A Literature Review of Proton Beam Therapy for Prostate Cancer in Japan
Abstract
1. Introduction
2. Results
2.1. Treatment Planning
2.2. Acute Toxicity
2.3. Late Toxicity
2.4. Biochemical Relapse and Survival Rate
2.5. Passive Scattered Proton Therapy
2.6. Intensity Modulated Proton Therapy
2.7. Comparison between IMPT and PSPT
2.8. Target Positioning and Organs at Risk
3. Discussion
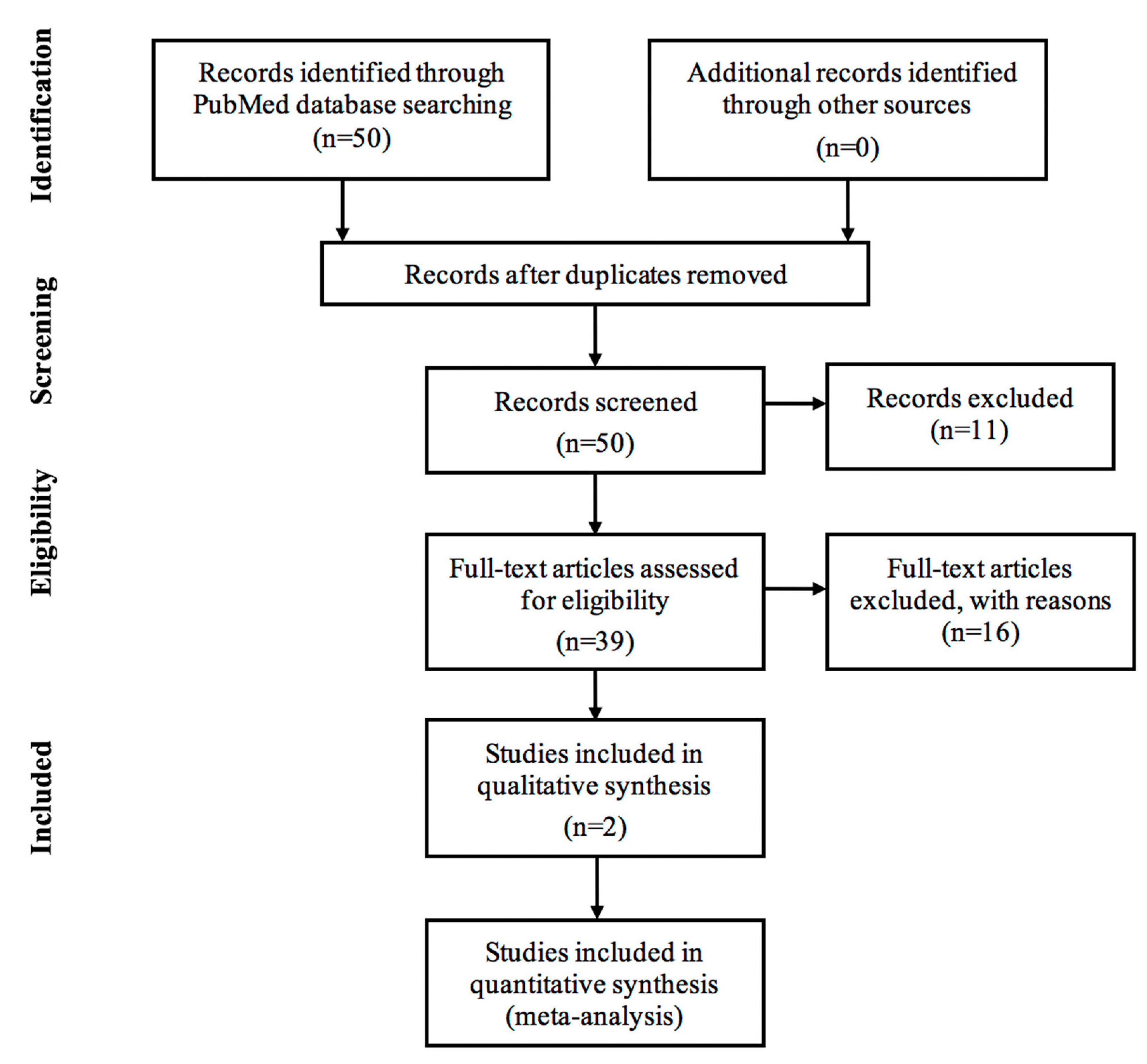
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Reasearch UK. World Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer (accessed on 21 June 2018).
- Ritch, C.R.; Punnen, S. Photodynamic therapy for low risk prostate cancer. BMJ 2017. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.A.C.; Navarro, F.B.G. Cancer de Prostata. SciELO 2016, 2, 279–284. [Google Scholar]
- Koupparis, A.; Gleave, M. Multimodal approaches to high-risk prostate cancer. Curr. Oncol. 2010, 17, S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Cancer Statistics in Japan; Table Download. Available online: https://ganjoho.jp/en/professional/statistics/table_download.html (accessed on 14 October 2018).
- World Population Review. Life Expectancy by Country 2017. Available online: http://worldpopulationreview.com/countries/life-expectancy-by-country/ (accessed on 28 July 2018).
- FIRST Program. Available online: http://www.jst.go.jp/first/en (accessed on 28 July 2018).
- Fiorino, C.; Sanguineti, G.; Cozzarini, C.; Fellin, G.; Foppiano, F.; Menegotti, L.; Piazzolla, A.; Vavassori, V.; Valdagni, R. Rectal dose-volume constraints in high-dose radiotherapy of localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 953–962. [Google Scholar] [CrossRef]
- Makishima, H.; Ishikawa, H.; Tanaka, K.; Mori, Y.; Mizumoto, M.; Ohnishi, K.; Aihara, T.; Fukumitsu, N.; Okumura, T.; Sakurai, H. A retrospective study of late adverse events in proton beam therapy for prostate cancer. Mol. Clin. Oncol. 2017, 7, 547–552. [Google Scholar] [CrossRef]
- Sakurai, H.; Ishikawa, H.; Okumura, T. Proton beam therapy in Japan: Current and future status. Jpn. J. Clin. Oncol. 2016, 46, 885–892. [Google Scholar] [CrossRef]
- Otake, T. April 1 marks start of Japan’s new medical fees and processes. Thejapantimes, 13 February 2018. [Google Scholar]
- Tian, X.; Liu, K.; Hou, Y.; Cheng, J.; Zhang, J. The evolution of proton beam therapy: Current and future status. Mol. Clin. Oncol. 2018, 8, 15–21. [Google Scholar] [CrossRef]
- Pugh, T.J.; Choi, S.; Nguyen, Q.N.; Gillin, M.T.; Zhu, X.R.; Palmer, M.B.; Lee, A.K. Proton beam therapy for the treatment of prostate cancer. Pract. Radiat. Oncol. 2013, 3, e87–e94. [Google Scholar] [CrossRef]
- Research Module 13: Treatment Volumes and Treatment Planning in Proton Therapy. Available online: https://www.oncolink.org/healthcare-professionals/oncolink-university/proton-therapy-professional-education/oncolink-proton-education-modules/module-13-treatment-volumes-and-treatment-planning-in-proton-therapy (accessed on 21 December 2018).
- Paganetti, H.; Jiang, H.; Parodi, K.; Slopsema, R.; Engelsman, M. Clinical implementation of full Monte Carlo dose calculation in proton beam therapy. Phys. Med. Biol. 2008, 53, 4825–4853. [Google Scholar] [CrossRef]
- Kohno, R.; Hotta, K.; Nishioka, S.; Matsubara, K.; Tansho, R.; Suzuki, T. Clinical implementation of a GPU-based simplified Monte Carlo method for a treatment planning system of proton beam therapy. Phys. Med. Biol. 2011, 56, N287–N294. [Google Scholar] [CrossRef]
- Nihei, K.; Ogino, T.; Onozawa, M.; Murayama, S.; Fuji, H.; Murakami, M.; Hishikawa, Y. Multi-institutional Phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Nihei, K.; Ogino, T.; Ishikura, S.; Kawashima, M.; Nishimura, H.; Arahira, S.; Onozawa, M. Phase II feasibility study of high-dose radiotherapy for prostate cancer using proton boost therapy: First clinical trial of proton beam therapy for prostate cancer in Japan. Jpn. J. Clin. Oncol. 2005, 35, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Khmelevsky, E.V.; Kancheli, I.N.; Khoroshkov, V.S.; Kaprin, A.D. Morbidity dynamics in proton–photon or photon radiation therapy for locally advanced prostate cancer. Rep. Pract. Oncol. Radiother. 2018, 23, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mayahara, H.; Murakami, M.; Kagawa, K.; Kawaguchi, A.; Oda, Y.; Miyawaki, D.; Sasaki, R.; Sugimura, K.; Hishikawa, Y. Acute morbidity of proton therapy for prostate cancer: The Hyogo Ion Beam Medical Center experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Kupelian, P.A.; Willoughby, T.R.; Reddy, C.A.; Klein, E.A.; Mahadevan, A. Hypofractionated Intensity-Modulated Radiotherapy (70 Gy at 2.5 Gy Per Fraction) for Localized Prostate Cancer: Cleveland Clinic Experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Cahlon, O.; Zelefsky, M.J.; Shippy, A.; Chan, H.; Fuks, Z.; Yamada, Y.; Hunt, M.; Greenstein, S.; Amols, H. Ultra-High Dose (86.4 Gy) IMRT for Localized Prostate Cancer: Toxicity and Biochemical Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Pei, X.; Yamada, J.; Kollmeier, M.A.; Cox, B.; Zelefsky, M.J. Long-term Survival and Toxicity in Patients Treated with High-Dose Intensity Modulated Radiation Therapy for Localized Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Iwata, H.; Ogino, H.; Hattori, Y.; Hashimoto, S.; Nakanishi, M.; Toshito, T.; Umemoto, Y.; Iwatsuki, S.; Shibamoto, Y.; et al. Acute toxicity of image-guided hypofractionated proton therapy for localized prostate cancer. Int. J. Clin. Oncol. 2018, 23, 353–360. [Google Scholar] [CrossRef]
- Takagi, M.; Demizu, Y.; Terashima, K.; Fujii, O.; Jin, D.; Niwa, Y.; Daimon, T.; Murakami, M.; Fuwa, N.; Okimoto, T. Long-term outcomes in patients treated with proton therapy for localized prostate cancer. Cancer Med. 2017, 6, 2234–2243. [Google Scholar] [CrossRef]
- Iwata, H.; Ishikawa, H.; Takagi, M.; Okimoto, T.; Murayama, S.; Akimoto, T.; Wada, H.; Arimura, T.; Sato, Y.; Araya, M.; et al. Long-term outcomes of proton therapy for prostate cancer in Japan: A multi-institutional survey of the Japanese Radiation Oncology Study Group. Cancer Med. 2018, 7, 677–689. [Google Scholar] [CrossRef]
- Arimura, T.; Yoshiura, T.; Matsukawa, K.; Kondo, N.; Kitano, I.; Ogino, T. Proton Beam Therapy Alone for Intermediate- or High-Risk Prostate Cancer: An Institutional Prospective Cohort Study. Cancers 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Dozono, H.; Yanazume, S.; Nakamura, H.; Etrych, T.; Chytil, P.; Ulbrich, K.; Fang, J.; Arimura, T.; Douchi, T.; Kobayashi, H.; et al. HPMA Copolymer-Conjugated Pirarubicin in Multimodal Treatment of a Patient with Stage IV Prostate Cancer and Extensive Lung and Bone Metastases. Target. Oncol. 2016, 11, 101–106. [Google Scholar] [CrossRef]
- Kase, Y.; Yamashita, H.; Fuji, H.; Yamamoto, Y.; Pu, Y.; Tsukishima, C.; Murayama, S. A treatment planning comparison of passive-scattering and intensity-modulated proton therapy for typical tumor sites. J. Radiat. Res. 2012, 53, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Hattori, Y.; Sugie, C.; Iwata, H.; Iwabuchi, M.; Shibamoto, Y. Comparison of multileaf collimator and conventional circular collimator systems in Cyberknife stereotactic radiotherapy. J. Radiat. Res. 2017, 58, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Kadoya, N.; Arai, K.; Takayama, Y.; Kato, T.; Kimura, K.; Ono, T.; Nakamura, T.; Wada, H.; Kikuchi, Y.; et al. Effect of DIR uncertainty on prostate passive-scattering proton therapy dose accumulation. Phys. Med. 2017, 39, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Maeda, K.; Sutherland, K.; Takayanagi, T.; Shimizu, S.; Takao, S.; Miyamoto, N.; Nihongi, H.; Toramatsu, C.; Nagamine, Y.; et al. Biological effect of dose distortion by fiducial markers in spot-scanning proton therapy with a limited number of fields: A simulation study. Med. Phys. 2012, 39, 5584–5591. [Google Scholar] [CrossRef] [PubMed]
- Fuji, H.; Murayama, S.; Niwakawa, M.; Yamaguchi, R.; Yamashita, R.; Matsui, T.; Yamashita, H.; Nishimura, T.; Tobisu, K. Changes in rectal volume and prostate localization due to placement of a rectum-emptying tube. Jpn. J. Radiol. 2009, 27, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Sato, Y.; Shibata, S.; Bou, S.; Yamamoto, K.; Tamamura, H.; Fuwa, N.; Takamatsu, S.; Sasaki, M.; Tameshige, Y.; et al. Effects of organ motion on proton prostate treatments, as determined from analysis of daily CT imaging for patient positioning. Med. Phys. 2018, 45, 1844–1856. [Google Scholar] [CrossRef]
- Maeda, Y.; Sato, Y.; Minami, H.; Yasukawa, Y.; Yamamoto, K.; Tamamura, H.; Shibata, S.; Bou, S.; Sasaki, M.; Tameshige, Y.; et al. Positioning accuracy and daily dose assessment for prostate cancer treatment using in-room CT image guidance at a proton therapy facility. Med. Phys. 2018, 45, 1832–1843. [Google Scholar] [CrossRef]
- Takamatsu, S.; Yamamoto, K.; Kawamura, M.; Sato, Y.; Asahi, S.; Kondou, T.; Tameshige, Y.; Maeda, Y.; Sasaki, M.; Kumano, T.; et al. Utility of an initial adaptive bladder volume control with ultrasonography for proton-beam irradiation for prostate cancer. Jpn. J. Radiol. 2014, 32, 618–622. [Google Scholar] [CrossRef]
- Singapore, N.C.C. New NCCS Building and Proton Therapy Center. Available online: https://www.nccs.com.sg (accessed on 29 June 2018).
- Shioyama, Y.; Tsuji, H.; Suefuji, H.; Sinoto, M.; Matsunobu, A.; Toyama, S.; Nakamura, K.; Kudo, S. Particle radiotherapy for prostate cancer. Int. J. Urol. 2015, 22, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kozak, K.R.; Smith, B.L.; Adams, J.; Kornmehl, E.; Katz, A.; Gadd, M.; Specht, M.; Hughes, K.; Gioioso, V.; Lu, H.M.; et al. Accelerated partial-breast irradiation using proton beams: Initial clinical experience. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Arimura, T.; Ogino, T.; Yoshiura, T.; Toi, Y.; Kawabata, M.; Chuman, I.; Wada, K.; Kondo, N.; Nagayama, S.; Hishikawa, Y. Effect of Film Dressing on Acute Radiation Dermatitis Secondary to Proton Beam Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 472–476. [Google Scholar] [CrossRef]
- Whaley, J.T.; Kirk, M.; Cengel, K.; McDonough, J.; Bekelman, J.; Christodouleas, J.P. Protective effect of transparent film dressing on proton therapy induced skin reactions. Radiat. Oncol. (Lond. Engl.) 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.M.; Purdy, J.A.; Winter, K.; Roach, M., 3rd; Vijayakumar, S.; Sandler, H.M.; Markoe, A.M.; Ritter, M.A.; Russell, K.J.; Sailer, S.; et al. Preliminary report of toxicity following 3D radiation therapy for prostate cancer on 3DOG/RTOG 9406. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 391–402. [Google Scholar] [CrossRef]
- Pollack, A.; Zagars, G.K. External beam radiotherapy dose response of prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 1011–1018. [Google Scholar] [CrossRef]
- Ryu, J.K.; Winter, K.; Michalski, J.M.; Purdy, J.A.; Markoe, A.M.; Earle, J.D.; Perez, C.A.; Roach, M., 3rd; Sandler, H.M.; Pollack, A.; et al. Interim report of toxicity from 3D conformal radiation therapy (3D-CRT) for prostate cancer on 3DOG/RTOG 9406, level III (79.2 Gy). Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1036–1046. [Google Scholar] [CrossRef]
- Slater, J.D.; Yonemoto, L.T.; Rossi, C.J., Jr.; Reyes-Molyneux, N.J.; Bush, D.A.; Antoine, J.E.; Loredo, L.N.; Schulte, R.W.; Teichman, S.L.; Slater, J.M. Conformal proton therapy for prostate carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 299–304. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Fuks, Z.; Hunt, M.; Lee, H.J.; Lombardi, D.; Ling, C.C.; Reuter, V.E.; Venkatraman, E.S.; Leibel, S.A. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J. Urol. 2001, 166, 876–881. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Fuks, Z.; Hunt, M.; Yamada, Y.; Marion, C.; Ling, C.C.; Amols, H.; Venkatraman, E.S.; Leibel, S.A. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1111–1116. [Google Scholar] [CrossRef]
- Hanks, G.E. External-beam radiation therapy for clinically localized prostate cancer: Patterns of care studies in the United States. NCI Monogr. 1988, 75–84. [Google Scholar]
- Pilepich, M.V.; Krall, J.M.; Sause, W.T.; Johnson, R.J.; Russ, H.H.; Hanks, G.E.; Perez, C.A.; Zinninger, M.; Martz, K.L. Prognostic factors in carcinoma of the prostate--analysis of RTOG study 75-06. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 339–349. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Ginor, R.X.; Fuks, Z.; Leibel, S.A. Efficacy of selective alpha-1 blocker therapy in the treatment of acute urinary symptoms during radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 567–570. [Google Scholar] [CrossRef]
- Valicenti, R.K.; Winter, K.; Cox, J.D.; Sandler, H.M.; Bosch, W.; Vijayakumar, S.; Michalski, J.; Purdy, J. RTOG 94-06: Is the addition of neoadjuvant hormonal therapy to dose-escalated 3D conformal radiation therapy for prostate cancer associated with treatment toxicity? Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 614–620. [Google Scholar] [CrossRef]
- Yoon, M.; Kim, D.; Shin, D.H.; Park, S.Y.; Lee, S.B.; Kim, D.Y.; Kim, J.Y.; Pyo, H.R.; Cho, K.H. Inter- and intrafractional movement-induced dose reduction of prostate target volume in proton beam treatment. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, N.P.; Hoppe, B.S.; Nichols, R.C.; Mendenhall, W.M.; Morris, C.G.; Li, Z.; Su, Z.; Williams, C.R.; Costa, J.; Henderson, R.H. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 596–602. [Google Scholar] [CrossRef]
- Mendenhall, N.P.; Li, Z.; Hoppe, B.S.; Marcus, R.B., Jr.; Mendenhall, W.M.; Nichols, R.C.; Morris, C.G.; Williams, C.R.; Costa, J.; Henderson, R. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 213–221. [Google Scholar] [CrossRef]
- Fujii, Y.; Matsuura, T.; Takao, S.; Matsuzaki, Y.; Fujii, T.; Miyamoto, N.; Umegaki, K.; Nishioka, K.; Shimizu, S.; Shirato, H. A simulation study on the dosimetric benefit of real-time motion compensation in spot-scanning proton therapy for prostate. J. Radiat. Res. 2017, 58, 591–597. [Google Scholar] [CrossRef]
- Antolak, J.A.; Rosen, I.I.; Childress, C.H.; Zagars, G.K.; Pollack, A. Prostate target volume variations during a course of radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 661–672. [Google Scholar] [CrossRef]
- Van Herk, M.; Bruce, A.; Kroes, A.P.; Shouman, T.; Touw, A.; Lebesque, J.V. Quantification of organ motion during conformal radiotherapy of the prostate by three dimensional image registration. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 1311–1320. [Google Scholar] [CrossRef]
- McGary, J.E.; Teh, B.S.; Butler, E.B.; Grant, W., 3rd. Prostate immobilization using a rectal balloon. J. Appl. Clin. Med. Phys. 2002, 3, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Wachter, S.; Gerstner, N.; Dorner, D.; Goldner, G.; Colotto, A.; Wambersie, A.; Potter, R. The influence of a rectal balloon tube as internal immobilization device on variations of volumes and dose-volume histograms during treatment course of conformal radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 91–100. [Google Scholar] [CrossRef]
- Van Lin, E.N.; van der Vight, L.P.; Witjes, J.A.; Huisman, H.J.; Leer, J.W.; Visser, A.G. The effect of an endorectal balloon and off-line correction on the interfraction systematic and random prostate position variations: A comparative study. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Wisenbaugh, E.S.; Andrews, P.E.; Ferrigni, R.G.; Schild, S.E.; Keole, S.R.; Wong, W.W.; Vora, S.A. Proton Beam Therapy for Localized Prostate Cancer 101: Basics, Controversies, and Facts. Rev. Urol. 2016, 16, 67–75. [Google Scholar]
- Mock, U.; Bogner, J.; Georg, D.; Auberger, T.; Potter, R. Comparative treatment planning on localized prostate carcinoma conformal photon- versus proton-based radiotherapy. Strahlentherapie und Onkologie 2005, 181, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.; Petersen, J.B.B.; Stokkevåg, C.H.; Ytre-Hauge, K.S.; Flampouri, S.; Li, Z.; Mendenhall, N.; Muren, L.P. Biological dose and complication probabilities for the rectum and bladder based on linear energy transfer distributions in spot scanning proton therapy of prostate cancer. Acta Oncol. 2017, 56, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Smith, T.L.; Henderson, R.H.; Hoppe, B.S.; Mendenhall, W.M.; Nichols, R.C.; Morris, C.G.; Williams, C.R.; Su, Z.; Li, Z.; et al. Five-Year Biochemical Results, Toxicity, and Patient-Reported Quality of Life After Delivery of Dose-Escalated Image Guided Proton Therapy for Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Lee, A.K.; Newhauser, W.D. Risk of secondary malignant neoplasms from proton therapy and intensity-modulated x-ray therapy for early-stage prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 616–622. [Google Scholar] [CrossRef]
- Kohno, R.; Takada, Y.; Sakae, T.; Terunuma, T.; Matsumoto, K.; Nohtomi, A.; Matsuda, H. Experimental evaluation of validity of simplified Monte Carlo method in proton dose calculations. Phys. Med. Biol. 2003, 48, 1277–1288. [Google Scholar] [CrossRef]
- Kohno, R.; Sakae, T.; Takada, Y.; Matsumoto, K.; Matsuda, H.; Nohtomi, A.; Terunuma, T.; Tsunashima, Y. Simplified Monte Carlo Dose Calculation for Therapeutic Proton Beams. Jpn. J. Appl. Phys. 2002, 41, L294–L297. [Google Scholar] [CrossRef]
- Yepes, P.P.; Mirkovic, D.; Taddei, P.J. A GPU implementation of a track-repeating algorithm for proton radiotherapy dose calculations. Phys. Med. Biol. 2010, 55, 7107–7120. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Kohno, R.; Takada, Y.; Hara, Y.; Tansho, R.; Himukai, T.; Kameoka, S.; Matsuura, T.; Nishio, T.; Ogino, T. Improved dose-calculation accuracy in proton treatment planning using a simplified Monte Carlo method verified with three-dimensional measurements in an anthropomorphic phantom. Phys. Med. Biol. 2010, 55, 3545–3556. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, C.M.; Gottschalk, A.R.; Lessard, E.; Nakamura, J.L.; Pinnaduwage, D.; Pouliot, J.; Sims, C.; Descovich, M. Investigating the clinical advantages of a robotic linac equipped with a multileaf collimator in the treatment of brain and prostate cancer patients. J. Appl. Clin. Med. Phys. 2015, 16, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Koch, N.C.; Fontenot, J.D.; Rosenthal, S.J.; Gombos, S.D.; Fitzek, M.M.; Mohan, R. Dosimetric impact of tantalum markers used in the treatment of uveal melanoma with proton beam therapy. Phys. Med. Biol. 2007, 52, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLos Med. 2009, 6, 7. [Google Scholar] [CrossRef] [PubMed]
| Study Type | Hospital | Year Pulished | Journal (Impact Factor) | Article Title | Author/s | Number of Patients | Treatment Planning Equipment (Proton Beam Irradiation Equipment) |
|---|---|---|---|---|---|---|---|
| Observational Study | |||||||
| Medipolis Proton Therapy and Research Center | 2018 | Cancers (5.326) | Proton Beam Therapy Alone for Intermediate- or High-Risk Prostate Cancer: An Institutional Prospective Cohort Study | Takeshi Arimura et al. | 218 | XiO-M CT based 3D treatment planning system, Elekta, Stockholm, Sweden | |
| Fukui Prefectural Hospital | 2018 | Medical Physics (2.617) | Effects of organ motion on proton prostate treatments, as determined from analysis of daily CT imaging for patient positioning | Yoshikazu Maeda et al. | 10 | XiO-N Proton Treatment Planning System (Elekta Corp., Stockholm, Sweden) | |
| Fukui Prefectural Hospital | 2018 | Medical Physics (2.617) | Positioning accuracy and daily dose assessment for prostate cancer treatment using in-room CT image guidance at a proton therapy facility | Yoshikazu Maeda et al. | 30 | XiO-N Proton Treatment Planning System (Elekta Corp., Stockholm, Sweden) | |
| National Cancer Center Hospital East, Shizuoka Cancer Center, Hyogo Ion Beam Medical Center, University of Tsukuba Faculty of Medicine, Southern TOHOKU Proton Therapy Center, Fukui Prefectural Hospital, Medipolis Proton Therapy and Research Center | 2018 | Cancer Medicine (3.362) | Long term outcomes of proton therapy for prostate cancer in Japan: a multi-institutional survey of the Japanese Radiation Oncology Study Group | Hiromitsu Iwata et al. | 1291 | FOCUS-M, CMS, St. Louis MO, Mitsubishi Electric Corp (Kobe, Japan) and VQA, Hitachi Ltd. (Tokyo, Japan), | |
| University of Tsukuba Department of Radiation Oncology and Proton Medical Research Center | 2017 | Molecular and Clinical Oncology | A retrospective study of late adverse events in proton beam therapy for prostate cancer | Hirokazu Makishima et al. | 93 | Hitachi 3D Treatment Planning System ver. 2.0, Hitachi Ltd. (Tokyo, Japan) | |
| Hyogo Ion Beam Medical Center | 2017 | Cancer Medicine (3.362) | Long-term outcomes in patients treated with proton therapy for localized prostate cancer | Masaru Takagi et al. | 1375 | Not Mentioned | |
| Southern Tohoku Proton Therapy Center | 2017 | Physica Medica (2.24) | Effect of DIR on prostate passive-scattering proton therapy dose accumulation | Yoshimoto Abe et al. | 10 | Proton Therapy System (Mitsubushi Electric, Kobe, Japan) Aquilion LB Treatment Planning CT simulation (Toshiba Medical Systems, Tokyo, Japan) Xio-M System PSPT treatment planning (Elekta, Stockholm, Sweden) | |
| Hokkaido University Hospital | 2017 | Journal of Radiation Research (2.031) | A simulation study on the dosimetric benefit of real-time motion compensation in spot-scanning proton therapy for prostate | Yusuke Fujii et al. | 9 | VQA, Hitachi Ltd. (Tokyo, Japan) | |
| Shizuoka Cancer Center | 2012 | Journal of Radiation Research (2.031) | A Treatment Planning Comparison of Passive Scattering and Intensity-Modulated Proton Therapy for Typical Tumor Sites | Yuki Kase et al. | 16 (4 PCa Patients) | Xio-M Elekta CMS Software (Elekta, Stockholm, Sweden and Mitsubishi Electric Corp., (Kobe, Japan) * | |
| National Cancer Center Hospital East, Shizuoka Cancer Center, Hyogo Ion Beam Medical Center | 2011 | International Journal of Radiation Oncology BiologyPhysics (5.554) | Multi-institutional Phase II Study of Proton Beam Therapy for Organ-confined Prostate Cancer Focusing on the Incidence of Late Rectal Toxicities | Keiji Nihei et al. | 151 | Not Mentioned | |
| Hyogo Ion Beam Medical Center | 2009 | International Journal of Radiation Oncology Biology Physics (5.554) | Physiologic Reactions after proton Beam Therapy in Patients with Prostate Cancer: Significance of Urinary Autoactivation | Masakazu Shimizu et al. | 59 | FOCUS-M, CMS Japan (Tokyo, Japan) and Mitsubishi Electric Corp. (Kobe, Japan) | |
| Hyogo Ion Beam Medical Center | 2007 | International Journal of Radiation Oncology Biology Physics (5.554) | Acute Morbidity of Proton Therapy for Prostate Cancer: The Hyogo Ion Beam Medical Center Experience | Hiroshi Mayahara et al. | 287 | FOCUS-M, CMS Japan (Tokyo, Japan) and Mitsubishi Electric Corp. (Kobe, Japan) | |
| National Cancer Center Hospital East | 2005 | Japanese Journal of Clinical Oncology (2.370) | Phase II Feasibility Study of High-Dose Radiotherapy for Prostate Cancer Using Proton Boost Therapy: First Clinical Trial of Proton Beam Therapy for Prostate Cancer in Japan | Keiji Nihei et al. | 30 | - | |
| Interventional Study | |||||||
| Nagoya City Hospital | 2018 | International Journal of Clinical Oncology (2.61) | Acute toxicity of image-guided hypofractionated proton therapy for localized prostate cancer | Koichiro Nakajima et al. | 526 | VQA Hitachi (PROBEAT III: Hitachi Ltd., Tokyo, Japan) | |
| Nagoya Proton Therapy Center | 2017 | Journal of Radiation Research (2.013) | Comparison of multileaf collimator and conventional circular collimator systems in Cyberknife stereostatic radiotherapy | Taro Murai et al. | 10 | Multiplan treatment planning system ver 5.1 (Accuray Inc., Sunnyvale, CA, USA) | |
| Medipolis Proton Therapy and Research Center | 2016 | International Journal of Radiation Oncology Biology Physics (5.554) | Effects of Film Dressing on Acute Radiation Dermatitis Secondary to Proton Beam Therapy | Takeshi Arimura et al. | 271 | Not Mentioned | |
| Medipolis Proton Therapy and Reseach Center | 2015 | Targeted Oncology (3.877) | HPMA Copolymer-Conjugated Pirarubicin in Multimodal Treatment of a Patient with Stage IV Prostate Cancer and Extensive Lung and Bone Metastases | Haruhiko Dozono et al. | 1 | XiO-M: CMS and Mitsubishi Electric Corp. (Tokyo, Japan) | |
| Fukui Prefectural Hospital | 2014 | Japanese Journal of Radiology (1.044) | Utility an initial adaptive bladder volume control with ultrasonography for proton-beam irradiation for prostate | Shigeyuki Takamatsu et al. | 75 | XiO-N: ELEKTA, Stockholm, Sweden & Mitsubishi Electric Corp., Kobe, Japan | |
| Hokkaido University Hospital | 2012 | Medical Physics (2.617) | Biological effect of dose distortion by fiducial markers in spot scanning proton therapy with a limited number of fields: A simulation study | Taeko Matsuura et al. | 10 | Hitachi (Hitachi Ltd., Tokyo, Japan) | |
| National Cancer Center Hospital East | 2011 | Physics in Medicine and Biology (2.811) | Clinical implementation of a GPU-based simplified Monte Carlo method for a treatment planning system of proton beam therapy | R Kohno et al. | 4 | Not Mentioned | |
| Shizuoka Cancer Center | 2009 | Japanese Journal of Radiology (1.044) | Changes in rectal volume and prostate localization due to placement of a rectum-emptying tube | Hiroshi Fuji et al. | 21 | Asterion Treatment planning system (Toshiba Medical Systems, Tokyo, Japan) XiO-M (Mitsubishi Electric Corp., Tokyo, Japan) | |
| Secondary Data Analysis | |||||||
| 2016 | Japanese Journal of Clinical Oncology (2.370) | Proton beam therapy in Japan: current and future status | Hideyaki Sakurai et al. | N/A | |||
| 2015 | International Journal of Urology (1.884) | Particle radiotherapy for prostate cancer | Yoshiyuki Shioyama et al. | N/A |
| Author | Year | EBRT | Patients | Dose | Acute Toxicity | Late Toxicity | 5-Year Biochemical Relapse Free Survival | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (GyE) | Genitourinary (Number) | Gastrointestinal | Genitourinary | Gastrointestinal | |||||||||||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Low Risk | Intermediate Risk | High Risk | |||||
| Nihei et al. [18] | 2005 | Photon + Proton | 30 | 50Gy + 26 | 66.7% (20) | 13.3% (4) | NR | 56.7% (17) | 0% | 0% | 6.67% (2) | 10% (3) | NR | 26.67% (8) | 10% (3) | NR | NR | ||
| Mayahara et al. [20] | 2007 | Proton | 287 | 60 | 54% (154) | 39% (111) | 1.1% (4) | NR | NR | NR | NR | ||||||||
| Kupelian et al. [21] | 2007 | IMRT | 770 | 70 | NR | NR | NR | 7% (36) | NR | NR | 6% (30) | NR | 94%% | 83% | 72% | ||||
| Cahlon et al. [22] | 2008 | IMRT | 478 | 86.4 | NR | 22% (105) | 0.6% (3) | NR | 8%(37) | 0% | NR | 13% (60) | <3% (12) | NR | 3% (16) | <1% (2) | 98% | 85% | 70% |
| Nihei et al. [17] | 2011 | Proton | 151 | 74 | NR | 12.7% (9) | 0% | NR | NR | 6% | 0% | NR | NR | ||||||
| Spratt et al. [23] | 2013 | IMRT | 1002 | 86.4 | NR | NR | NR | 21.1% (211) | 2.2%(22) | NR | 4.4% (44) | 0.7% (7) | 98.8% * | 85.6 * | 67.9 * | ||||
| Nakajima et al. [24] | 2017 | Proton | 526 | ||||||||||||||||
| CPT | 254 | 74 (LR) | NR | 15% (38) | NR | 0.8% (2) | NR | NR | NR | NR | NR | ||||||||
| 78 (HR) | |||||||||||||||||||
| HFPT | 272 | 60 (LR) | 5.9% (16) | 0.7% (2) | NR | NR | NR | ||||||||||||
| 63 (HR) | |||||||||||||||||||
| Takagi et al. [25] | 2017 | Proton | 1375 | 74 | NR | NR | 8.7% (119) | 2.4% (33) | 0.07% (1) | 6% (82) | 4% (53) | 0.07% (1) | 99% | 91% | 86% | ||||
| Makishima et al. [9] | 2017 | Proton | 93 | 74 (LR) | NR | NR | 0% | 5.4% (5) | 1.08% (1) | NR | 4.3% (4) | NR | NR | 99% | NR | ||||
| 78 (IR, HR) | |||||||||||||||||||
| Iwata et al. [26] | 2018 | Proton | 1291 | 70-80 | NR | NR | 4.1% (53) | 0.5% (6) | 4.0% (52) | 0.3% (4) | 97% | 91.10% | 83.10% | ||||||
| Arimura et al. [27] | 2018 | Proton | 218 | 70 | 13.1%% | 0% | 0.00% | NR | 97% | 83% | |||||||||
| 74 | NR | 27.90% | NR | NR | NR | 2.3% (5) | NR | NR | 2.30% | NR | |||||||||
| 78 | 28.10% | 10.5% (23) | 10.50% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshina, R.M.; Matsuura, T.; Umegaki, K.; Shimizu, S. A Literature Review of Proton Beam Therapy for Prostate Cancer in Japan. J. Clin. Med. 2019, 8, 48. https://doi.org/10.3390/jcm8010048
Hoshina RM, Matsuura T, Umegaki K, Shimizu S. A Literature Review of Proton Beam Therapy for Prostate Cancer in Japan. Journal of Clinical Medicine. 2019; 8(1):48. https://doi.org/10.3390/jcm8010048
Chicago/Turabian StyleHoshina, Rika Maglente, Taeko Matsuura, Kikuo Umegaki, and Shinichi Shimizu. 2019. "A Literature Review of Proton Beam Therapy for Prostate Cancer in Japan" Journal of Clinical Medicine 8, no. 1: 48. https://doi.org/10.3390/jcm8010048
APA StyleHoshina, R. M., Matsuura, T., Umegaki, K., & Shimizu, S. (2019). A Literature Review of Proton Beam Therapy for Prostate Cancer in Japan. Journal of Clinical Medicine, 8(1), 48. https://doi.org/10.3390/jcm8010048





