FAK is Required for Tumor Metastasis-Related Fluid Microenvironment in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Parallel-Plate Flow Chamber and Circulatory System
2.2. Cell Culture
2.3. Reagents
2.4. Western Blotting
2.5. RNA Interference and Plasmid Transfection
2.6. Cell Proliferation Assays
2.7. Cell Cycle Analysis
2.8. Wound-Healing Assays
2.9. Cell Invasion Assays
2.10. Immunofluorescence Staining
2.11. Specimens
2.12. Immunohistochemistry (IHC) Staining
2.13. Scoring
2.14. Statistical Analysis
3. Results
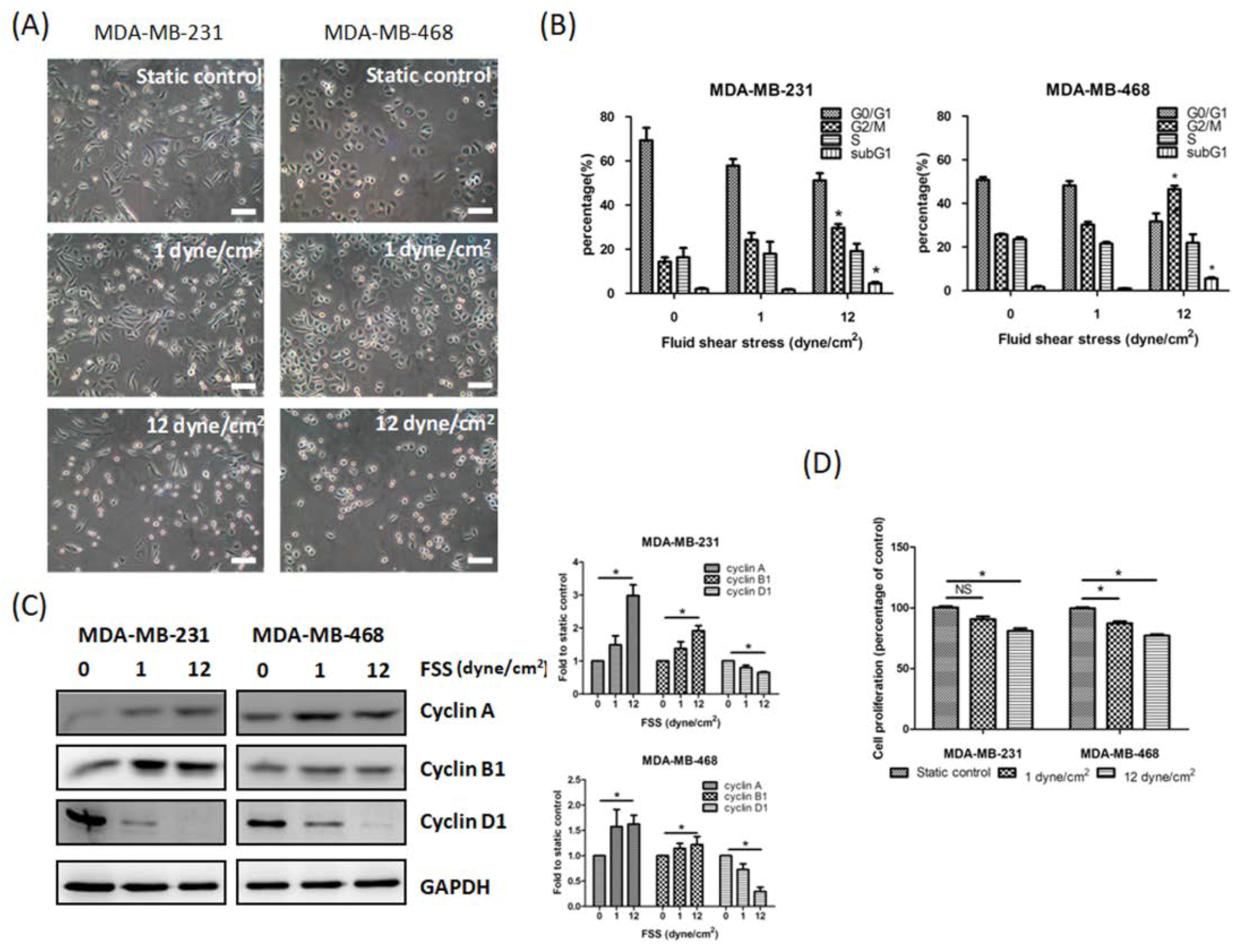
3.1. FSS Alters the Morphology, Cell Cycle, Proliferation and Survival of TNBC Cells
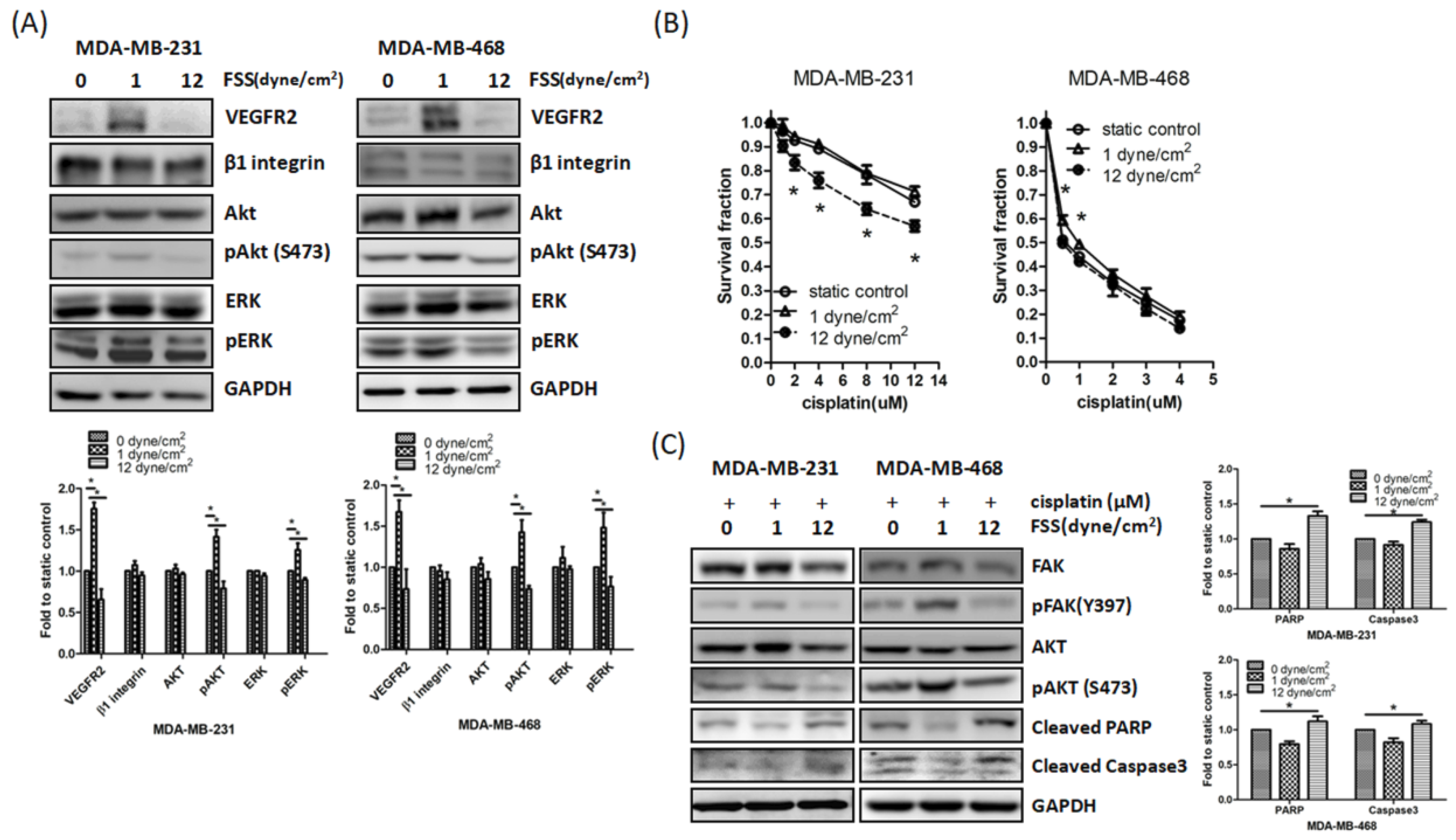
3.2. FSS Regulates Cell Motility by Altering The Expression of EMT-Related Proteins and FAK in TNBC Cells
3.3. Downregulation of FAK by FSS Correlates with Enhanced Cisplatin Sensitivity
3.4. FSS Modulated Cell Motility and Cisplatin-Induced Cell Death via FAK-VEGFR2 Axis Pathway
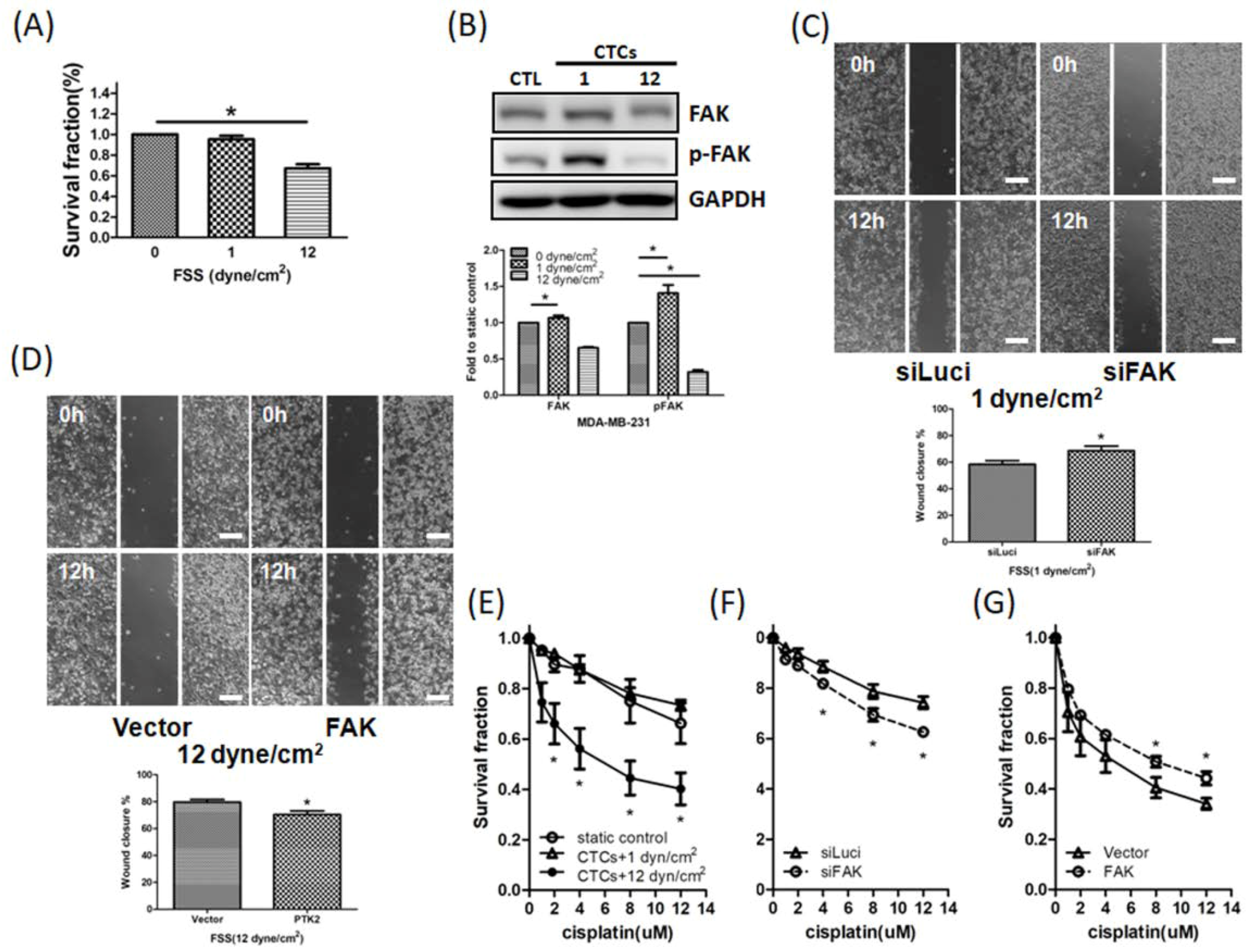
3.5. FSS Affects CTCs via the FAK Signaling Pathway
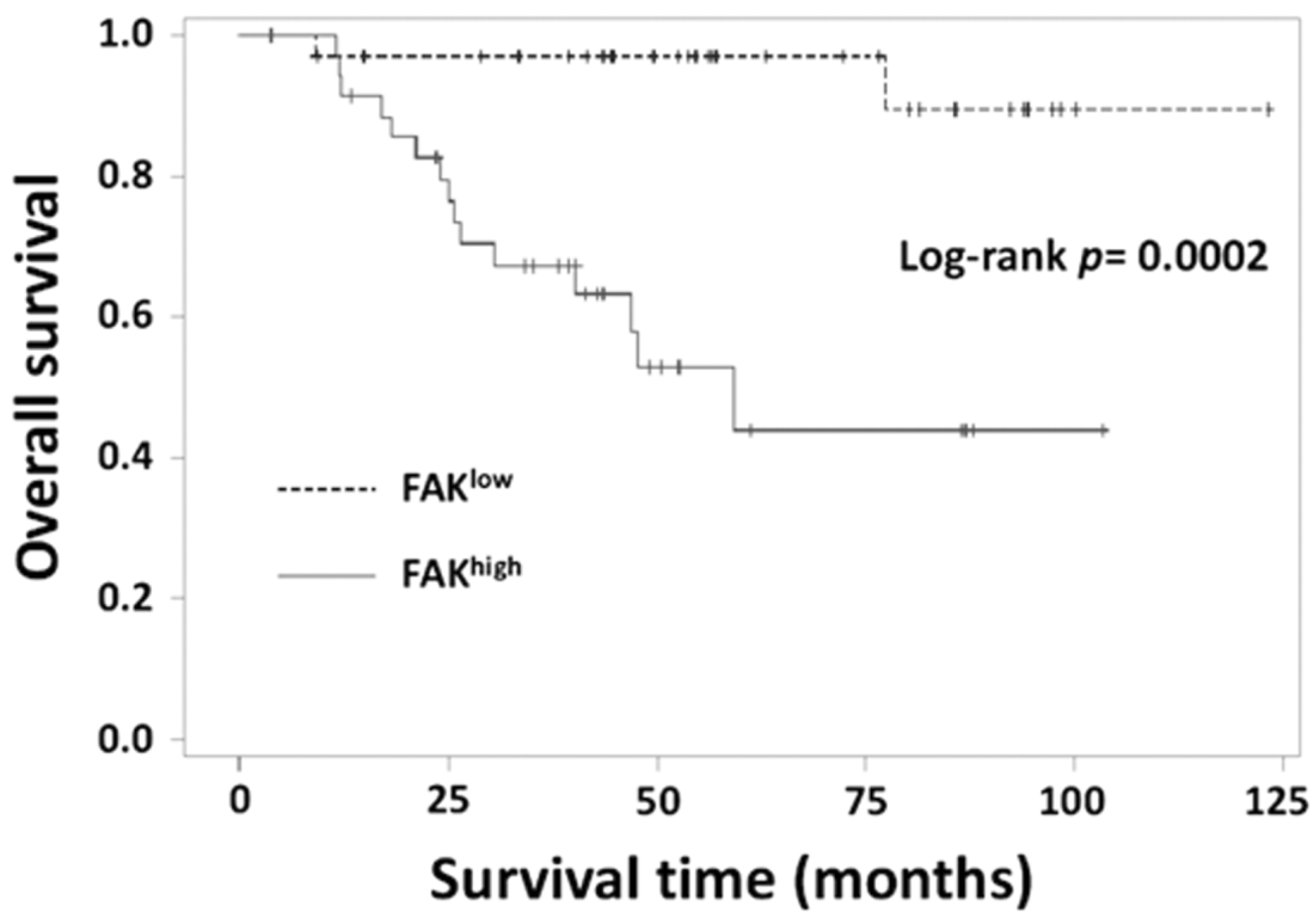
3.6. Relationship between FAK Levels and Clinicopathological Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Swartz, M.A. A driving force for change: Interstitial flow as a morphoregulator. Trends Cell Biol. 2007, 17, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Diaz, M.F.; Price, K.M.; Ozuna, J.A.; Zhang, S.; Sevick-Muraca, E.M.; Hagan, J.P.; Wenzel, P.L. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 2017, 8, 14122. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Delivery of molecular and nanoscale medicine to tumors: Transport barriers and strategies. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 281–298. [Google Scholar] [CrossRef]
- Shields, J.D.; Fleury, M.E.; Yong, C.; Tomei, A.A.; Randolph, G.J.; Swartz, M.A. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007, 11, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Lund, A.W. Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 2012, 12, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Chang, C.A.; Lee, D.Y.; Lee, P.L.; Yeh, Y.M.; Yeh, C.R.; Cheng, C.K.; Chien, S.; Chiu, J.J. Tumor cell cycle arrest induced by shear stress: Roles of integrins and Smad. Proc. Natl. Acad. Sci. USA 2008, 105, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.C.; Chang, S.F.; Lee, P.L.; Wei, S.Y.; Chang, M.D.; Chang, J.Y.; Chiu, J.J. Mechanical regulation of cancer cell apoptosis and autophagy: Roles of bone morphogenetic protein receptor, Smad1/5, and p38 MAPK. Biochim. Biophys. Acta 2013, 1833, 3124–3133. [Google Scholar] [CrossRef] [PubMed]
- Hyler, A.R.; Baudoin, N.C.; Brown, M.S.; Stremler, M.A.; Cimini, D.; Davalos, R.V.; Schmelz, E.M. Fluid shear stress impacts ovarian cancer cell viability, subcellular organization, and promotes genomic instability. PLoS ONE 2018, 13, e0194170. [Google Scholar] [CrossRef]
- Luo, C.W.; Wu, C.C.; Ch’ang, H.J. Radiation sensitization of tumor cells induced by shear stress: The roles of integrins and FAK. Biochim. Biophys. Acta 2014, 1843, 2129–2137. [Google Scholar] [CrossRef]
- Ma, S.; Fu, A.; Chiew, G.G.; Luo, K.Q. Hemodynamic shear stress stimulates migration and extravasation of tumor cells by elevating cellular oxidative level. Cancer Lett. 2017, 388, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Fu, A.; Luo, K.Q. High Shear Stresses under Exercise Condition Destroy Circulating Tumor Cells in a Microfluidic System. Sci. Rep. 2017, 7, 39975. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.L.; Wu, C.C.; Lin, C.H.; Chai, C.Y.; Hou, M.F.; Chang, S.J.; Tsai, H.P.; Hung, W.C.; Pan, M.R.; Luo, C.W. beta1 Integrin as a Prognostic and Predictive Marker in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1432. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-negative breast cancer: Is there a treatment on the horizon? Oncotarget 2017, 8, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef]
- Jain, R.K.; Tong, R.T.; Munn, L.L. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 2007, 67, 2729–2735. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Xie, M.; Corbett, C.; Singhal, S.; Dai, B.; Wang, J.; Ding, Q.; Lu, Q.; Wang, Y. Downregulating Heparanase-Induced Vascular Normalization: A New Approach To Increase the Bioavailability of Chemotherapeutics in Solid Tumors. Mol. Pharm. 2018, 15, 4303–4309. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, B.; Zhang, J. New strategies in achieving antiangiogenic effect: Multiplex inhibitors suppressing compensatory activations of RTKs. Med. Res. Rev. 2018, 38, 1674–1705. [Google Scholar] [CrossRef] [PubMed]
- El Alaoui-Lasmaili, K.; Faivre, B. Antiangiogenic therapy: Markers of response, “normalization” and resistance. Crit. Rev. Oncol. Hematol. 2018, 128, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Taliaferro-Smith, L.; Oberlick, E.; Liu, T.; McGlothen, T.; Alcaide, T.; Tobin, R.; Donnelly, S.; Commander, R.; Kline, E.; Nagaraju, G.P.; et al. FAK activation is required for IGF1R-mediated regulation of EMT, migration, and invasion in mesenchymal triple negative breast cancer cells. Oncotarget 2015, 6, 4757–4772. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. BioMed Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef] [PubMed]
- Hsia, D.A.; Mitra, S.K.; Hauck, C.R.; Streblow, D.N.; Nelson, J.A.; Ilic, D.; Huang, S.; Li, E.; Nemerow, G.R.; Leng, J.; et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 2003, 160, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.M. Focal adhesion kinase as a cancer therapy target. Anticancer Agents Med. Chem. 2010, 10, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hua, Z.C. FAK expression regulation and therapeutic potential. Adv. Cancer Res. 2008, 101, 45–61. [Google Scholar] [CrossRef]
- Agochiya, M.; Brunton, V.G.; Owens, D.W.; Parkinson, E.K.; Paraskeva, C.; Keith, W.N.; Frame, M.C. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene 1999, 18, 5646–5653. [Google Scholar] [CrossRef]
- Owens, L.V.; Xu, L.; Dent, G.A.; Yang, X.; Sturge, G.C.; Craven, R.J.; Cance, W.G. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann. Surg. Oncol. 1996, 3, 100–105. [Google Scholar] [CrossRef]
- Golubovskaya, V.M.; Ylagan, L.; Miller, A.; Hughes, M.; Wilson, J.; Wang, D.; Brese, E.; Bshara, W.; Edge, S.; Morrison, C.; et al. High focal adhesion kinase expression in breast carcinoma is associated with lymphovascular invasion and triple-negative phenotype. BMC Cancer 2014, 14, 769. [Google Scholar] [CrossRef]
- Kolev, V.N.; Tam, W.F.; Wright, Q.G.; McDermott, S.P.; Vidal, C.M.; Shapiro, I.M.; Xu, Q.; Wicha, M.S.; Pachter, J.A.; Weaver, D.T. Inhibition of FAK kinase activity preferentially targets cancer stem cells. Oncotarget 2017, 8, 51733–51747. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Luo, Q.; Liu, L.; Song, G. Low-level shear stress promotes migration of liver cancer stem cells via the FAK-ERK1/2 signalling pathway. Cancer Lett. 2018, 427, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Li, S.; Tang, K.; Bai, H.; Peng, Y.; Yang, H.; Wu, C.; Liu, Y. Involvement of caveolin-1 in low shear stress-induced breast cancer cell motility and adhesion: Roles of FAK/Src and ROCK/p-MLC pathways. Biochim. Biophys. Acta 2017, 1864, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Young, S.R.; Gerard-O’Riley, R.; Kim, J.B.; Pavalko, F.M. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J. Bone Miner. Res. 2009, 24, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Pan, M.R.; Wei, Y.C.; Lin, C.H.; Yang, S.F.; Tsai, H.P.; Luo, C.W.; Chai, C.Y. CHD4 as a Potential Biomarker in Differentiating Between Cellular Schwannoma and Malignant Peripheral Nerve Sheath Tumor. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Nicholes, K.; Bustos, D.; Lin, E.; Song, Q.; Stephan, J.P.; Kirkpatrick, D.S.; Settleman, J. Overcoming EMT-associated resistance to anti-cancer drugs via Src/FAK pathway inhibition. Oncotarget 2014, 5, 7328–7341. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Geng, Y.; Peyton, S.; Mercurio, A.M.; Rotello, V.M. Biochemical and biomechanical drivers of cancer cell metastasis, drug response and nanomedicine. Drug Discov. Today 2016, 21, 1489–1494. [Google Scholar] [CrossRef]
- Malik, R.; Lelkes, P.I.; Cukierman, E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015, 33, 230–236. [Google Scholar] [CrossRef]
- Fan, R.; Emery, T.; Zhang, Y.; Xia, Y.; Sun, J.; Wan, J. Circulatory shear flow alters the viability and proliferation of circulating colon cancer cells. Sci. Rep. 2016, 6, 27073. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.H.; Hung, W.C.; Paul, C.; Zhu, F.; Guan, P.P.; Huso, D.L.; Kontrogianni-Konstantopoulos, A.; Konstantopoulos, K. Fluid shear promotes chondrosarcoma cell invasion by activating matrix metalloproteinase 12 via IGF-2 and VEGF signaling pathways. Oncogene 2015, 34, 4558–4569. [Google Scholar] [CrossRef]
- Zhao, F.; Li, L.; Guan, L.; Yang, H.; Wu, C.; Liu, Y. Roles for GP IIb/IIIa and alphavbeta3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction. Cancer Lett. 2014, 344, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; King, M.R. Fluid Shear Stress Sensitizes Cancer Cells to Receptor-Mediated Apoptosis via Trimeric Death Receptors. New J. Phys. 2013, 15, 015008. [Google Scholar] [CrossRef] [PubMed]
- Shyy, J.Y.; Chien, S. Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 2002, 91, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Wahba, H.A.; El-Hadaad, H.A. Current approaches in treatment of triple-negative breast cancer. Cancer Biol. Med. 2015, 12, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Gnani, D.; Romito, I.; Artuso, S.; Chierici, M.; De Stefanis, C.; Panera, N.; Crudele, A.; Ceccarelli, S.; Carcarino, E.; D’Oria, V.; et al. Focal adhesion kinase depletion reduces human hepatocellular carcinoma growth by repressing enhancer of zeste homolog 2. Cell Death Differ. 2017, 24, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Bess, E.; Fisslthaler, B.; Hartel, F.V.; Noll, T.; Busse, R.; Fleming, I. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc. Res. 2008, 77, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Mehta, S.; Na, N.; Ciechanover, A.; Goldman, R.D.; Ridge, K.M. Ubiquitin-proteasome-mediated degradation of keratin intermediate filaments in mechanically stimulated A549 cells. J. Biol. Chem. 2008, 283, 25348–25355. [Google Scholar] [CrossRef]
- Alt-Holland, A.; Sowalsky, A.G.; Szwec-Levin, Y.; Shamis, Y.; Hatch, H.; Feig, L.A.; Garlick, J.A. Suppression of E-cadherin function drives the early stages of Ras-induced squamous cell carcinoma through upregulation of FAK and Src. J. Investig. Dermatol. 2011, 131, 2306–2315. [Google Scholar] [CrossRef]
- Pei, G.; Lan, Y.; Chen, D.; Ji, L.; Hua, Z.C. FAK regulates E-cadherin expression via p-SrcY416/p-ERK1/2/p-Stat3Y705 and PPARgamma/miR-125b/Stat3 signaling pathway in B16F10 melanoma cells. Oncotarget 2017, 8, 13898–13908. [Google Scholar] [CrossRef]
- Lv, P.C.; Jiang, A.Q.; Zhang, W.M.; Zhu, H.L. FAK inhibitors in Cancer, a patent review. Expert Opin. Ther. Pat. 2018, 28, 139–145. [Google Scholar] [CrossRef]
- Tiede, S.; Meyer-Schaller, N.; Kalathur, R.K.R.; Ivanek, R.; Fagiani, E.; Schmassmann, P.; Stillhard, P.; Hafliger, S.; Kraut, N.; Schweifer, N.; et al. The FAK inhibitor BI 853520 exerts anti-tumor effects in breast cancer. Oncogenesis 2018, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Basu, M.; Roy, S.S. ETS-1 protein regulates vascular endothelial growth factor-induced matrix metalloproteinase-9 and matrix metalloproteinase-13 expression in human ovarian carcinoma cell line SKOV-3. J. Biol. Chem. 2012, 287, 15001–15015. [Google Scholar] [CrossRef] [PubMed]
- Kopparapu, P.K.; Boorjian, S.A.; Robinson, B.D.; Downes, M.; Gudas, L.J.; Mongan, N.P.; Persson, J.L. Expression of VEGF and its receptors VEGFR1/VEGFR2 is associated with invasiveness of bladder cancer. Anticancer Res. 2013, 33, 2381–2390. [Google Scholar] [PubMed]
- Zhu, X.; Zhou, W. The Emerging Regulation of VEGFR-2 in Triple-Negative Breast Cancer. Front. Endocrinol. (Lausanne) 2015, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Chiorean, R.; Irimie, A.; Chira, S.; Tomuleasa, C.; Neagoe, E.; Paradiso, A.; Achimas-Cadariu, P.; Lazar, V.; Berindan-Neagoe, I. Novel insight into triple-negative breast cancers, the emerging role of angiogenesis, and antiangiogenic therapy. Expert Rev. Mol. Med. 2016, 18, e18. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Colbert, L.S.; Fuller, M.; Zhang, Y.; Gonzalez-Perez, R.R. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim. Biophys. Acta 2010, 1806, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wu, H.J.; Guan, J.L. Nuclear FAK and its kinase activity regulate VEGFR2 transcription in angiogenesis of adult mice. Sci. Rep. 2018, 8, 2550. [Google Scholar] [CrossRef] [PubMed]


| Parameters | n | FAK, n (%) | p-Value | |
|---|---|---|---|---|
| Low | High | |||
| Total | 69 | 33 (47.83) | 36 (52.17) | |
| Age, n (%) | 0.8769 | |||
| ≤40 years | 8 | 4 (14.29) | 4 (12.90) | |
| >40 years | 51 | 24 (85.71) | 27 (87.10) | |
| Size, n (%) | 0.3458 | |||
| ≤2.0 cm | 23 | 13 (39.39) | 10 (28.57) | |
| >2.0 cm | 45 | 20 (60.61) | 25 (71.43) | |
| Grade, n (%) | 0.8793 | |||
| I/II | 29 | 12 (48.00) | 17 (50.00) | |
| III | 30 | 13 (52.00) | 17 (50.00) | |
| Tumor stage, n (%) | 0.4298 | |||
| T1 | 28 | 15 (45.45) | 13 (36.11) | |
| T2/T3 | 41 | 18 (54.55) | 23 (63.89) | |
| Nodal stage, n (%) | 0.015 * | |||
| N0 | 42 | 25 (75.76) | 17 (47.22) | |
| N1/N2/N3 | 27 | 8 (24.24) | 19 (52.78) | |
| Metastatic stage, n (%) | 0.8817 | |||
| M0 | 59 | 28 (84.85) | 31 (86.11) | |
| M1 | 10 | 5 (15.15) | 5 (13.89) | |
| Tumor recurrent, n (%) | 0.3348 | |||
| Absent | 68 | 33 (100.00) | 35 (97.22) | |
| Present | 1 | 0 (0.00) | 1 (2.78) | |
| Survival status, n (%) | <0.01 * | |||
| Survival | 52 | 31 (93.94) | 21 (58.33) | |
| Death | 17 | 2 (6.06) | 15 (41.67) | |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| FAK expression | 0.0188 * | 0.0445 * | ||
| Low | 1.0 | 1.0 | ||
| High | 6.174 (1.352–28.195) | 5.393 (1.042–27.917) | ||
| Age, n (%) | 0.4307 | 0.1774 | ||
| ≤40 years | 1.0 | 1.0 | ||
| >40 years | 2.277 (0.294–17.646) | 4.508 (0.505–40.200) | ||
| Size, n (%) | 0.0846 | |||
| ≤2.0 cm | 1.0 | |||
| >2.0 cm | 6.038 (0.783–46.581) | |||
| Grade | 0.0263 * | 0.0156 * | ||
| I/II | 1.0 | 1.0 | ||
| III | 4.386 (1.191–16.158) | 6.470 (1.425–29.388) | ||
| Tumor stage, n (%) | 0.0494 * | 0.7186 | ||
| T1 | 1.0 | 1.0 | ||
| T2/T3 | 4.533 (1.004–20.464) | 1.397 (0.227–8.620) | ||
| Nodal stage, n (%) | 0.0107 * | 0.0443 * | ||
| N0 | 1.0 | 1.0 | ||
| N1/N2/N3 | 5.368 (1.476–19.515) | 4.688 (1.040–21.128) | ||
| Metastatic stage, n (%) | 0.5432 | 0.3667 | ||
| M0 | 1.0 | 1.0 | ||
| M1 | 1.495 (0.409–5.460) | 0.506 (0.115–2.221) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.-R.; Hou, M.-F.; Ou-Yang, F.; Wu, C.-C.; Chang, S.-J.; Hung, W.-C.; Yip, H.-K.; Luo, C.-W. FAK is Required for Tumor Metastasis-Related Fluid Microenvironment in Triple-Negative Breast Cancer. J. Clin. Med. 2019, 8, 38. https://doi.org/10.3390/jcm8010038
Pan M-R, Hou M-F, Ou-Yang F, Wu C-C, Chang S-J, Hung W-C, Yip H-K, Luo C-W. FAK is Required for Tumor Metastasis-Related Fluid Microenvironment in Triple-Negative Breast Cancer. Journal of Clinical Medicine. 2019; 8(1):38. https://doi.org/10.3390/jcm8010038
Chicago/Turabian StylePan, Mei-Ren, Ming-Feng Hou, Fu Ou-Yang, Chun-Chieh Wu, Shu-Jyuan Chang, Wen-Chun Hung, Hon-Kan Yip, and Chi-Wen Luo. 2019. "FAK is Required for Tumor Metastasis-Related Fluid Microenvironment in Triple-Negative Breast Cancer" Journal of Clinical Medicine 8, no. 1: 38. https://doi.org/10.3390/jcm8010038
APA StylePan, M.-R., Hou, M.-F., Ou-Yang, F., Wu, C.-C., Chang, S.-J., Hung, W.-C., Yip, H.-K., & Luo, C.-W. (2019). FAK is Required for Tumor Metastasis-Related Fluid Microenvironment in Triple-Negative Breast Cancer. Journal of Clinical Medicine, 8(1), 38. https://doi.org/10.3390/jcm8010038





