Effect of Size and Location of Nevi on Postoperative Pain and Emergence Agitation in Children Undergoing Nevi Excision
Abstract
:1. Introduction
2. Materials and Methods
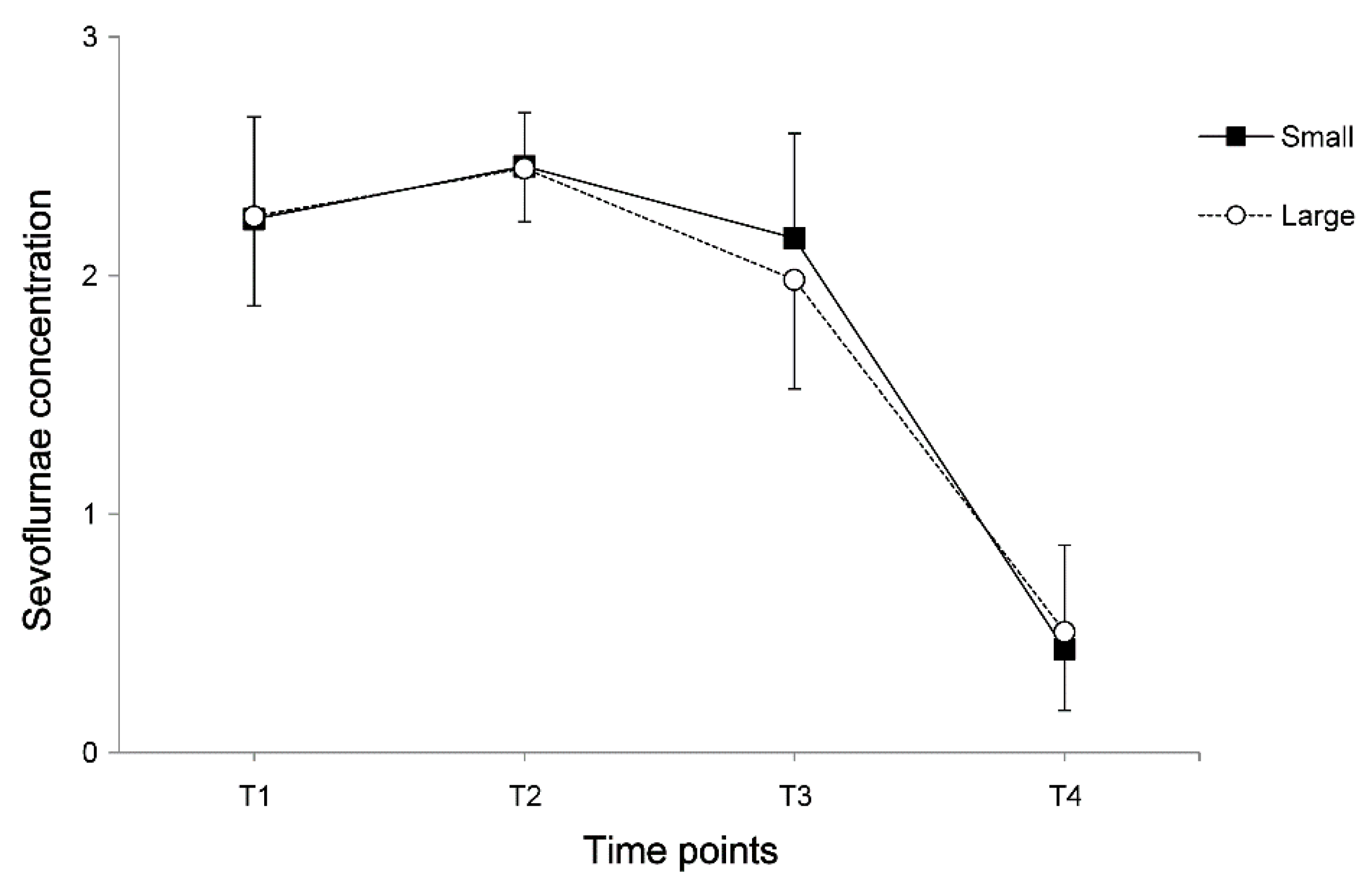
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Price, H.N.; Schaffer, J.V. Congenital melanocytic nevi—When to worry and how to treat: Facts and controversies. Clin. Dermatol. 2010, 28, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Roldan, F.A.; Hernando, A.B.; Cuadrado, A.; Blanco, C.C.; Fernandez, R.S.; Hermosa, J.M.; Ochaita, P.L. Small and medium-sized congenital nevi in children: A comparison of the costs of excision and long-term follow-up. Dermatol. Surg. 2009, 35, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.A.; MacLaren, J.E.; Martin, S.R.; Perret-Karimi, D.; Kain, Z.N. Pediatric pain after ambulatory surgery: Where’s the medication? Pediatrics 2009, 124, e588–e595. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, C.B.; Rabbitts, J.A.; Schroeder, D.R.; Harrison, T.E. Prevalence of moderate-severe pain in hospitalized children. Paediatr. Anaesth. 2012, 22, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Brennan, T.J. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology 2010, 112, 153–164. [Google Scholar] [CrossRef]
- Meier, P.M.; Berde, C.B.; DiCanzio, J.; Zurakowski, D.; Sethna, N.F. Quantitative assessment of cutaneous thermal and vibration sensation and thermal pain detection thresholds in healthy children and adolescents. Muscle Nerve 2001, 24, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, A.; Isik, D.; Bekerecioglu, M. Comparison of classification systems for congenital melanocytic nevi. Dermatol. Surg. 2010, 36, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Guler, G.; Akin, A.; Tosun, Z.; Ors, S.; Esmaoglu, A.; Boyaci, A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr. Anaesth. 2005, 15, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Woodforth, I.J.; Hicks, R.G.; Crawford, M.R.; Stephen, J.P.; Burke, D.J. Electroencephalographic evidence of seizure activity under deep sevoflurane anesthesia in a nonepileptic patient. Anesthesiology 1997, 87, 1579–1582. [Google Scholar] [CrossRef]
- Isik, B.; Arslan, M.; Tunga, A.D.; Kurtipek, O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr. Anaesth. 2006, 16, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, G.W.; Park, D.H.; Ahn, H.E.; Chang, M.Y.; Kim, J.Y. Effects of scalp nerve block on pain and emergence agitation after paediatric nevus surgery: A clinical trial. Acta Anaesthesiol. Scand. 2017, 61, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, S.; Stany, I.; Brasher, C.; Lejeune, C.; Bruneau, B.; Wood, C.; Nivoche, Y.; Constant, I.; Murat, I. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: A meta-analysis of published studies. Br. J. Anaesth. 2010, 104, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Aono, J.; Ueda, W.; Mamiya, K.; Takimoto, E.; Manabe, M. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology 1997, 87, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Voepel-Lewis, T.; Malviya, S.; Tait, A.R. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth. Analg. 2003, 96, 1625–1630. [Google Scholar] [CrossRef]
- Dahmani, S.; Delivet, H.; Hilly, J. Emergence delirium in children: An update. Curr. Opin. Anaesthesiol. 2014, 27, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Johr, M.; Berger, T.M. Paediatric anaesthesia and inhalation agents. Best Pract. Res. Clin. Anaesthesiol. 2005, 19, 501–522. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.T.; Finkel, J.C.; Hannallah, R.S.; Hummer, K.A.; Patel, K.M. Rapid emergence does not explain agitation following sevoflurane anaesthesia in infants and children: A comparison with propofol. Paediatr. Anaesth. 2003, 13, 63–67. [Google Scholar] [CrossRef]
- Costi, D.; Cyna, A.M.; Ahmed, S.; Stephens, K.; Strickland, P.; Ellwood, J.; Larsson, J.N.; Chooi, C.; Burgoyne, L.L.; Middleton, P. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst. Rev. 2014, 9, Cd007084. [Google Scholar] [CrossRef]
- Bajwa, S.A.; Costi, D.; Cyna, A.M. A comparison of emergence delirium scales following general anesthesia in children. Paediatr. Anaesth. 2010, 20, 704–711. [Google Scholar] [CrossRef]
- Dorr, L.D.; Maheshwari, A.V.; Long, W.T.; Wan, Z.; Sirianni, L.E. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty: A prospective, randomized, blinded study. J. Bone Jt. Surg. Am. 2007, 89, 1153–1160. [Google Scholar] [CrossRef]
- Ogonda, L.; Wilson, R.; Archbold, P.; Lawlor, M.; Humphreys, P.; O’Brien, S.; Beverland, D. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes: A prospective, randomized, controlled trial. J. Bone Jt. Surg. Am. 2005, 87, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Prager, J.P.; Csete, M. An unusual cause of pain after nevus excision: Complex regional pain syndrome. J. Am. Acad. Dermatol. 1997, 37, 652–653. [Google Scholar] [CrossRef]
- Oztas, P.; Ilhan, M.N.; Polat, M.; Alli, N. Clinical and dermoscopic characteristics of melanocytic nevi in turkish children and their relationship with environmental and constitutional factors. Dermatol. Surg. 2007, 33, 607–613. [Google Scholar] [PubMed]
- Defrin, R.; Shachal-Shiffer, M.; Hadgadg, M.; Peretz, C. Quantitative somatosensory testing of warm and heat-pain thresholds: The effect of body region and testing method. Clin. J. Pain 2006, 22, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Meh, D.; Denislic, M. Quantitative assessment of thermal and pain sensitivity. J. Neurol. Sci. 1994, 127, 164–169. [Google Scholar] [CrossRef]
- Dyck, P.J.; Zimmerman, I.; Gillen, D.A.; Johnson, D.; Karnes, J.L.; O’Brien, P.C. Cool, warm, and heat-pain detection thresholds: Testing methods and inferences about anatomic distribution of receptors. Neurology 1993, 43, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Hagander, L.G.; Midani, H.A.; Kuskowski, M.A.; Parry, G.J. Quantitative sensory testing: Effect of site and skin temperature on thermal thresholds. Clin. Neurophysiol. 2000, 111, 17–22. [Google Scholar] [CrossRef]

| Variables | Pain | Agitation | |||||
|---|---|---|---|---|---|---|---|
| Low (n = 50) | High (n = 50) | p-Value | Low (n = 50) | High (n = 50) | p-Value | ||
| Parameters for size | long diameter | 3.07 ± 2.44 | 7.78 ± 17.59 | 0.066 | 3.10 ± 2.48 | 7.65 ± 17.43 | 0.071 |
| area before resection | 4.10 ± 5.40 | 10.68 ± 17.76 | 0.015 | 5.34 ± 12.91 | 9.36 ± 13.83 | 0.137 | |
| area of resection | 3.39 ± 4.20 | 6.11 ± 7.35 | 0.026 | 3.33 ± 4.01 | 6.12 ± 7.38 | 0.021 | |
| proportion | 0.06 ± 0.08 | 0.11 ± 0.13 | 0.021 | 0.05 ± 0.07 | 0.11 ± 0.14 | 0.009 | |
| Nevus location | trunk | 8 (16%) | 3 (6%) | 0.110 | 6 (12%) | 5 (10%) | 0.697 |
| face | 26 (52%) | 24 (48%) | 0.689 | 26 (53%) | 24 (47%) | 0.548 | |
| scalp | 7 (14%) | 13 (26%) | 0.134 | 9 (18%) | 11 (22%) | 0.689 | |
| extremities | 9 (18%) | 10 (20%) | 0.799 | 8 (16%) | 11 (22%) | 0.504 | |
| Variables | Small Group (n = 50) | Large Group (n = 50) | p-Value |
|---|---|---|---|
| Age (month) | 34 (12–103) | 31 (10–74) | 0.338 |
| Gender (M/F) | 17/33 | 20/30 | 0.679 |
| Height (cm) | 93.4 (75–131) | 92 (12.5–133) | 0.242 |
| Weight (kg) | 14.5 (8.9–28) | 13.8 (8–25) | 0.471 |
| BSA (cm2) | 6120 (4460–10,094) | 5879 (2946–9218) | 0.275 |
| Operation time (min) | 15 (8–55) | 30 (15–85) | <0.001 |
| Anesthesia time (min) | 55 (15–110) | 67.5 (20–115) | <0.001 |
| Long diameter (cm) | 1.7 (0.5–11.7) | 4.7 (0.5–93) | <0.001 |
| Area before resection (cm2) | 0.95 (0.25–3.25) | 6.75 (2.5–88.5) | <0.001 |
| Area of resection (cm2) | 0.875 (0.25–3.25) | 5.625 (2.25–33.5) | <0.001 |
| Proportion (%) | 0.014 (0.003–0.041) | 0.097 (0.043–0.560) | <0.001 |
| Variables | Small Group (n = 50) | Large Group (n = 50) | p-Value |
|---|---|---|---|
| Highest pain score | 5.5 (0–10) | 7.5 (0–10) | 0.057 |
| Highest agitation score | 6 (0–20) | 12.5 (0–20) | 0.021 |
| Vomiting | 0 | 2 (4%) | 0.495 |
| Patients receiving analgesic | 26 (52%) | 21 (41%) | 0.423 |
| Duration of PACU stay (min) | 60 (30–110) | 62.5 (30–120) | 0.258 |
| Variables | β | SE | p-Value | |
|---|---|---|---|---|
| Pain | age | −0.049 | 0.014 | 0.001 |
| proportion | 7.913 | 2.709 | 0.004 | |
| Agitation | age | −0.097 | 0.031 | 0.002 |
| proportion | 15.803 | 6.061 | 0.011 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Lee, H.S.; Park, D.H.; Seok, S.; Kim, T.K.; Lee, H.S.; Kim, J.E. Effect of Size and Location of Nevi on Postoperative Pain and Emergence Agitation in Children Undergoing Nevi Excision. J. Clin. Med. 2019, 8, 106. https://doi.org/10.3390/jcm8010106
Kim J-S, Lee HS, Park DH, Seok S, Kim TK, Lee HS, Kim JE. Effect of Size and Location of Nevi on Postoperative Pain and Emergence Agitation in Children Undergoing Nevi Excision. Journal of Clinical Medicine. 2019; 8(1):106. https://doi.org/10.3390/jcm8010106
Chicago/Turabian StyleKim, Jin-Soo, Hye Sun Lee, Dong Ha Park, Suhyun Seok, Tae Kwang Kim, Hye Seon Lee, and Ji Eun Kim. 2019. "Effect of Size and Location of Nevi on Postoperative Pain and Emergence Agitation in Children Undergoing Nevi Excision" Journal of Clinical Medicine 8, no. 1: 106. https://doi.org/10.3390/jcm8010106
APA StyleKim, J.-S., Lee, H. S., Park, D. H., Seok, S., Kim, T. K., Lee, H. S., & Kim, J. E. (2019). Effect of Size and Location of Nevi on Postoperative Pain and Emergence Agitation in Children Undergoing Nevi Excision. Journal of Clinical Medicine, 8(1), 106. https://doi.org/10.3390/jcm8010106





