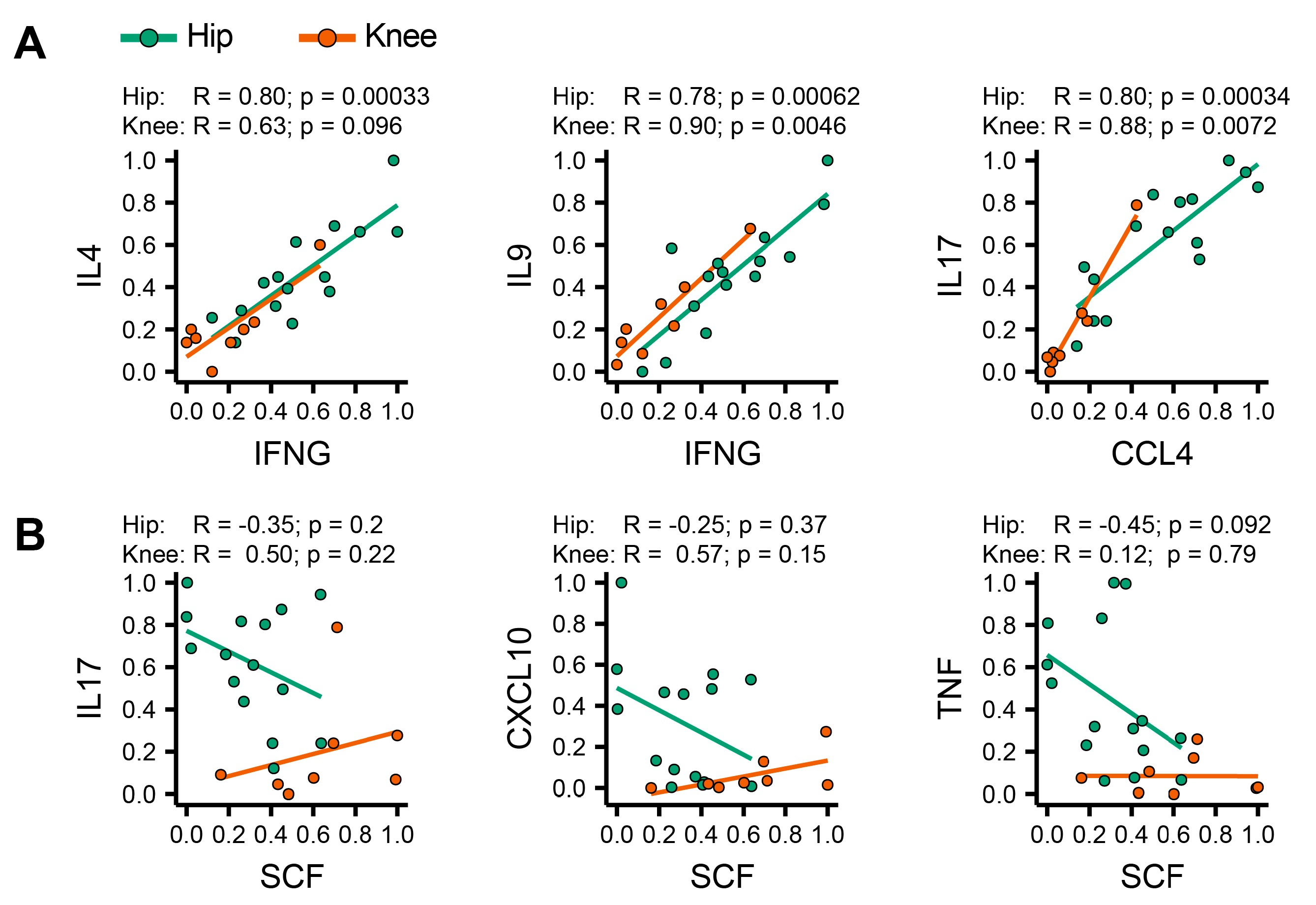
3.5. Identification of Coordinated Cytokine Expression Patterns in Hip and Knee Osteoarthritis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. “Chronic Rheumatic Conditions.” Chronic Diseases and Health Promotion (Internet). 2018. Available online: http://www.who.int/chp/topics/rheumatic/en/ (accessed on 1 September 2019).
- Maetzel, A.; Li, L.C.; Pencharz, J.; Tomlinson, G.; Bombardier, C. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: A comparative study. Ann. Rheum. Dis. 2004, 63, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Lajeunesse, D.; Fahmi, H.; Tardif, G.; Pelletier, J.-P. New thoughts on the pathophysiology of osteoarthritis: One more step toward new therapeutic targets. Curr. Rheumatol. Rep. 2006, 8, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Lu, W.W.; Chiu, K.Y. Importance of subchondral bone in the pathogenesis and management of osteoarthritis from bench to bed. J. Orthop. Transl. 2014, 2, 16–25. [Google Scholar] [CrossRef]
- Weber, A.; Chan, P.M.B.; Wen, C. Do immune cells lead the way in subchondral bone disturbance in osteoarthritis? Prog. Biophys. Mol. Biol. 2017. [Google Scholar] [CrossRef]
- Little, C.B.; Barai, A.; Burkhardt, D.; Smith, S.M.; Fosang, A.J.; Werb, Z.; Shah, M.; Thompson, E.W. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009, 60, 3723–3733. [Google Scholar] [CrossRef]
- Little, C.B.; Meeker, C.T.; Golub, S.B.; Lawlor, K.E.; Farmer, P.J.; Smith, S.M.; Fosang, A.J. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J. Clin. Investig. 2007, 117, 1627–1636. [Google Scholar] [CrossRef]
- Le Marshall, K.F.; Yee, B.; Dieppe, P.A.; Leung, A.; Page, C.; Choong, P.F.; Dowsey, M.; Lim, K.K. Differences between patients with hip and knee osteoarthritis. Osteoarthr. Cartil. 2013, 21, S139. [Google Scholar] [CrossRef]
- Moradi, B.; Rosshirt, N.; Tripel, E.; Kirsch, J.; Barie, A.; Zeifang, F.; Gotterbarm, T.; Hagmann, S. Unicompartmental and bicompartmental knee osteoarthritis show different patterns of mononuclear cell infiltration and cytokine release in the affected joints. Clin. Exp. Immunol. 2015, 180, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Lutz, I.; Railton, P.; Wiley, J.P.; McAllister, J.; Powell, J.; Krawetz, R.J. Serum and synovial fluid cytokine profiling in hip osteoarthritis: Distinct from knee osteoarthritis and correlated with pain. BMC Musculoskelet. Disord. 2018, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D. The normal synovium. Open Rheumatol. J. 2011, 5, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Klein-Wieringa, I.R.; De Lange-Brokaar, B.J.; Yusuf, E.; Andersen, S.N.; Kwekkeboom, J.C.; Kroon, H.M.; Van Osch, G.J.; Zuurmond, A.-M.; Stojanovic-Susulic, V.; Nelissen, R.G.; et al. Inflammatory Cells in Patients with Endstage Knee Osteoarthritis: A Comparison between the Synovium and the Infrapatellar Fat Pad. J. Rheumatol. 2016, 43, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Yoshino, S.; Kato, T.; Tsuruha, J.; Nishioka, K. T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthr. Cartil. 1999, 7, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Madrid, F.; Karvonen, R.L.; Teitge, R.A.; Miller, P.R.; An, T.; Negendank, W.G. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn. Reson. Imaging 1995, 13, 177–183. [Google Scholar] [CrossRef]
- Ishii, H.; Tanaka, H.; Katoh, K.; Nakamura, H.; Nagashima, M.; Yoshino, S. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthr. Cartil. 2002, 10, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Penatti, A.; Facciotti, F.; De-Matteis, R.; Larghi, P.; Paroni, M.; Murgo, A. Differences in serum and synovial CD4+ T cells and cytokine profiles to stratify patients with inflammatory osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Arayssi, T.; Duray, P.; Schumacher, H.R. Immunohistochemistry of normal human knee synovium: A quantitative study. Ann. Rheum. Dis. 2004, 63, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hao, B.; Yang, X.F.; Chen, W.Q. Decreased CD200R expression on monocyte-derived macrophages correlates with Th17/Treg imbalance and disease activity in rheumatoid arthritis patients. Inflamm. Res. 2014, 63, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Daghestani, H.N.; Pieper, C.F.; Kraus, V.B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015, 67, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, F.; Slowikowski, K.; Wei, K.; Marshall, J.L.; Rao, D.A.; Chang, S.K.; Nguyen, H.N.; Noss, E.H.; Turner, J.D.; Earp, B.E.; et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 2018, 9, 789. [Google Scholar] [CrossRef]
- Croft, A.P.; Campos, J.; Jansen, K.; Turner, J.D.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.J.; Kemble, S.; et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019, 570, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Rosshirt, N.; Hagmann, S.; Tripel, E.; Gotterbarm, T.; Kirsch, J.; Zeifang, F. A predominant Th1 polarization is present in synovial fluid of end-stage osteoarthritic knee joints: Analysis of peripheral blood, synovial fluid and synovial membrane. Clin. Exp. Immunol. 2019, 195, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Mabey, T.; Honsawek, S. Cytokines as biochemical markers for knee osteoarthritis. World J. Orthop. 2015, 6, 95–105. [Google Scholar] [CrossRef]
- Botha-Scheepers, S.; Watt, I.; Slagboom, E.; Craen, A.J.; Meulenbelt, I.; Rosendaal, F.R. Innate production of tumour necrosis factor alpha and interleukin 10 is associated with radiological progression of knee osteoarthritis. Ann. Rheum. Dis. 2008, 67, 1165–1169. [Google Scholar] [CrossRef]
- Goekoop, R.J.; Kloppenburg, M.; Kroon, H.M.; Frolich, M.; Huizinga, T.W.; Westendorp, R.G. Low innate production of interleukin-1beta and interleukin-6 is associated with the absence of osteoarthritis in old age. Osteoarthr. Cartil. 2010, 18, 942–947. [Google Scholar] [CrossRef]
- Miller, R.E.; Miller, R.J.; Malfait, A.-M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef]
- Fukushima, K.; Inoue, G.; Fujimaki, H.; Uchida, K.; Miyagi, M.; Nagura, N.; Uchiyama, K.; Takahira, N.; Takaso, M. The cytokine expression in synovial membrane and the relationship with pain and pathological findings at hip arthroscopy. J. Exp. Orthop. 2017, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Heard, B.J.; Fritzler, M.J.; Wiley, J.P.; McAllister, J.; Martin, L.; El-Gabalawy, H.; Hart, D.A.; Frank, C.B.; Krawetz, R. Intraarticular and Systemic Inflammatory Profiles May Identify Patients with Osteoarthritis. J. Rheumatol. 2013, 40, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Deng, Y.; Tan, Y.; Qin, J.; Chen, L.B. Association between severity of knee osteoarthritis and serum and synovial fluid interleukin 17 concentrations. J. Int. Med. Res. 2014, 42, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Liu, Y.; Peng, H.; Meng, Z. Correlation of IL-17 Level in Synovia and Severity of Knee Osteoarthritis. Med. Sci. Monit. 2015, 21, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Abed, E.; Bouvard, B.; Martineau, X.; Jouzeau, J.-Y.; Reboul, P.; Lajeunesse, D. Elevated hepatocyte growth factor levels in osteoarthritis osteoblasts contribute to their altered response to bone morphogenetic protein-2 and reduced mineralization capacity. Bone 2015, 75, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Haywood, L.; McWilliams, D.F.; Pearson, C.I.; Gill, S.E.; Ganesan, A.; Wilson, D.; Walsh, D.A.; McWilliams, D. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003, 48, 2173–2177. [Google Scholar] [CrossRef]
- Nagao, M.; Hamilton, J.L.; Kc, R.; Berendsen, A.D.; Duan, X.; Cheong, C.W.; Li, X.; Im, H.-J.; Olsen, B.R. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci. Rep. 2017, 7, 13027. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Yang, P.; Tan, J.; Yuan, Z.; Meng, G.; Bi, L.; Liu, J. Expression profile of cytokines and chemokines in osteoarthritis patients: Proinflammatory roles for CXCL8 and CXCL11 to chondrocytes. Int. Immunopharmacol. 2016, 40, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, Z.H.; Song, Y.W. The interaction between CXCL10 and cytokines in chronic inflammatory arthritis. Autoimmun. Rev. 2013, 12, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Daghestani, H.N.; Kraus, V.B. Inflammatory biomarkers in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Ioan-Facsinay, A. Initiating pain in osteoarthritis (OA): Is it the mast cell? Osteoarthr. Cartil. 2018, 26, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Vangsness, C.T.; Burke, W.S.; Narvy, S.J.; Macphee, R.D.; Fedenko, A.N. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis—A pilot study. Bull. NYU Hosp. Jt. Dis. 2011, 69, 122–127. [Google Scholar]
- Pietschmann, P.; Gollob, E.; Brosch, S.; Hahn, P.; Kudlacek, S.; Willheim, M.; Woloszczuk, W.; Peterlik, M.; Tragl, K.H. The effect of age and gender on cytokine production by human peripheral blood mononuclear cells and markers of bone metabolism. Exp. Gerontol. 2003, 38, 1119–1127. [Google Scholar] [CrossRef]
- Pawlowska, J.; Mikosik, A.; Soroczynska-Cybula, M.; Jozwik, A.; Luczkiewicz, P.; Mazurkiewicz, S. Different distribution of CD4 and CD8 T cells in synovial membrane and peripheral blood of rheumatoid arthritis and osteoarthritis patients. Folia Histochem. Cytobiol. 2009, 47, 627–632. [Google Scholar] [CrossRef]
- Huch, K. Knee and ankle: Human joints with different susceptibility to osteoarthritis reveal different cartilage cellularity and matrix synthesis in vitro. Arch. Orthop. Trauma Surg. 2001, 121, 301–306. [Google Scholar] [CrossRef]
- Nelson, A.E.; Smith, M.W.; Golightly, Y.M.; Jordan, J.M. “Generalized osteoarthritis”: A systematic review. Semin. Arthritis Rheum. 2014, 43, 713–720. [Google Scholar] [CrossRef]
| Total Study Population | Hip OA | Knee OA | p-Value | |
|---|---|---|---|---|
| Number of patients (n) | 50 | 34 | 16 | |
| Gender Male/Female | M = 27 (54.0%) F = 23 (46.0%) | M = 18 (52.9%) F = 16 (47.1%) | M = 9 (56.2%) F = 7 (43.8%) | n.s. |
| Age at surgery (Years) Mean ± SD (range) | 66.0 ± 11.3 (38–88) | 63.2 ± 12.2 (38–88) | 71.9 ± 5.6 (62–83) | 0.0001 * |
| Operation side (n) (%) Right/Left | R = 29 (58.0%) L = 21 (42.0%) | R = 20 (58.8%) L = 14 (41.2%) | R = 9 (56.2%) L = 7 (43.8%) | n.s. |
| BMI (kg/m2) mean ± SD (range) | 27.2 ± 5.4 (16.9–43.2) | 26.0 ± 4.6 (16.9–36.0) | 29.7 ± 6.2 (19.8–43.2) | 0.022 * |
| Leukocytes (cells/nl) mean ± SD (range) | 7.0 ± 1.6 (4.1–10.1) | 7.1 ± 1.7 (4.1–10.1) | 6.8 ± 1.3 (5.0–10.1) | n.s. |
| C-reactive protein (mg/l) mean ± SD (range) | 3.6 ± 3.7 (2.0–17.8) | 3.5 ± 3.7 (2.0–17.8) | 3.8 ± 3.9 (2.0–16.5) | n.s. |
| ESR (mm/h) mean ± SD (range) | 13.6 ± 12.4 (1–77) | 13.4 ± 13.6 (1–77) | 14.0 ± 9.0 (2–30) | n.s. |
| K&L Score (n) (%) 3/4 | K&L 3 = 36 (72.0%) K&L 4 = 14 (28.0%) | K&L 3 = 22 (64.7%) K&L 4 = 12 (25.3%) | K&L 3 = 14 (87.5%) K&L 4 = 2 (12.5%) | n.s. |
| Hip OA | Knee OA | p-Value | |
|---|---|---|---|
| Number of patients (n) | 19 | 8 | |
| Sample volume (g) | 1.75 ± 0.17 (0.83–3.62) | 1.46 ± 0.64 (1.23–1.78) | 0.5755 |
| Total mononuclear cells (cells/mg) | 213.9 ± 118,3 (10.43–2321) | 827.4 ± 254.1 (11.55–1998) | 0,0662 |
| CD14+ macrophages (% of mononuclear cells) | 16.83 ± 3.55 (1.53–46.17) | 14.89 ± 3.39 (4.57–30.40) | 0.6878 |
| CD14+ macrophages (cells/mg) | 29.45 ± 15.88 (0.83–306.4) | 116.1 ± 48.42 (1.75–414.6) | 0.0293 * |
| CD4+ T cells (% of mononuclear cells) | 10.63 ± 3.11 (0.52–51.30) | 7.79 ± 2.47 (0.79–22.00) | 0.8966 |
| CD4+ T cells (cells/mg) | 21.37 ± 9.9 (0 – 165) | 80.88 ± 52.25 (1–440) | 0.1795 |
| CD8+ T cells (% of mononuclear cells) | 1.15 ± 0.21 (0.19–3.70) | 1.08 ± 0.33 (0.34–2.82) | 0.8966 |
| CD8+ T cells (cells/mg) | 2.59 ± 1.83 (0.16–35.13) | 7.88 ± 2.67 (0.07–20.52) | 0.0863 |
| CD19+ B cells (% of mononuclear cells) | 1.74 ± 0.41 (0.42–6.13) | 3.58 ± 1.43 (0.2–12.30) | 0.2332 |
| CD19+ B cells (cells/mg) | 9.14 ± 7.43 (0.05–142.3) | 40.79 ± 29.55 (0.23–245.8) | 0.0749 |
| CD16+ CD56+ NK cells (% of mononuclear cells) | 0.152 ± 0.093 (0.000–1.760) | 0.012 ± 0.005 (0.000–0.038) | 0.5042 |
| CD16+ CD56+ NK cells (cells/mg) | 0.132 ± 0.058 (0.000–0.910) | 0.071 ± 0.040 (0.000–0.280) | 0.9077 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grieshaber-Bouyer, R.; Kämmerer, T.; Rosshirt, N.; Nees, T.A.; Koniezke, P.; Tripel, E.; Schiltenwolf, M.; Kirsch, J.; Hagmann, S.; Moradi, B. Divergent Mononuclear Cell Participation and Cytokine Release Profiles Define Hip and Knee Osteoarthritis. J. Clin. Med. 2019, 8, 1631. https://doi.org/10.3390/jcm8101631
Grieshaber-Bouyer R, Kämmerer T, Rosshirt N, Nees TA, Koniezke P, Tripel E, Schiltenwolf M, Kirsch J, Hagmann S, Moradi B. Divergent Mononuclear Cell Participation and Cytokine Release Profiles Define Hip and Knee Osteoarthritis. Journal of Clinical Medicine. 2019; 8(10):1631. https://doi.org/10.3390/jcm8101631
Chicago/Turabian StyleGrieshaber-Bouyer, Ricardo, Till Kämmerer, Nils Rosshirt, Timo A. Nees, Philipp Koniezke, Elena Tripel, Marcus Schiltenwolf, Johannes Kirsch, Sébastien Hagmann, and Babak Moradi. 2019. "Divergent Mononuclear Cell Participation and Cytokine Release Profiles Define Hip and Knee Osteoarthritis" Journal of Clinical Medicine 8, no. 10: 1631. https://doi.org/10.3390/jcm8101631
APA StyleGrieshaber-Bouyer, R., Kämmerer, T., Rosshirt, N., Nees, T. A., Koniezke, P., Tripel, E., Schiltenwolf, M., Kirsch, J., Hagmann, S., & Moradi, B. (2019). Divergent Mononuclear Cell Participation and Cytokine Release Profiles Define Hip and Knee Osteoarthritis. Journal of Clinical Medicine, 8(10), 1631. https://doi.org/10.3390/jcm8101631






