Pirfenidone, an Anti-Fibrotic Drug, Suppresses the Growth of Human Prostate Cancer Cells by Inducing G1 Cell Cycle Arrest
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Cell Cycle Analysis
2.5. Apoptosis Assay
2.6. ELISA
2.7. Preparation of Cell Lysates
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
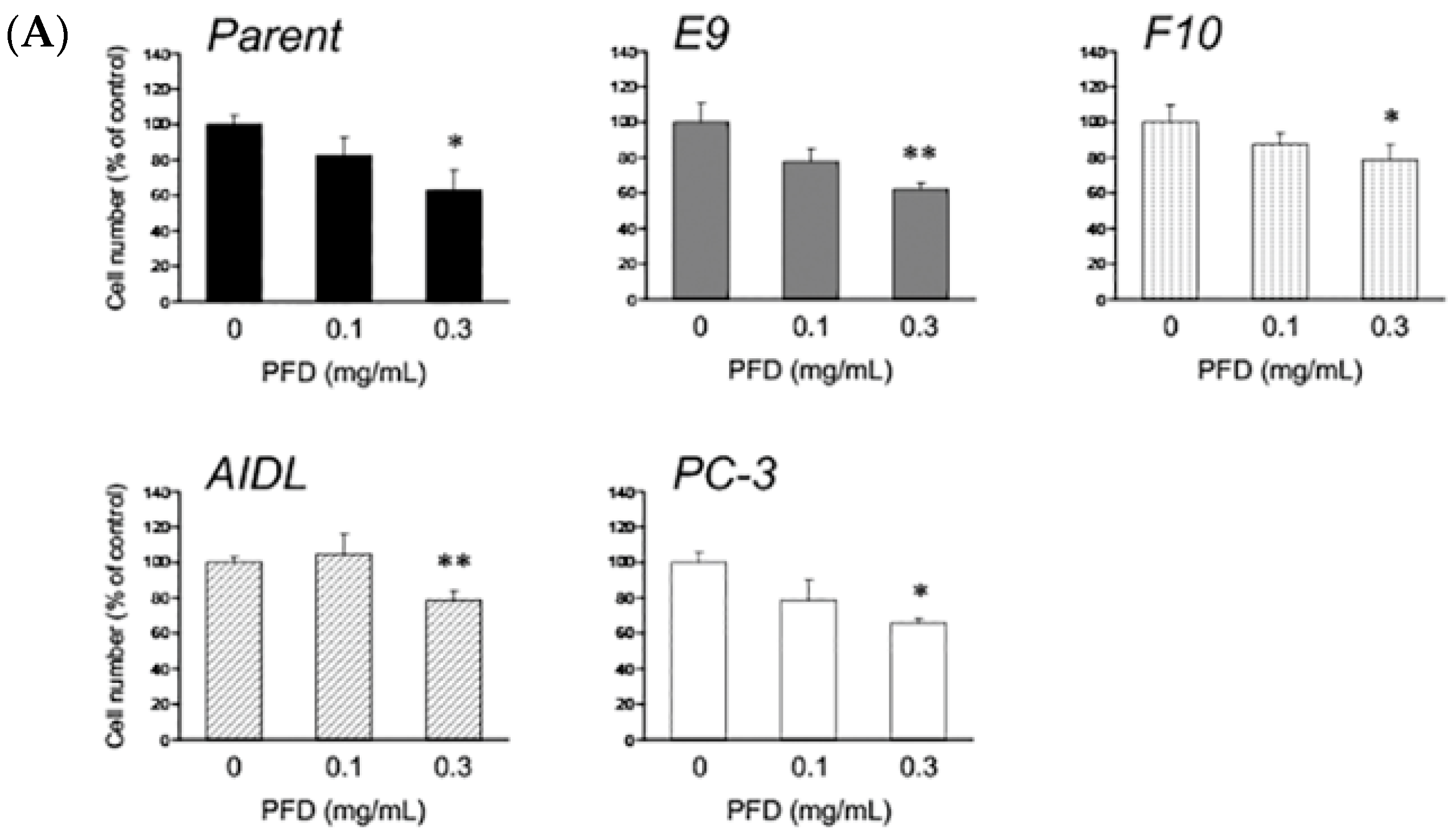
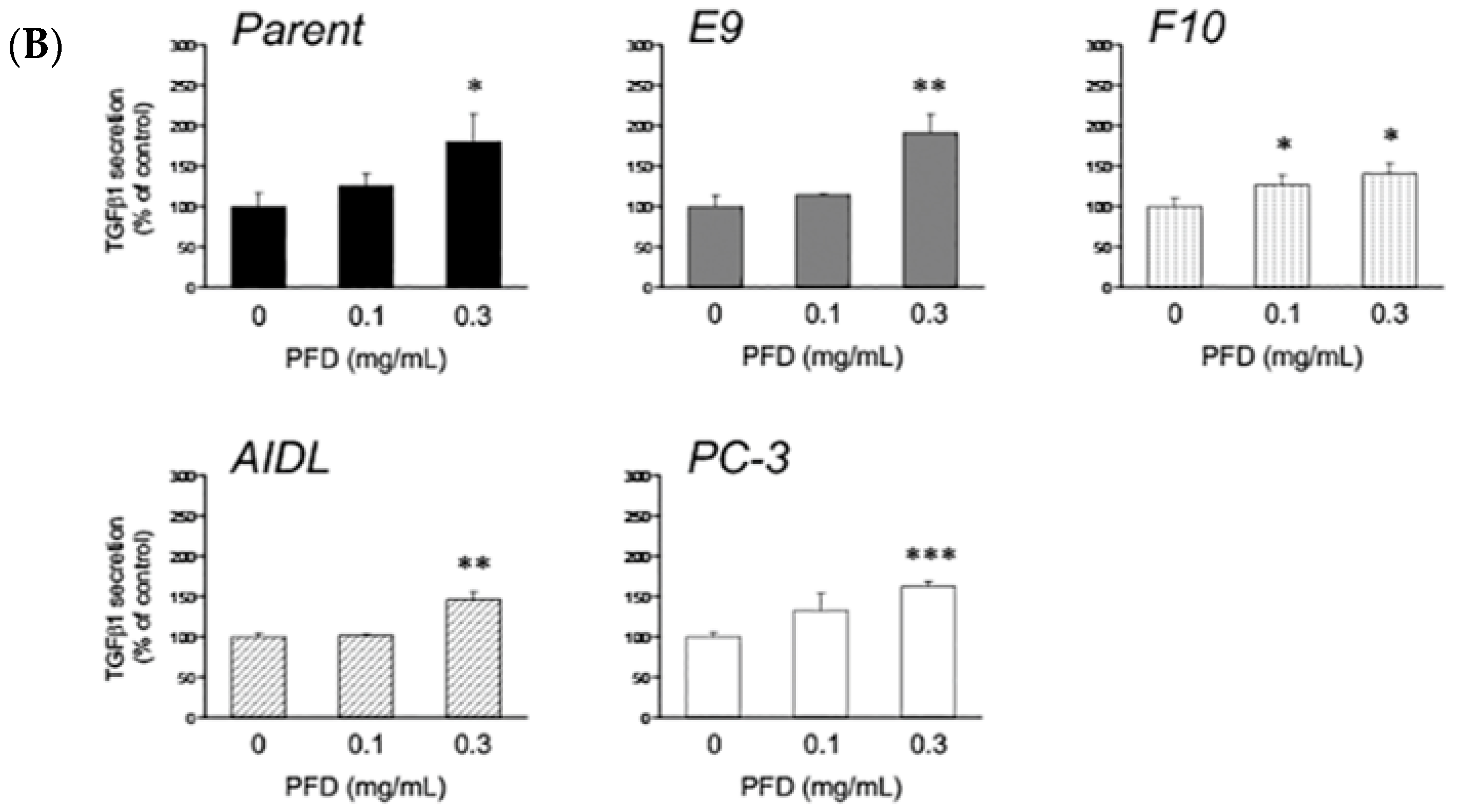
3.1. Effects of Pirfenidone Treatment on the Growth of Prostate Cancer Cells (LNCaP, LNCaP Sublines, and PC-3)
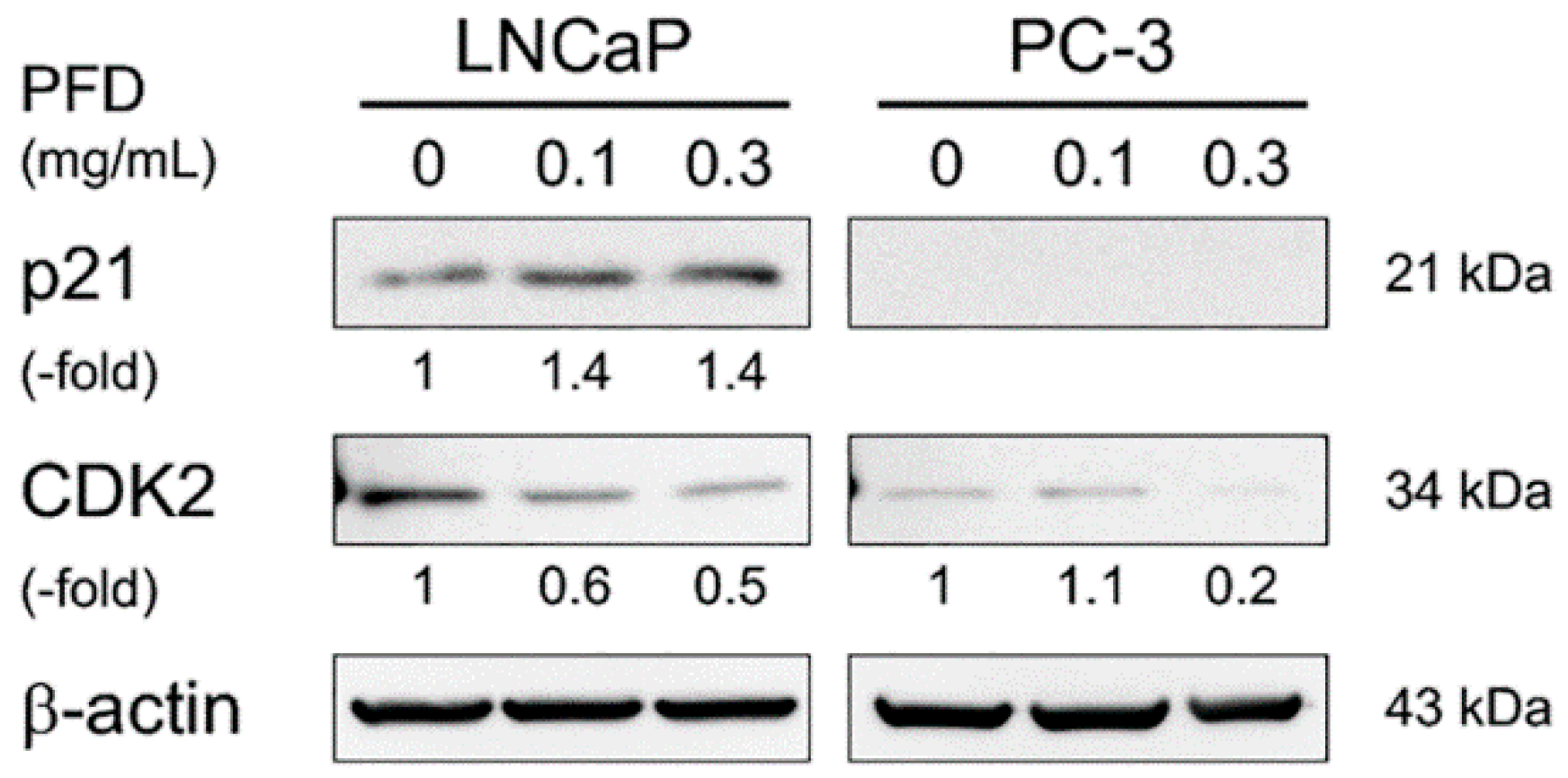
3.2. Pirfenidone Antiproliferative Mechanisms in LNCaP and PC-3 Cells
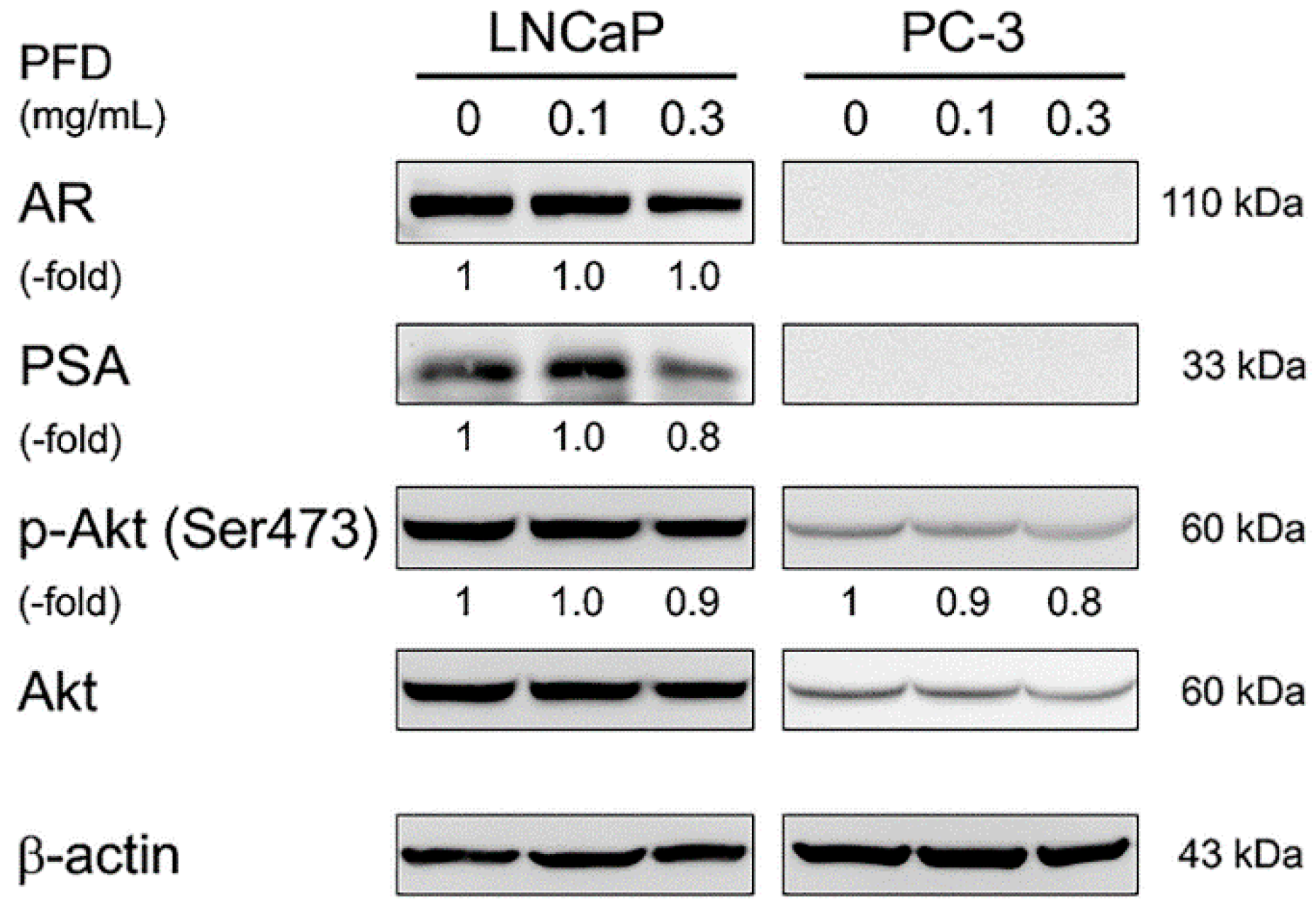
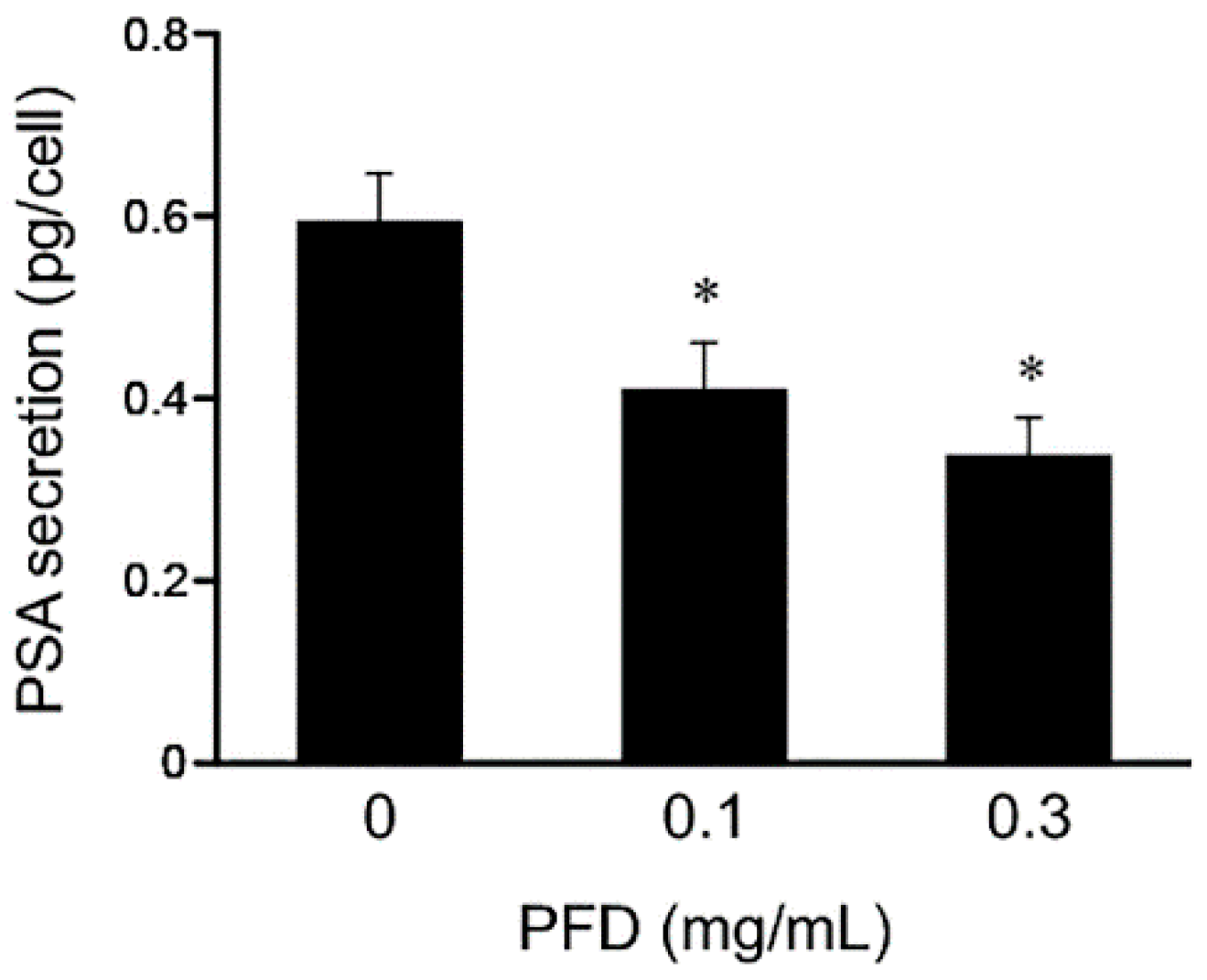
3.3. Effects of Pirfenidone Treatment on Androgen Receptor Signaling-Related Protein Levels in LNCaP and PC-3 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gronberg, H. Prostate cancer epidemiology. Lancet 2003, 361, 859–864. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J. Urol. 2002, 168, 9–12. [Google Scholar] [CrossRef]
- Fizazi, K.; Higano, C.S.; Nelson, J.B.; Gleave, M.; Miller, K.; Morris, T.; Nathan, F.E.; McIntosh, S.; Pemberton, K.; Moul, J.W. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2013, 31, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, D.; Omlin, A.; Pezaro, C.; Shamseddine, A.; de Bono, J. Metastatic castration-resistant prostate cancer (CRPC): Preclinical and clinical evidence for the sequential use of novel therapeutics. Cancer Metast. Rev. 2014, 33, 555–566. [Google Scholar] [CrossRef]
- Nevedomskaya, E.; Baumgart, S.J.; Haendler, B. Recent Advances in Prostate Cancer Treatment and Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1359. [Google Scholar] [CrossRef]
- Shim, J.S.; Liu, J.O. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int. J. Biol. Sci. 2014, 10, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Hisatake, J.I.; Ikezoe, T.; Carey, M.; Holden, S.; Tomoyasu, S.; Koeffler, H.P. Down-Regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor gamma in human prostate cancer. Cancer Res. 2000, 60, 5494–5498. [Google Scholar]
- Uemura, H.; Ishiguro, H.; Nakaigawa, N.; Nagashima, Y.; Miyoshi, Y.; Fujinami, K.; Sakaguchi, A.; Kubota, Y. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: A possibility of tyrosine kinase inhibitor of growth factor. Mol. Cancer Ther. 2003, 2, 1139–1147. [Google Scholar] [PubMed]
- Kanda, H.; Ishii, K.; Ogura, Y.; Imamura, T.; Kanai, M.; Arima, K.; Sugimura, Y. Naftopidil, a selective alpha-1 adrenoceptor antagonist, inhibits growth of human prostate cancer cells by G1 cell cycle arrest. Int. J. Cancer 2008, 122, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Mizokami, A.; Li, Y.Q.; Narimoto, K.; Sugimoto, K.; Kadono, Y.; Kitagawa, Y.; Konaka, H.; Koh, E.; Keller, E.T.; et al. Tranilast inhibits hormone refractory prostate cancer cell proliferation and suppresses transforming growth factor beta1-associated osteoblastic changes. Prostate 2009, 69, 1222–1234. [Google Scholar] [CrossRef]
- Olgen, S.; Kotra, L. Drug Repurposing in the Development of Anticancer Agents. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Selman, M. Nintedanib and pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am. J. Respir. Crit. Care Med. 2015, 191, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Koli, K.; Myllarniemi, M.; Vuorinen, K.; Salmenkivi, K.; Ryynanen, M.J.; Kinnula, V.L.; Keski-Oja, J. Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am. J. Pathol. 2006, 169, 61–71. [Google Scholar] [CrossRef]
- Xiang, X.H.; Jiang, T.P.; Zhang, S.; Song, J.; Li, X.; Yang, J.Y.; Zhou, S. Pirfenidone inhibits proliferation, arrests the cell cycle, and downregulates heat shock protein-47 and collagen type I in rat hepatic stellate cells in vitro. Mol. Med. Rep. 2015, 12, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yu, M.; Wu, K.; Yuan, H.; Zhong, H. Effects of pirfenidone on proliferation, migration, and collagen contraction of human Tenon’s fibroblasts in vitro. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3763–3770. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Y.; Liu, L.; Liu, X.; Xu, J.; Wu, K.; Yu, M. Pirfenidone Induces G1 Arrest in Human Tenon’s Fibroblasts In Vitro Involving AKT and MAPK Signaling Pathways. J. Ocul. Pharmacol. Ther. 2017, 33, 366–374. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.; Lin, X.; Wu, K.; Yu, M. Inhibition of pirfenidone on TGF-β2 induced proliferation, migration and epithlial-mesenchymal transition of human lens epithelial cells line SRA01/04. PLoS ONE 2013, 8, e56837. [Google Scholar] [CrossRef]
- Zou, W.J.; Huang, Z.; Jiang, T.P.; Shen, Y.P.; Zhao, A.S.; Zhou, S.; Zhang, S. Pirfenidone Inhibits Proliferation and Promotes Apoptosis of Hepatocellular Carcinoma Cells by Inhibiting the Wnt/beta-Catenin Signaling Pathway. Med. Sci. Monit. 2017, 23, 6107–6113. [Google Scholar] [CrossRef]
- Miura, Y.; Saito, T.; Tanaka, T.; Takoi, H.; Yatagai, Y.; Inomata, M.; Nei, T.; Saito, Y.; Gemma, A.; Azuma, A. Reduced incidence of lung cancer in patients with idiopathic pulmonary fibrosis treated with pirfenidone. Respir. Investig. 2018, 56, 72–79. [Google Scholar] [CrossRef]
- Ishii, K.; Imamura, T.; Iguchi, K.; Arase, S.; Yoshio, Y.; Arima, K.; Hirano, K.; Sugimura, Y. Evidence that androgen-independent stromal growth factor signals promote androgen-insensitive prostate cancer cell growth in vivo. Endocr. Relat. Cancer 2009, 16, 415–428. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Chu, T.M.; Wajsman, Z.L.; Friedman, M.; Papsidero, L.; Kim, U.; Chai, L.S.; Kakati, S.; Arya, S.K.; et al. The LNCaP cell line—A new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 1980, 37, 115–132. [Google Scholar] [PubMed]
- Iguchi, K.; Ishii, K.; Nakano, T.; Otsuka, T.; Usui, S.; Sugimura, Y.; Hirano, K. Isolation and characterization of LNCaP sublines differing in hormone sensitivity. J. Androl. 2007, 28, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Hayakawa, Y.; Ishii, K.; Matsumoto, K.; Usui, S.; Sugimura, Y.; Hirano, K. Characterization of the low pH/low nutrient-resistant LNCaP cell subline LNCaP-F10. Oncol. Rep. 2012, 28, 2009–2015. [Google Scholar] [CrossRef]
- Onishi, T.; Yamakawa, K.; Franco, O.E.; Kawamura, J.; Watanabe, M.; Shiraishi, T.; Kitazawa, S. Mitogen-activated protein kinase pathway is involved in alpha6 integrin gene expression in androgen-independent prostate cancer cells: Role of proximal Sp1 consensus sequence. Biochim. Biophys. Acta 2001, 1538, 218–227. [Google Scholar] [CrossRef]
- Ishii, K.; Sasaki, T.; Iguchi, K.; Kajiwara, S.; Kato, M.; Kanda, H.; Hirokawa, Y.; Arima, K.; Mizokami, A.; Sugimura, Y. Interleukin-6 induces VEGF secretion from prostate cancer cells in a manner independent of androgen receptor activation. Prostate 2018, 78, 849–856. [Google Scholar] [CrossRef]
- Ishii, K.; Matsuoka, I.; Kajiwara, S.; Sasaki, T.; Miki, M.; Kato, M.; Kanda, H.; Arima, K.; Shiraishi, T.; Sugimura, Y. Additive naftopidil treatment synergizes docetaxel-induced apoptosis in human prostate cancer cells. J. Cancer Res. Clin. Oncol. 2018, 144, 89–98. [Google Scholar] [CrossRef]
- Thomas, B.J.; Kan, O.K.; Loveland, K.L.; Elias, J.A.; Bardin, P.G. In the Shadow of Fibrosis: Innate Immune Suppression Mediated by Transforming Growth Factor-beta. Am. J. Respir. Cell Mol. Biol. 2016, 55, 759–766. [Google Scholar] [CrossRef]
- Conte, E.; Gili, E.; Fagone, E.; Fruciano, M.; Iemmolo, M.; Vancheri, C. Effect of pirfenidone on proliferation, TGF-beta-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur. J. Pharm. Sci. 2014, 58, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kozono, S.; Ohuchida, K.; Eguchi, D.; Ikenaga, N.; Fujiwara, K.; Cui, L.; Mizumoto, K.; Tanaka, M. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013, 73, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Kelynack, K.J.; Tait, M.G.; Martic, M.; Jones, C.L.; Margolin, S.B.; Becker, G.J. Pirfenidone reduces in vitro rat renal fibroblast activation and mitogenesis. J. Nephrol. 2001, 14, 453–460. [Google Scholar] [PubMed]
- Shi, Q.; Liu, X.; Bai, Y.; Cui, C.; Li, J.; Li, Y.; Hu, S.; Wei, Y. In vitro effects of pirfenidone on cardiac fibroblasts: Proliferation, myofibroblast differentiation, migration and cytokine secretion. PLoS ONE 2011, 6, e28134. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Katsuno, Y.; Koinuma, D.; Ehata, S.; Morikawa, M. Intracellular and extracellular TGF-beta signaling in cancer: Some recent topics. Front. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.M.; Massague, J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A Two-Faced Genome Guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef]
- Lin, H.K.; Hu, Y.C.; Yang, L.; Altuwaijri, S.; Chen, Y.T.; Kang, H.Y.; Chang, C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J. Biol. Chem. 2003, 278, 50902–50907. [Google Scholar] [CrossRef]
- Iguchi, K.; Fukami, K.; Ishii, K.; Otsuka, T.; Usui, S.; Sugimura, Y.; Hirano, K. Low androgen sensitivity is associated with low levels of Akt phosphorylation in LNCaP-E9 cells. J. Androl. 2012, 33, 660–666. [Google Scholar] [CrossRef]
- Nakanishi, H.; Kaibori, M.; Teshima, S.; Yoshida, H.; Kwon, A.H.; Kamiyama, Y.; Nishizawa, M.; Ito, S.; Okumura, T. Pirfenidone inhibits the induction of iNOS stimulated by interleukin-1beta at a step of NF-kappaB DNA binding in hepatocytes. J. Hepatol. 2004, 41, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.S.; Down, L.; Davey Smith, G.; Donovan, J.L.; Athene Lane, J.; Hamdy, F.C.; Neal, D.E.; Martin, R.M. Associations of aspirin, nonsteroidal anti-inflammatory drug and paracetamol use with PSA-detected prostate cancer: Findings from a large, population-based, case-control study (the ProtecT study). Int. J. Cancer 2011, 128, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, A.; Shiota, M.; Kashiwagi, E.; Kuroiwa, K.; Tatsugami, K.; Inokuchi, J.; Takeuchi, A.; Naito, S. Statins reduce the androgen sensitivity and cell proliferation by decreasing the androgen receptor protein in prostate cancer cells. Prostate 2011, 71, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Hashimoto, M.; Kubota, M.; Yamashita, S.; Nakamura, M.; Usui, S.; Sugiyama, T.; Hirano, K. Effects of 14 frequently used drugs on prostate-specific antigen expression in prostate cancer LNCaP cells. Oncol. Lett. 2014, 7, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Takahashi, S.; Sugimura, Y.; Watanabe, M. Role of Stromal Paracrine Signals in Proliferative Diseases of the Aging Human Prostate. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef]
- Franco, O.E.; Jiang, M.; Strand, D.W.; Peacock, J.; Fernandez, S.; Jackson, R.S., 2nd; Revelo, M.P.; Bhowmick, N.A.; Hayward, S.W. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011, 71, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Kiskowski, M.A.; Jackson, R.S., 2nd; Banerjee, J.; Li, X.; Kang, M.; Iturregui, J.M.; Franco, O.E.; Hayward, S.W.; Bhowmick, N.A. Role for stromal heterogeneity in prostate tumorigenesis. Cancer Res. 2011. [Google Scholar] [CrossRef]
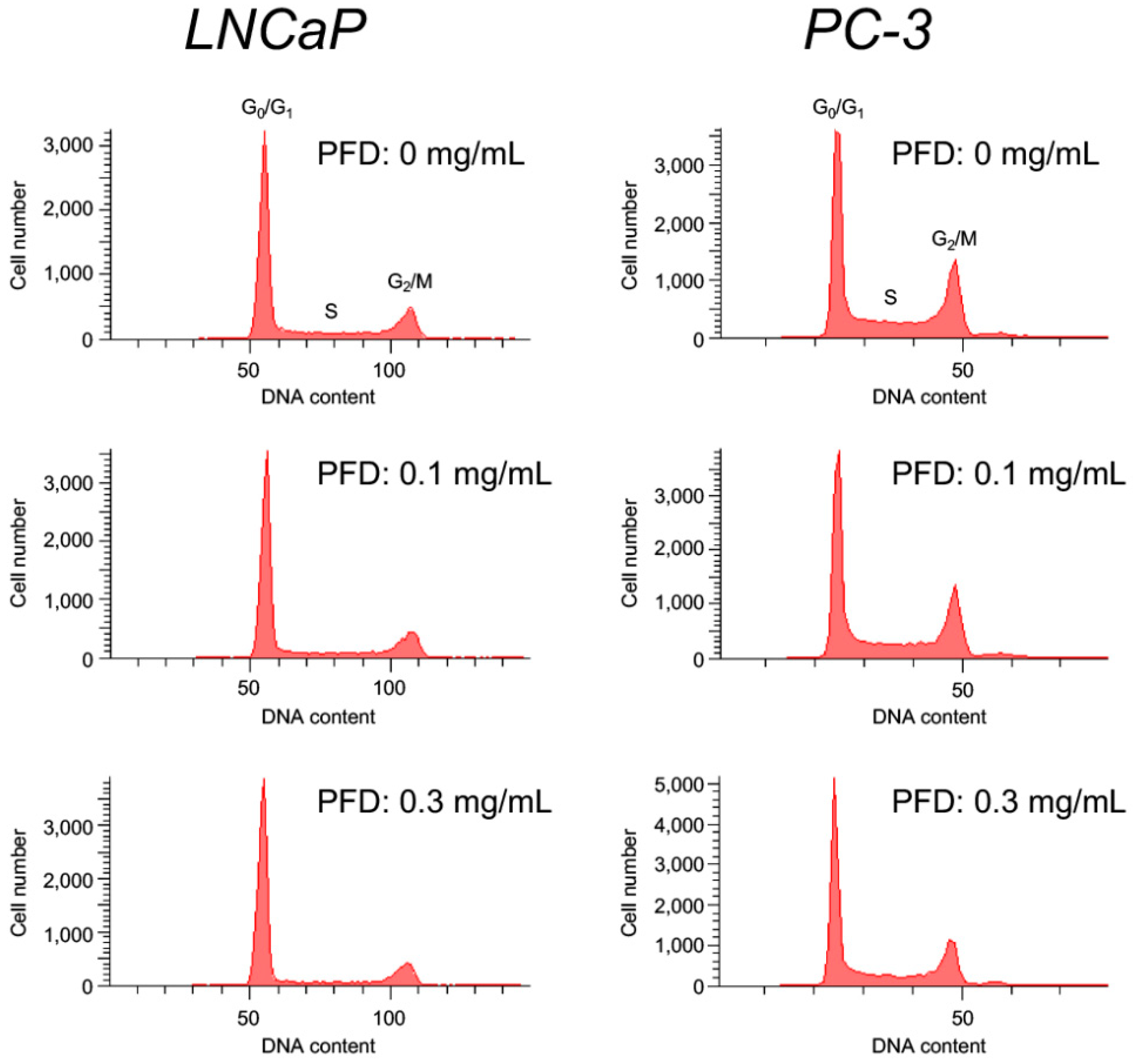
| PFD (mg/mL) | Phase (%) | ||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| 0 | 61.8 ± 0.9 | 19.2 ± 0.3 | 17.8 ± 0.5 |
| 0.1 | 64.9 ± 0.7 ** | 16.8 ± 0.7 * | 17.2 ± 0.5 |
| 0.3 | 72.3 ± 0.7 *** | 12.0 ± 0.2 *** | 14.7 ± 0.5 ** |
| PFD (mg/mL) | Phase (%) | ||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| 0 | 45.5 ± 1.3 | 20.5 ± 0.4 | 26.7 ± 0.7 |
| 0.1 | 51.1 ± 0.7 ** | 16.1 ± 0.1 ** | 25.5 ± 0.3 |
| 0.3 | 55.6 ± 0.6 ** | 15.2 ± 0.9 ** | 23.0 ± 0.9 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, K.; Sasaki, T.; Iguchi, K.; Kato, M.; Kanda, H.; Hirokawa, Y.; Arima, K.; Watanabe, M.; Sugimura, Y. Pirfenidone, an Anti-Fibrotic Drug, Suppresses the Growth of Human Prostate Cancer Cells by Inducing G1 Cell Cycle Arrest. J. Clin. Med. 2019, 8, 44. https://doi.org/10.3390/jcm8010044
Ishii K, Sasaki T, Iguchi K, Kato M, Kanda H, Hirokawa Y, Arima K, Watanabe M, Sugimura Y. Pirfenidone, an Anti-Fibrotic Drug, Suppresses the Growth of Human Prostate Cancer Cells by Inducing G1 Cell Cycle Arrest. Journal of Clinical Medicine. 2019; 8(1):44. https://doi.org/10.3390/jcm8010044
Chicago/Turabian StyleIshii, Kenichiro, Takeshi Sasaki, Kazuhiro Iguchi, Manabu Kato, Hideki Kanda, Yoshifumi Hirokawa, Kiminobu Arima, Masatoshi Watanabe, and Yoshiki Sugimura. 2019. "Pirfenidone, an Anti-Fibrotic Drug, Suppresses the Growth of Human Prostate Cancer Cells by Inducing G1 Cell Cycle Arrest" Journal of Clinical Medicine 8, no. 1: 44. https://doi.org/10.3390/jcm8010044
APA StyleIshii, K., Sasaki, T., Iguchi, K., Kato, M., Kanda, H., Hirokawa, Y., Arima, K., Watanabe, M., & Sugimura, Y. (2019). Pirfenidone, an Anti-Fibrotic Drug, Suppresses the Growth of Human Prostate Cancer Cells by Inducing G1 Cell Cycle Arrest. Journal of Clinical Medicine, 8(1), 44. https://doi.org/10.3390/jcm8010044







