Clinical and Rehabilitative Predictors of Peak Oxygen Uptake Following Cardiac Transplantation
Abstract
1. Introduction
2. Experimental Section
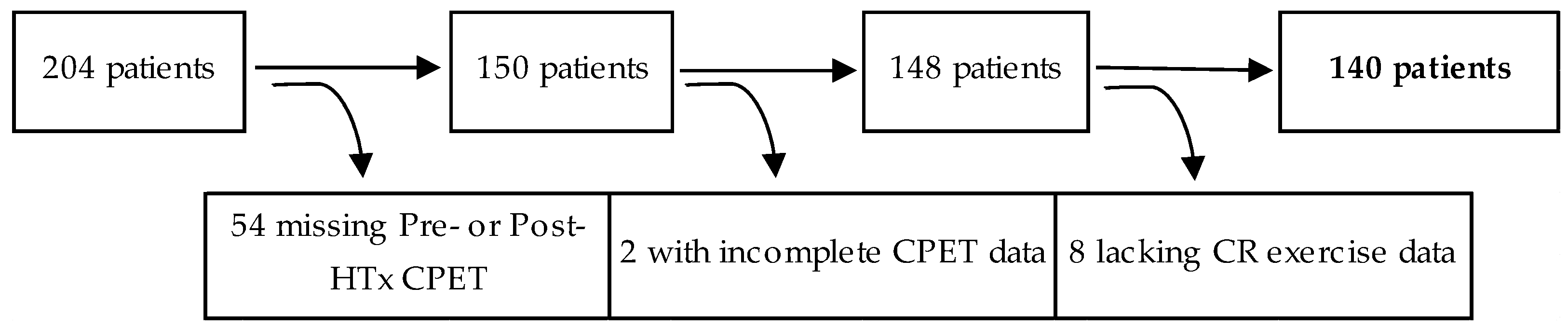
2.1. Participants and Study Design
2.2. Clinical Characteristics
2.3. Cardiac Rehabilitation Participation
2.4. Cardiopulmonary Exercise Testing Procedures
2.5. Statistical Analysis
3. Results
3.1. Patient Population and Clinical Characteristics
3.2. Exercise Testing Data Prior to and Following Transplant
3.3. Predictors of VO2peak
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, S.; Goldberg, L.R.; Allen, M.T.; Kao, A. Effects of gender on peak oxygen consumption and the timing of cardiac transplantation. J. Am. Coll. Cardiol. 2006, 47, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiol. Pol. 2016, 74, 1037–1147. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R.; Schechtman, K.B.; Ewald, G.A.; Geltman, E.M.; Meyer, T.; Krekeler, P.; Rogers, J.G. The effect of beta-adrenergic blockers on the prognostic value of peak exercise oxygen uptake in patients with heart failure. J. Heart Lung Transplant. 2003, 22, 70–77. [Google Scholar] [CrossRef]
- Keteyian, S.J.; Patel, M.; Kraus, W.E.; Brawner, C.A.; McConnell, T.R.; Pina, I.L.; Leifer, E.S.; Fleg, J.L.; Blackburn, G.; Fonarow, G.C.; et al. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J. Am. Coll. Cardiol. 2016, 67, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Swank, A.M.; Horton, J.; Fleg, J.L.; Fonarow, G.C.; Keteyian, S.; Goldberg, L.; Wolfel, G.; Handberg, E.M.; Bensimhon, D.; Illiou, M.C.; et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: Results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ. Heart Fail. 2012, 5, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, T.; Mertens, D.J.; Shephard, R.J.; Beyene, J.; Kennedy, J.; Campbell, R.; Sawyer, P.; Yacoub, M. Long-term cardiorespiratory results of exercise training following cardiac transplantation. Am. J. Cardiol. 2003, 91, 190–194. [Google Scholar] [CrossRef]
- Yardley, M.; Havik, O.E.; Grov, I.; Relbo, A.; Gullestad, L.; Nytroen, K. Peak oxygen uptake and self-reported physical health are strong predictors of long-term survival after heart transplantation. Clin. Transplant. 2016, 30, 161–169. [Google Scholar] [CrossRef]
- Branco, C.F.; Viamonte, S.; Matos, C.; Magalhaes, S.; Cunha, I.; Barreira, A.; Fernandes, P.; Torres, S. Predictors of changes in functional capacity on a cardiac rehabilitation program. Rev. Port. Cardiol. 2016, 35, 215–224. [Google Scholar] [CrossRef]
- Douard, H.; Parrens, E.; Billes, M.A.; Labbe, L.; Baudet, E.; Broustet, J.P. Predictive factors of maximal aerobic capacity after cardiac transplantation. Eur. Heart J. 1997, 18, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Hammond, H.K.; Kelly, T.L.; Froelicher, V.F.; Pewen, W. Use of clinical data in predicting improvement in exercise capacity after cardiac rehabilitation. J. Am. Coll. Cardiol. 1985, 6, 19–26. [Google Scholar] [CrossRef]
- Leung, T.C.; Ballman, K.V.; Allison, T.G.; Wagner, J.A.; Olson, L.J.; Frantz, R.P.; Edwards, B.S.; Dearani, J.A.; Daly, R.C.; McGregor, C.G.; et al. Clinical predictors of exercise capacity 1 year after cardiac transplantation. J. Heart Lung Transplant. 2003, 22, 16–27. [Google Scholar] [CrossRef]
- Oliveira Carvalho, V.; Guimaraes, G.V.; Vieira, M.L.; Catai, A.M.; Oliveira-Carvalho, V.; Ayub-Ferreira, S.M.; Bocchi, E.A. Determinants of peak VO2 in heart transplant recipients. Rev. Bras. Cir. Cardiovasc. 2015, 30, 9–15. [Google Scholar] [CrossRef]
- Anderson, L.; Nguyen, T.T.; Dall, C.H.; Burgess, L.; Bridges, C.; Taylor, R.S. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst. Rev. 2017, 4, CD012264. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Spina, R.J.; Martin, W.H., 3rd; Kohrt, W.M.; Schechtman, K.B.; Holloszy, J.O.; Ehsani, A.A. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 1992, 86, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Derleth, C.; Stratton, J.R.; Levy, W.C. The influence of age, gender, and training on exercise efficiency. J. Am. Coll. Cardiol. 2006, 47, 1049–1057. [Google Scholar] [CrossRef]
- Nytroen, K.; Rustad, L.A.; Gude, E.; Hallen, J.; Fiane, A.E.; Rolid, K.; Holm, I.; Aakhus, S.; Gullestad, L. Muscular exercise capacity and body fat predict VO(2peak) in heart transplant recipients. Eur. J. Prev. Cardiol. 2014, 21, 21–29. [Google Scholar] [CrossRef]
- Hsu, C.J.; Chen, S.Y.; Su, S.; Yang, M.C.; Lan, C.; Chou, N.K.; Hsu, R.B.; Lai, J.S.; Wang, S.S. The effect of early cardiac rehabilitation on health-related quality of life among heart transplant recipients and patients with coronary artery bypass graft surgery. Transplant. Proc. 2011, 43, 2714–2717. [Google Scholar] [CrossRef]
- Rosenbaum, A.N.; Kremers, W.K.; Schirger, J.A.; Thomas, R.J.; Squires, R.W.; Allison, T.G.; Daly, R.C.; Kushwaha, S.S.; Edwards, B.S. Association between early cardiac rehabilitation and long-term survival in cardiac transplant recipients. Mayo Clin. Proc. 2016, 91, 149–156. [Google Scholar] [CrossRef]
- Yawn, B.P.; Yawn, R.A.; Geier, G.R.; Xia, Z.; Jacobsen, S.J. The impact of requiring patient authorization for use of data in medical records research. J. Fam. Pract. 1998, 47, 361–365. [Google Scholar] [PubMed]
- Squires, R.W.; Allison, T.G.; Johnson, B.D.; Gau, G.T. Non-physician supervision of cardiopulmonary exercise testing in chronic heart failure: Safety and results of a preliminary investigation. J. Cardiopulm. Rehabil. 1999, 19, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.M.; Ball, C.A.; Hebl, V.B.; Ong, K.C.; Siontis, K.C.; Olson, T.P.; Ackerman, M.J.; Ommen, S.R.; Allison, T.G.; Geske, J.B. Effect of body mass index on exercise capacity in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kaminsky, L.A.; Lima, R.; Christle, J.W.; Ashley, E.; Arena, R. A reference equation for normal standards for VO2 Max: Analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry). Prog. Cardiovasc. Dis. 2017, 60, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Humphrey, R.; Peberdy, M.A. Prognostic ability of VE/VCO2 slope calculations using different exercise test time intervals in subjects with heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2003, 10, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.P.; Shamim, W.; Davies, L.C.; Piepoli, M.F.; Ponikowski, P.; Anker, S.D.; Coats, A.J. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value from VE/VCO2 slope and peak VO2. Eur. Heart J. 2000, 21, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Medina-Inojosa, J.R.; Layrisse, V.; Ommen, S.R.; Olson, T.P. Predictors of exercise capacity in patients with hypertrophic obstructive cardiomyopathy. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef]
- Pierson, L.M.; Miller, L.E.; Herbert, W.G. Predicting exercise training outcome from cardiac rehabilitation. J. Cardiopulm. Rehabil. 2004, 24, 113–118. [Google Scholar] [CrossRef]
- McKee, G.; Kerins, M.; Fitzgerald, G.; Spain, M.; Morrison, K. Factors that influence obesity, functional capacity, anxiety and depression outcomes following a Phase III cardiac rehabilitation programme. J. Clin. Nurs. 2013, 22, 2758–2767. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Sharman, J.; Prins, J.B.; Marwick, T.H. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes Care 2005, 28, 1643–1648. [Google Scholar] [CrossRef]
- Higgins, J.; Pflugfelder, P.W.; Kostuk, W.J. Increased morbidity in diabetic cardiac transplant recipients. Can. J. Cardiol. 2009, 25, e125–e129. [Google Scholar] [CrossRef]
- Horwich, T.B.; Hamilton, M.A.; Maclellan, W.R.; Fonarow, G.C. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J. Card Fail. 2002, 8, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Rauchhaus, M.; Clark, A.L.; Doehner, W.; Davos, C.; Bolger, A.; Sharma, R.; Coats, A.J.; Anker, S.D. The relationship between cholesterol and survival in patients with chronic heart failure. J. Am. Coll. Cardiol. 2003, 42, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.J.; Beaudry, R.I.; Samuel, T.J.; Nelson, M.D.; Halle, M.; Baggish, A.L.; Haykowsky, M.J. Performance limitations in heart transplant recipients. Exerc. Sport Sci. Rev. 2018, 46, 144–151. [Google Scholar] [CrossRef]
- Georgiadou, P.; Adamopoulos, S. Skeletal muscle abnormalities in chronic heart failure. Curr. Heart Fail. Rep. 2012, 9, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Lampert, E.; Mettauer, B.; Hoppeler, H.; Charloux, A.; Charpentier, A.; Lonsdorfer, J. Structure of skeletal muscle in heart transplant recipients. J. Am. Coll. Cardiol. 1996, 28, 980–984. [Google Scholar] [CrossRef]
- Kobashigawa, J.A.; Leaf, D.A.; Lee, N.; Gleeson, M.P.; Liu, H.; Hamilton, M.A.; Moriguchi, J.D.; Kawata, N.; Einhorn, K.; Herlihy, E.; et al. A controlled trial of exercise rehabilitation after heart transplantation. N. Engl. J. Med. 1999, 340, 272–277. [Google Scholar] [CrossRef]
- Daida, H.; Squires, R.W.; Allison, T.G.; Johnson, B.D.; Gau, G.T. Sequential assessment of exercise tolerance in heart transplantation compared with coronary artery bypass surgery after phase II cardiac rehabilitation. Am. J. Cardiol. 1996, 77, 696–700. [Google Scholar] [CrossRef]
- Pina, I.L.; Bittner, V.; Clare, R.M.; Swank, A.; Kao, A.; Safford, R.; Nigam, A.; Barnard, D.; Walsh, M.N.; Ellis, S.J.; et al. Effects of exercise training on outcomes in women with heart failure: Analysis of HF-ACTION (Heart Failure-A Controlled Trial Investigating Outcomes of Exercise TraiNing) by sex. JACC Heart Fail. 2014, 2, 180–186. [Google Scholar] [CrossRef]
- Sarullo, F.M.; Fazio, G.; Brusca, I.; Fasullo, S.; Paterna, S.; Licata, P.; Novo, G.; Novo, S.; Di Pasquale, P. Cardiopulmonary exercise testing in patients with chronic heart failure: Prognostic comparison from peak VO2 and VE/VCO2 Slope. Open Cardiovasc. Med. J. 2010, 4, 127–134. [Google Scholar] [CrossRef]
- Givertz, M.M.; Hartley, L.H.; Colucci, W.S. Long-term sequential changes in exercise capacity and chronotropic responsiveness after cardiac transplantation. Circulation 1997, 96, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.C.; Van Trigt, P., 3rd; Shaeffer-McCall, G.S.; Shaw, J.P.; Kuzil, B.B.; Page, R.D.; Higginbotham, M.B. Central and peripheral limitations to upright exercise in untrained cardiac transplant recipients. Circulation 1994, 89, 2605–2615. [Google Scholar] [CrossRef] [PubMed]

| n | 140 |
|---|---|
| Age (years) | 52 ± 12 |
| Sex (Female) | 41 (29) |
| Height (cm) | 172 ± 14 |
| Weight (kg) | 83 ± 19 |
| BSA (m2) | 2.0 ± 0.2 |
| BMI (kg/m2) | 27.1 ± 4.6 |
| History of Diabetes | 31 (22.1) |
| History of Smoking | 52 (37.1) |
| History of Dyslipidemia | 74 (52.9) |
| History of Hypertension | 63 (45) |
| Previous LVAD | 27 (19.3) |
| Indication for Heart Transplant | |
| Restrictive Cardiomyopathy | 29 (20.7) |
| Dilated Cardiomyopathy | 53 (37.9) |
| Hypertrophic Cardiomyopathy | 8 (5.6) |
| Ischemic Cardiomyopathy | 25 (17.9) |
| Other | 25 (17.9) |
| Labs | |
| Hemoglobin (g/dL) | 12.0 ± 2.0 |
| Hematocrit (%) | 36 ± 6 |
| White Blood Cell Count (109/L) | 7.5 ± 3.0 |
| Creatinine (mg/dL) | 1.4 ± 1.0 |
| Medications * | |
| ACE Inhibitor | 52 (37.1) |
| Amiodarone | 12 (8.6) |
| Aspirin | 65 (46.4) |
| Beta Blocker | 103 (73.6) |
| Calcium Channel Blocker | 3 (2.1) |
| Diuretic | 92 (65.7) |
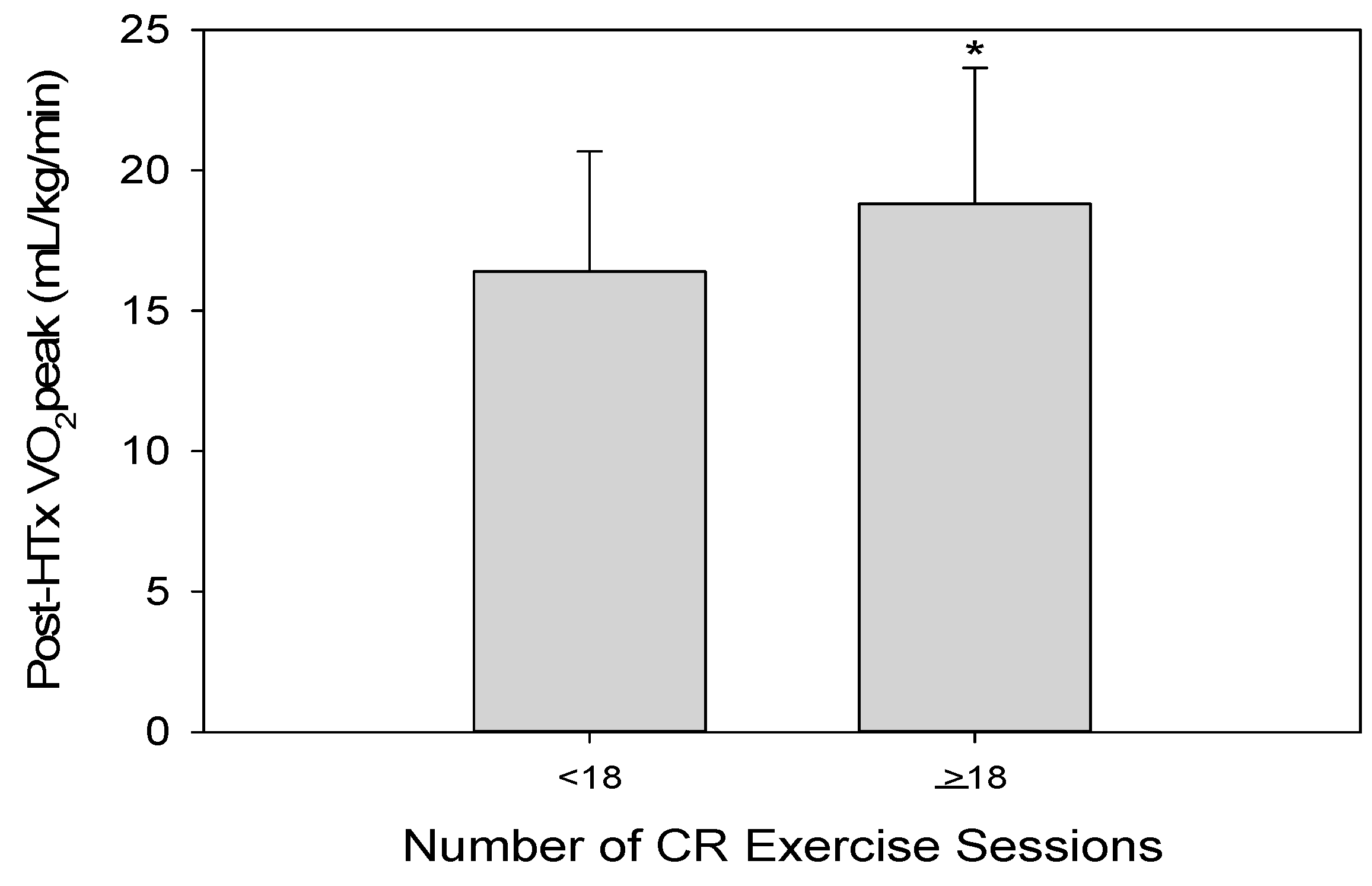
| Number of CR Exercise Sessions | 18 ± 9 |
| Pre-HTx | Post-HTx | p-Value | |
|---|---|---|---|
| n | 140 | 140 | |
| Exercise time (min) | 5.3 ± 1.7 | 6.7 ± 1.7 | <0.001 |
| METS | 4.9 ± 1.8 | 6.4 ± 1.8 | <0.001 |
| Absolute VO2peak (L/min) | 1.1 ± 0.4 | 1.4 ± 0.4 | <0.001 |
| VO2peak % Predicted (%) | 42 ± 14 | 58 ± 17 | <0.001 |
| Relative VO2peak (mL/kg/min) | 12.9 ± 4.4 | 17.5 ± 4.7 | <0.001 |
| VE/VCO2 slope | 42 ± 12 | 37 ± 6 | <0.001 |
| VCO2 (L/min) | 1.2 ± 0.5 | 1.7 ± 0.5 | <0.001 |
| RER | 1.15 ± 0.13 | 1.21 ± 0.12 | <0.001 |
| SBP (mmHg) | 105 ± 25 | 145 ± 30 | <0.001 |
| DBP (mmHg) | 60 ± 10 | 67 ± 11 | <0.001 |
| HR (bpm) | 110 ± 21 | 125 ± 20 | <0.001 |
| Variable | Univariate Analysis | ||
|---|---|---|---|
| OR | 95% CI | p-Value | |
| Age (years) | 0.978 | 0.949–1.009 | 0.170 |
| Sex (female) | 0.457 | 0.173–1.209 | 0.115 |
| BMI (kg/m2) | 0.896 | 0.815–0.985 | 0.022 |
| History of Diabetes | 0.174 | 0.039–0.772 | 0.021 |
| History of Smoking | 0.805 | 0.354–1.831 | 0.605 |
| History of Hypertension | 0.741 | 0.335–1.641 | 0.460 |
| History of Dyslipidemia | 0.415 | 0.185–0.929 | 0.032 |
| History of LVAD | 1.171 | 0.446–3.076 | 0.748 |
| Beta Blocker Medication | 0.642 | 0.275–1.499 | 0.306 |
| Indication for HTx | |||
| Restrictive Cardiomyopathy | 0.812 | 0.299–2.201 | 0.682 |
| Dilated Cardiomyopathy | 0.920 | 0.409–2.066 | 0.840 |
| Hypertrophic Cardiomyopathy | 2.040 | 0.461–9.035 | 0.348 |
| Ischemic Cardiomyopathy | 0.565 | 0.179–1.782 | 0.330 |
| Other | 1.694 | 0.655–4.380 | 0.277 |
| Labs | |||
| Hemoglobin (g/dL) | 1.294 | 1.041–1.608 | 0.020 |
| Hematocrit (%) | 1.063 | 0.985–1.146 | 0.115 |
| White Blood Cell Count (109/L) | 0.806 | 0.662–0.982 | 0.033 |
| Creatinine (mg/dL) | 0.402 | 0.155–1.039 | 0.060 |
| Pre-HTx CPET Data | |||
| Peak SBP (mmHg) | 0.998 | 0.971–1.005 | 0.158 |
| Heart Rate Recovery (bpm) | 1.021 | 0.987–1.056 | 0.225 |
| Relative VO2peak (mL/kg/min) | 1.174 | 1.070–1.289 | 0.001 |
| CR Exercise Sessions | 1.095 | 1.041–1.152 | <0.001 |
| Variable | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age (years) | 0.968 | 0.932–1.006 | 0.094 | 0.970 | 0.933–1.009 | 0.130 | 0.962 | 0.922–1.004 | 0.077 |
| Sex (female) | 0.395 | 0.128–1.217 | 0.106 | 0.284 | 0.080–1.010 | 0.052 | 0.292 | 0.087–0.986 | 0.047 |
| BMI (kg/m2) | 0.889 | 0.791–0.999 | 0.049 | 0.097 | 0.811–1.018 | 0.097 | |||
| History of Diabetes | 3.050 | 0.602–15.450 | 0.178 | 2.760 | 0.635–11.995 | 0.176 | |||
| History of Dyslipidemia | 0.880 | 0.315–2.465 | 0.807 | ||||||
| Labs | |||||||||
| Hemoglobin (g/dL) | 1.172 | 0.930–1.478 | 0.178 | 1.045 | 0.829–1.31 | 0.710 | |||
| White Blood Cell Count (109/L) | 0.796 | 0.620–1.021 | 0.072 | ||||||
| Pre-HTx CPET Data | |||||||||
| Relative VO2peak (mL/kg/min) | 1.145 | 1.037–1.264 | 0.007 | 1.110 | 1.002–1.231 | 0.047 | 1.206 | 1.068–1.361 | 0.002 |
| Peak HR (bpm) | 0.979 | 0.951–1.007 | 0.147 | ||||||
| HRR (bpm) | 1.008 | 0.959–1.059 | 0.764 | ||||||
| CR Exercise Sessions | 1.102 | 1.037–1.264 | <0.001 | 1.095 | 1.037–1.157 | 0.001 | 1.103 | 1.042–1.167 | 0.001 |
| Study Group | n | Age (yrs) | Predictors of VO2peak | Time from Transplant | Post-Transplant VO2peak (mL/kg/min) |
|---|---|---|---|---|---|
| Douard et al. 1997 [11] | 85 | 52 ± 12 | Chronotropic reserve, time from transplantation, age of donor, age of patient | 1–100 months | 21.1 ± 6.0 |
| Leung et al. 2003 [13] | 95 | 48 ± 14 | Age, sex, height, and weight (alternatively, body mass index) | 12 months | 19.9 ± 4.8 |
| Nytrøen et al. 2012 [18] | 51 | 52 ± 16 | Muscular exercise capacity and body fat | 1–8 years | Group 1: 23.1 ± 3.7; Group 2: 32.6 ± 4.4 |
| Carvalho et al. 2015 [14] | 60 | 48 ± 15 | Age, sex, body mass index, heart rate reserve, and left atrium diameter | 64 ± 54 months | unspecified |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uithoven, K.E.; Smith, J.R.; Medina-Inojosa, J.R.; Squires, R.W.; Van Iterson, E.H.; Olson, T.P. Clinical and Rehabilitative Predictors of Peak Oxygen Uptake Following Cardiac Transplantation. J. Clin. Med. 2019, 8, 119. https://doi.org/10.3390/jcm8010119
Uithoven KE, Smith JR, Medina-Inojosa JR, Squires RW, Van Iterson EH, Olson TP. Clinical and Rehabilitative Predictors of Peak Oxygen Uptake Following Cardiac Transplantation. Journal of Clinical Medicine. 2019; 8(1):119. https://doi.org/10.3390/jcm8010119
Chicago/Turabian StyleUithoven, Katelyn E., Joshua R. Smith, Jose R. Medina-Inojosa, Ray W. Squires, Erik H. Van Iterson, and Thomas P. Olson. 2019. "Clinical and Rehabilitative Predictors of Peak Oxygen Uptake Following Cardiac Transplantation" Journal of Clinical Medicine 8, no. 1: 119. https://doi.org/10.3390/jcm8010119
APA StyleUithoven, K. E., Smith, J. R., Medina-Inojosa, J. R., Squires, R. W., Van Iterson, E. H., & Olson, T. P. (2019). Clinical and Rehabilitative Predictors of Peak Oxygen Uptake Following Cardiac Transplantation. Journal of Clinical Medicine, 8(1), 119. https://doi.org/10.3390/jcm8010119






