Increased Systemic Malondialdehyde Levels and Decreased Mo/Co, Co/Fe2+ Ratios in Patients with Long-Term Dental Titanium Implants and Amalgams
Abstract
1. Introduction
1.1. Aim
1.2. Specific Aims
2. Materials and Methods
2.1. Inclusion Criteria: Size Sample
2.2. Exclusion Criteria
2.3. Thiobarbituric Acid Assay (TBARS): Malondialdehyde Levels (MDA) as an Index of Lipoperoxidation
2.4. Statistical Analysis
3. Results
3.1. Comparative Heavy Metals/Oligoelement Levels in Patients with Long-Term Dental Titanium Implants and/or Amalgams Alone
3.2. Molybdenum (Mo) and Vanadium (V) Levels Were Significantly Lower in Patients with Long-Term Dental Amalgam and Titanium Implants (A + I) Than in Those with Long-Term Dental Amalgams Alone (A) or Controls
3.3. Iron (Fe2+): Patients Who Have Long-Term Amalgams and Titanium Dental Implants (A + I) Have Significantly Higher Iron Levels than Those in with Long-Term Dental Amalgams Alone (A)
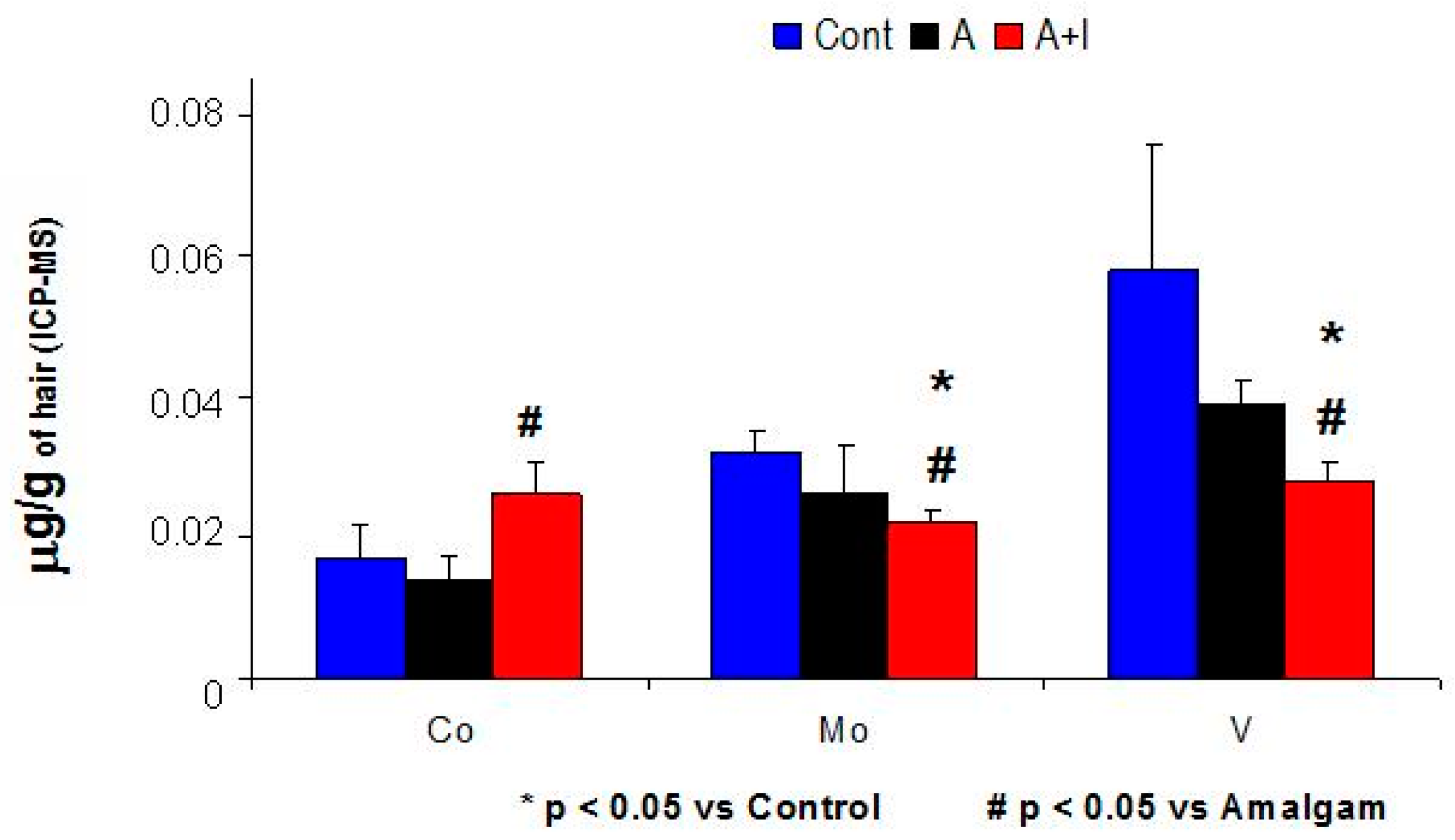
3.4. Low Molybdenum (Mo) Levels as well as Lower Mo/Co and Mo/Fe2+ Ratios in Patients Who Have Long-Term Dental Titanium Implants and Amalgams (A + I) than in Those with Long-Term Dental Amalgams Alone (A)
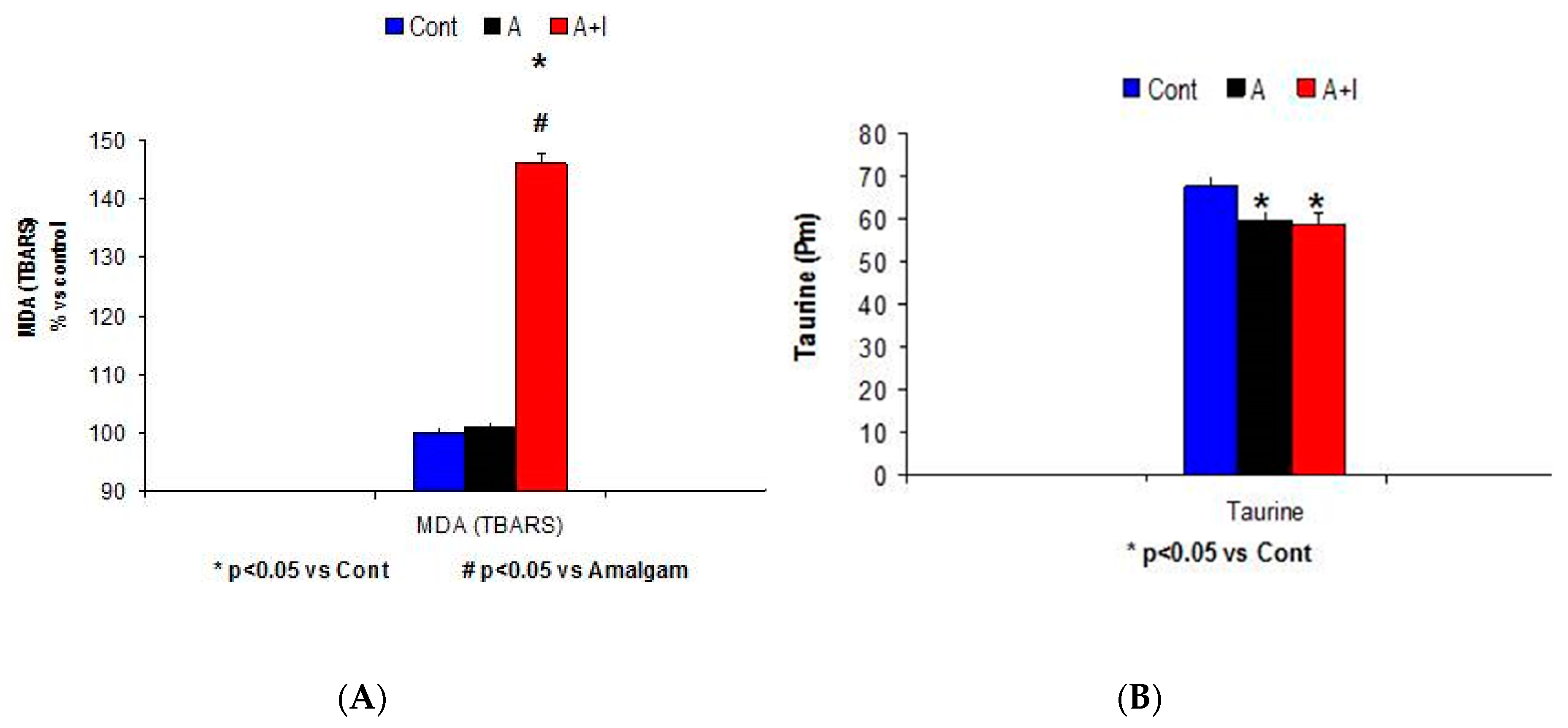
3.5. Systemic Malondialdehyde (MDA) Rises among Patients with Long-Term Dental Titanium Implants (A + I) as Compared to Those with Long-Term Dental Amalgams Alone (A)
Taurine Levels
3.6. Oligoelement Levels Found in Patients with Long-Term Titanium Implant (A + I) and/or Dental Amalgams alone (A) (Ca2+, Sr)
3.6.1. Calcium (Ca2+)
3.6.2. Strontium (Sr)
3.6.3. Sulfur (S)
3.7. Metals of Environmental Exposure
3.7.1. Barium (Ba): patients with Long-Term Titanium Dental Implant and Amalgams Have Significantly Higher Ba Levels than Those with Long-Term Dental Amalgams Alone
3.7.2. Cadmium (Cd), and Lead (Pb) Levels
3.8. Correlations between Certain Oligoelements (Ca2+, Mo, Fe2+, Co, V, Cr, Co) and Heavy Metal Levels (Hg2+) in Patients with Long-Term Titanium Implants and Dental Amalgams (A + I Group)
4. Discussion
The Rise in Cobalt (Co) and Nickel (Ni) in Hair of Patients with Long-Term Titanium Implants and Amalgam Fillings as Compared to Patients with Long-Term Amalgams Only
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shraim, A.; Alsuhaimi, A.; Al-Thakafy, J.T. Dental clinics: A point pollution source, not only of mercury but also of other amalgam constituents. Chemosphere 2011, 21, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, M.A.; Abbas, M.S. Mercury poisoning dentistry: High-level indoor air mercury contamination at selected dental sites. Rev. Environ. Health 2014, 29, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Puchyr, R.F.; Bass, D.A.; Gajewski, R.; Calvin, M.; Marquardt, W.; Urek, K.; Druyan, M.E.; Quig, D. Preparation of hair for measurement of elements by inductively coupled plasma-mass spectrometry (ICP-MS). Biol. Trace Elem. Res. 1998, 62, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Bravo-González, L.A.; Kyung, H.M.; Merino, J.J. Increased Zn/Glutathione Levels and Higher Superoxide Dismutase-1 Activity as Biomarkers of Oxidative Stress in Women with Long-Term Dental Amalgam Fillings: Correlation between Mercury/Aluminium Levels (in Hair) and Antioxidant Systems in Plasma. PLoS ONE 2015, 10, e0126339. [Google Scholar] [CrossRef] [PubMed]
- Mutter, J. Is dental amalgam safe for humans? The opinion of the scientific committee of the European Commission. J. Occup. Med. Toxicol. 2011, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Taut, C. 2013. Dental amalgam: Is this the end? J. Ir. Dent. Assoc. 2013, 59, 311–317. [Google Scholar] [PubMed]
- Homme, K.G.; Kern, J.K.; Haley, B.E.; Geier, D.A.; King, P.G.; Sykes, L.K. New science challenges old notion that mercury dental amalgam is safe. Biometals 2014, 27, 19–24. [Google Scholar] [CrossRef]
- Arinola, O.G. Evaluation of antioxidant levels and trace elements status in Nigerian cassava processors. Pak. J. Nutr. 2008, 7, 770–772. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Otewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Merk, V.M. The Merck Veterinary Manual. In A Handbook of Diagnosis, Theraphy and Disease Prevention and Control for the Veterinarian, 6th ed.; Merve and Co. Inc.: Rahway, NJ, USA, 1986. [Google Scholar]
- Cabaña Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Cabaña-Parmigiani, J.M.; Merino, J.J. Safe removal of dental amalgams by using nasal filters and phytoteraphy. IJSR Int. J. Sci. Res. 2015, 4, 2391–2395. [Google Scholar]
- Barrett, R.D.; Bishara, S.E.; Quinn, J.K. Biodegradation of orthodontic appliances. Part I. Biodegradation of nickel and chromium in vitro. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 8–14. [Google Scholar] [CrossRef]
- Lim, S.D.; Takada, Y.; Kim, K.H.; Okuno, O. Ions released from dental amalgams in contact with titanium. Dent. Mater. J. 2003, 22, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.; Tasat, D.; Duffo, G.; Cabrini, R.; Guglielmotti, M. Systemic and local tissue response to titanium corrosion. Book Chapter Intech. 2012. [Google Scholar] [CrossRef]
- Zaffe, D.; Bertoldi, C.; Consolo, U. Element release from titanium devices used in oral and maxillofacial surgery. Biomaterials 2003, 24, 1093–1099. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Trachtenberg, F.; Barregard, L.; Tavares, M.; Cernichiari, E.; Daniel, D. Neuropsychological and renal effects of dental amalgam in children: A randomized clinical trial. JAMA 2006, 295, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Lai, M.B.; Jandhyam, S.; Dukkande, V.V.; Bhushan, A.; Daniels, C.K. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. Int. J. Nanomed. 2008, 3, 533–545. [Google Scholar]
- Merino, J.J.; Arce, C.; Naddaf, A.; Bellver-Landete, V.; Oset-Gasque, M.J.; González, M.P. The nitric oxide donor SNAP-induced amino acid neurotransmitter release in cortical neurons: Effects of blockers of voltaje-dependent sodium and calcium channels. PLoS ONE 2014, 5, e90703. [Google Scholar] [CrossRef] [PubMed]
- Twari, V.; Chopra, K. Resveratrol prevents alcohol-induced cognitive deficits and brain damage by blocking inflammatory signaling and cell death cascade in neonatal brain. J. Neurochem. 2011, 1174, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.W.; Kretzer, J.P.; Reinders, J.; Streit, M.R.; Bruckner, T.; Gotterbarm, T.; Aldinger, P.R.; Merle, C. In vivo serum titanium ion levels following modular neck hip arthroplasty—10 year results in 67 patients. Acta Biomater. 2013, 9, 6278–6282. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T.; Yuko, K.; Sachiko, H.; Akiko, Y. Effects of Biological Factors on the Repassivation Current of Titanium. Mater. Trans. 2004, 45, 1635–1639. [Google Scholar] [CrossRef]
- Camacho Alonso, F.; Sánchez-Siles, M.; Gilbel-del Águila, O. No evidence of genotoxic damage in a group of patients with titanium dental implants and different metal restorations in the oral cavity. Clin. Implant. Dent. Relat. Res. 2015, 17, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Agaoglu, G.; Arun, T.; Izgu, B.; Yarat, A. Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod. 2001, 71, 375–379. [Google Scholar] [PubMed]
- Pigatto, P.D.; Minoia, C.; Ronchi, A.; Brambilla, L.; Ferrucci, S.M.; Spadari, F.; Passoni, M.; Somalvico, F.; Bombeccaria, G.P.; Guzzi, G. Allergological and Toxicological aspects in a multiple chemical sensitivity cohort. Oxid. Med. Cell Longev. 2013, 2013, 356235. [Google Scholar] [CrossRef] [PubMed]
- Björkman, L.; Lundekvam, B.F.; Laegreid, T.; Bertelsen, B.I.; Morild, I.; Lilleng, P.; Lind, B.; Palm, B.; Vahther, M. Mercury in human brain, blood, muscle and toenails in relation to exposure: An autopsy study. Environ. Health. 2007, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.C.; Ganser, M.A.; Warren, J.S.; Basu, N.; Wang, L.; Zick, S.M.; Park, S.K. Mercury Exposure and Antinuclear Antibodies among Females of Reproductive Age in the United States: NHANES. Environ. Health Perspect. 2015, 123, 792–798. [Google Scholar] [CrossRef]
- Fakour, H.; Esmaili-Sari, A.; Zayeri, F. Scalp hair and saliva as biomarkers in determination of mercury levels in Iranian women: Amalgam as a determinant of exposure. J. Hazard. Mater. 2010, 177, 109–113. [Google Scholar] [CrossRef]
- Babi, D.; Vasjari, M.; Celo, V.; Koroveshi, M. Some results on Hg content in hair in different populations in Albania. Sci. Total Environ. 2000, 259, 55–60. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Al-Sedairi, A.; Elkhatib, R. Effect of mercury (Hg) dental amalgam fillings on renal and oxidative stress biomarkers in children. Sci. Total Environ. 2012, 431, 88–96. [Google Scholar] [CrossRef]
- Geier, D.A.; Carmody, T.; Kern, J.K.; King, P.G.; Geier, M.R. A significant dose-dependent relationship between mercury exposure from dental amalgams and kidney integrity biomarkers: A further assessment. Hum. Exp. Toxicol. 2013, 32, 434–440. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleos. Nucleot. Nucl. 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Anjun, S.A.; Lawrence, H.; Deehan, D.J.; Tyson-Capper, A.J. Effect of cobalt mediated Toll-like receptor 4 activation on inflammatory response in endothelial cells. Oncotarget 2016, 7, 76471–76478. [Google Scholar]
- Food and Nutrition Board, Institute of Medicine. Molybdenum. Dietary Reference Intakes for Vitamin A, Vitamin K, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2011; pp. 420–441. [Google Scholar]
- Murray, R.K.; Granner, D.K.; Mayes, P.A.; Rodwell, W. Harper’s Biochemistry, 25th ed.; McGraw-Hill, Health Profession Division: New York, NY, USA, 2000. [Google Scholar]
- Pierson, R.E.; Aenes, W.A. Treatment of copper poisoning in sheep. J. Am. Med. Ass. 1958, 133, 307–311. [Google Scholar]
- Mendel, R.R. Metabolism of molybdenum. Met. Ions. Life Sci. 2013, 12, 503–528. [Google Scholar] [PubMed]
- Abumrad, N.N.; Schneider, A.J.; Steel, D.; Rogers, L.S. Amino acid intolerance during prolonged total parenteral nutrition reversed by molybdate theraphy. Am. J. Clin. Nutr. 1981, 34, 2551–2559. [Google Scholar] [CrossRef]
- Vyskocill, A.; Viau, C. Assessment of molybdenum toxicity in humans. J. Appl. Toxicol. 1999, 19, 185–192. [Google Scholar] [CrossRef]
- Conesa, H.M.; Faz, A.; Arnaldes, E. Heavy metal accumulation in plants from mine tailings of the semiarid Cartagena La Unión mining district (SE Spain). Sci. Total Environ. 2006, 366, 1–11. [Google Scholar] [CrossRef]
- European Commission. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A52000DC0001 (accessed on 7 January 2019).
- Mortazavi, G.; Mortazavi, S.M. Increased mercury release from dental amalgam restorations after exposure to electromagnetic fields as a potential hazard for hypersensitive people and pregnant women. Rev. Environ. Health 2015, 30, 287–292. [Google Scholar] [CrossRef]

| Metal (µg/g Hair) | Control (Cont) | Amalgams (A) | Amalgams+ Implants (A + I) | p-Value (H or F) |
|---|---|---|---|---|
| Hg2+ | 1.28 ± 0.51 | 2.72 ± 0.24 * | 2.67 ± 0.29 * | H = 8.4, p = 0.015 |
| Sn | 0.3 ± 0.18 | 0.9 ± 0.32 | 0.57 ± 0.19 | H = 1.84, p = 0.44; n.s. |
| Cu2+ | 29.1 ± 7.4 | 41.8 ± 12 | 29.1 ± 5.7 | H = 3.93, p = 0.14; n.s. |
| Zn2+ | 185 ± 9.3 | 211 ± 12.8 * | 217 ± 7.4 * | H = 5.8, p = 0.05 |
| Ti | 0.44 ± 0.037 | 0.47 ± 0.07 | 0.48 ± 0.065 | H = 1.02 p = 0.6; n.s. |
| Al | 2.08 ± 0.1 | 4.88 ± 0.82 * | 3.37 ± 0.4 * | F = 14.5, p < 0.001 |
| V | 0.058 ± 0.018 | 0.039 ± 0.0033 * | 0.028 ± 0.003 *,# | H = 11.63, p = 0.003 |
| Ni | 0.13 ± 0.06 | 0.159 ± 0.04 | 0.39 ± 0.18 *,# | H = 6.6, p = 0.037 |
| Co | 0.017 ± 0.0045 | 0.014 ± 0.0034 | 0.026 ± 0.0049 # | H = 13.9, p < 0.001 |
| Cr | 0.35 ± 0.13 | 0.35 ± 0.013 | 0.35 ± 0.01 | F = 0.12, p = 0.88; n.s. |
| Metal (µg/g Hair) | Control (Cont) | Amalgams (A) | Implants + Amalgams (A + I) | p |
|---|---|---|---|---|
| Mo | 0.032 ± 0.0033 | 0.026 ± 0.007 | 0.024 ± 0.002 *,# | H = 9.14, p = 0.01 |
| Fe2+ | 7.73 ± 0.58 | 7.42 ± 0.43 | 8.89 ± 0.5 # | H = 5.83, p = 0.05 |
| Mo/Hg2+ | 0.026 ± 0.0045 | 0.017 ± 0.0022 * | 0.016 ± 0.003 * | H = 11.9, p = 0.003 |
| Mo/Co | 5.13 ± 1.27 | 4.77 ± 0.59 | 2.17 ± 0.4 *,# | H = 10.89, p = 0.004 |
| Mo/Fe2+ | 0.048 ± 0.037 | 0.042 ± 0.0017 | 0.015 ± 0.002 *,# | H = 15.13, p < 0.001 |
| Metal (µg/g Hair) | Control (Cont) | Amalgams (A) | Implants + Amalgams (A + I) | p-Value |
|---|---|---|---|---|
| Ca2+ | 722 ± 142 | 1096 ± 191 | 1695 ± 233 *, # | H = 9.51, p = 0.009 |
| Sr | 8.7 ± 3.7 | 12 ± 3.3 | 17 ± 2.88 * | H = 6.09, p = 0.047 |
| S | 48088 ± 426 | 47151 ± 386 | 47684 ± 286 | H = 3.83, p = 0.14; n.s. |
| Mn2+ | 0.07 ± 0.06 | 0.094 ± 0.018 | 0.093 ± 0.007 | H = 0.12, p = 0.85; n.s. |
| Ge | 0.028 ± 0.0011 | 0.029 ± 0.0006 | 0.03 ± 0.0007 | H = 0.94, p = 0.62; n.s. |
| I | 1.03 ± 0.24 | 0.75 ± 0.16 | 0.87 ± 0.23 | H = 1.44, p = 0.48; n.s. |
| P | 189 ± 7.26 | 182.2 ± 7.03 | 179 ± 4.61 | H = 79, p = 0.5; n.s. |
| Metal (µg/g Hair) | Control (Cont) | Amalgams (A) | Implants + Amalgams (A + I) H (A + I, KW) | p-Value |
|---|---|---|---|---|
| Ba | 0.28 ± 0.04 | 0.57 ± 0.13 * | 0.92 ± 0.13 *,# | H = 15.77, p < 0.05 |
| Pb | 0.2 ± 0.066 | 0.29 ± 0.063 | 0.7 ± 0.23 | H = 1.07, p = 0.58; n.s. |
| Cd | 0.012 ± 0.0025 | 0.009 ± 0.0004 | 0.026 ± 0.015 | H = 3.18, p = 0.2; n.s. |
| Sb | 0.01 ± 0.0046 | 0.012 ± 0.0027 | 0.013 ± 0.001 | H = 0.81, p = 0.6; n.s. |
| As | 0.030 ± 0.0028 | 0.031 ± 0.0026 | 0.029 ± 0.004 | H = 1.6, p = 0.33; n.s. |
| Pt | 0.06 ± 0.0091 | 0.064 ± 0.0077 | 0.068 ± 0.0026 | p > 0.05, n.s. |
| Tl | 0.0010 ± 0.0006 | 0.0010 ± 0.0004 | 0.0011 ± 0.0006 | p > 0.05, n.s. |
| Th | 0.0011 ± 0.0045 | 0.0010 ± 0.0085 | 0.0011 ± 0.005 | p > 0.05, n.s. |
| U | 0.001 ± 0.0004 | 0.0011 ± 0.0008 | 0.0011 ± 0.0008 | p > 0.05, n.s. |
| Correlations r Pearson/Spearman p-Value | Mo/Co | Mo/Fe2+ |
|---|---|---|
| Mo/Hg2+ (A + I) | r = 0.45 p = 0.005 | r = 0.62 p = 0.000048 |
| Ca++ | Fe2+ | |
| Mo (A + I) | r = −0.37 p = 0.034 | r = −0.4 p = 0.025 |
| Mn++ | Cr | |
| Hg2+ (A + I) | r = −0.34 p = 0.048 | r = 0.49 p = 0.016 |
| Cr | Cu2+ | |
| V | r = 0.56 p = 0.004 | r = 0.49 p = 0.0034 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Camacho Alonso, F.; Merino, J.J. Increased Systemic Malondialdehyde Levels and Decreased Mo/Co, Co/Fe2+ Ratios in Patients with Long-Term Dental Titanium Implants and Amalgams. J. Clin. Med. 2019, 8, 86. https://doi.org/10.3390/jcm8010086
Cabaña-Muñoz ME, Parmigiani-Izquierdo JM, Camacho Alonso F, Merino JJ. Increased Systemic Malondialdehyde Levels and Decreased Mo/Co, Co/Fe2+ Ratios in Patients with Long-Term Dental Titanium Implants and Amalgams. Journal of Clinical Medicine. 2019; 8(1):86. https://doi.org/10.3390/jcm8010086
Chicago/Turabian StyleCabaña-Muñoz, María Eugenia, José María Parmigiani-Izquierdo, Fabio Camacho Alonso, and José Joaquín Merino. 2019. "Increased Systemic Malondialdehyde Levels and Decreased Mo/Co, Co/Fe2+ Ratios in Patients with Long-Term Dental Titanium Implants and Amalgams" Journal of Clinical Medicine 8, no. 1: 86. https://doi.org/10.3390/jcm8010086
APA StyleCabaña-Muñoz, M. E., Parmigiani-Izquierdo, J. M., Camacho Alonso, F., & Merino, J. J. (2019). Increased Systemic Malondialdehyde Levels and Decreased Mo/Co, Co/Fe2+ Ratios in Patients with Long-Term Dental Titanium Implants and Amalgams. Journal of Clinical Medicine, 8(1), 86. https://doi.org/10.3390/jcm8010086






