Clopidogrel versus Ticagrelor for Secondary Prevention after Coronary Artery Bypass Grafting
Abstract
1. Introduction
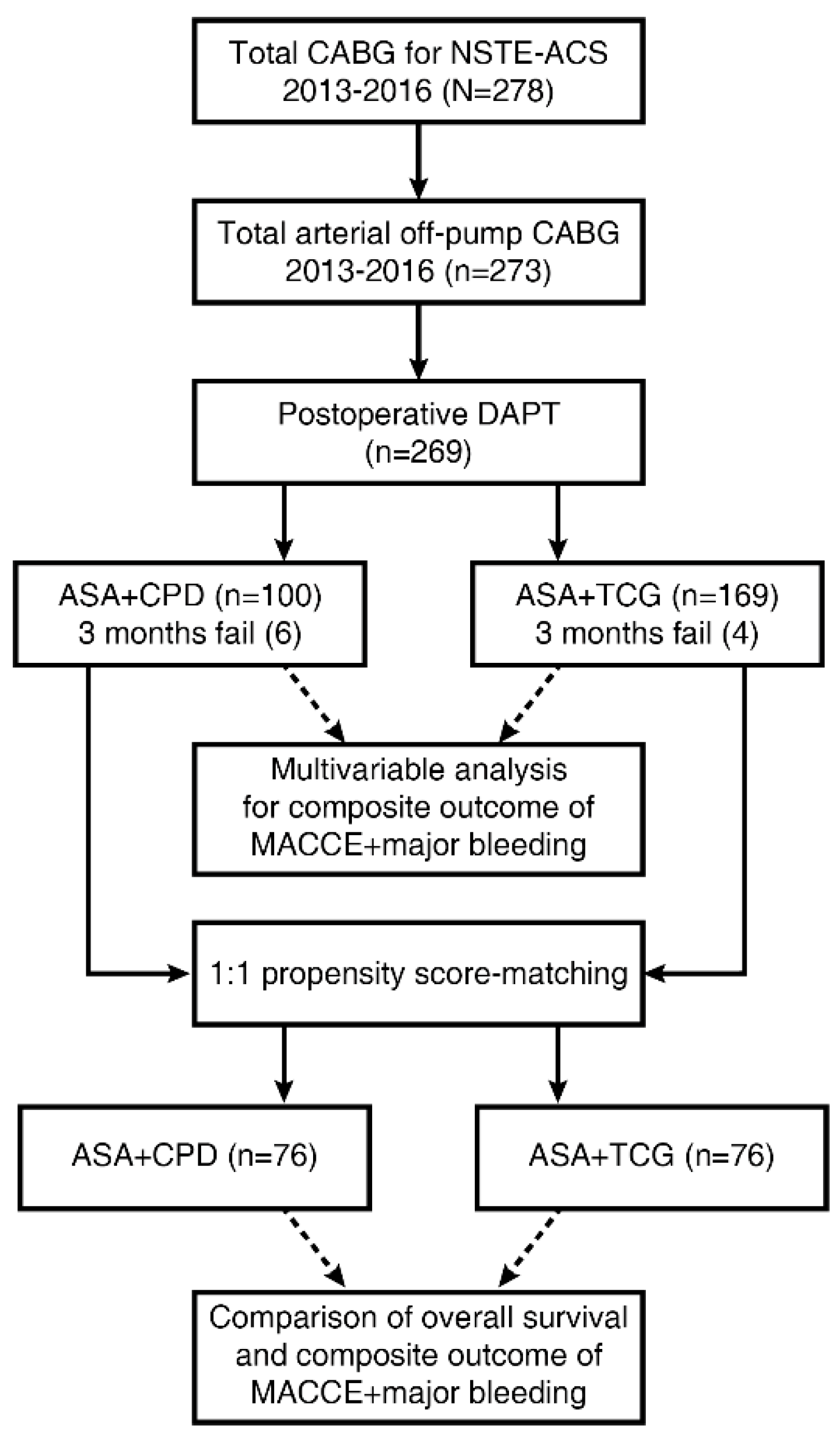
2. Material and Methods
2.1. Study Design
2.2. Surgical Techniques
2.3. Perioperative Management
2.4. Postoperative Dual Antiplatelet Therapy
2.5. Follow-Up
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics and Operative Data
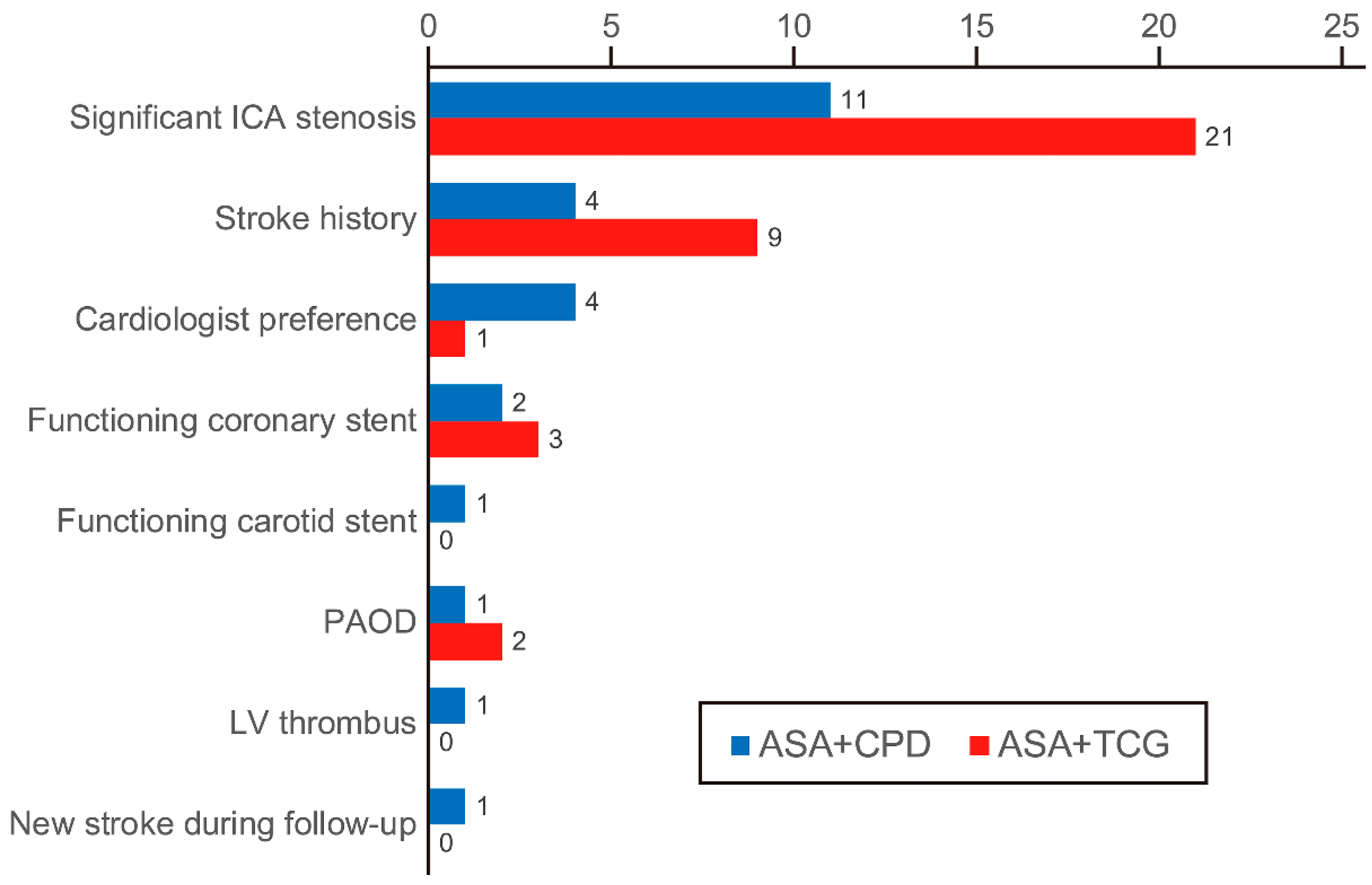
3.2. Postoperative Dual Antiplatelet Therapy
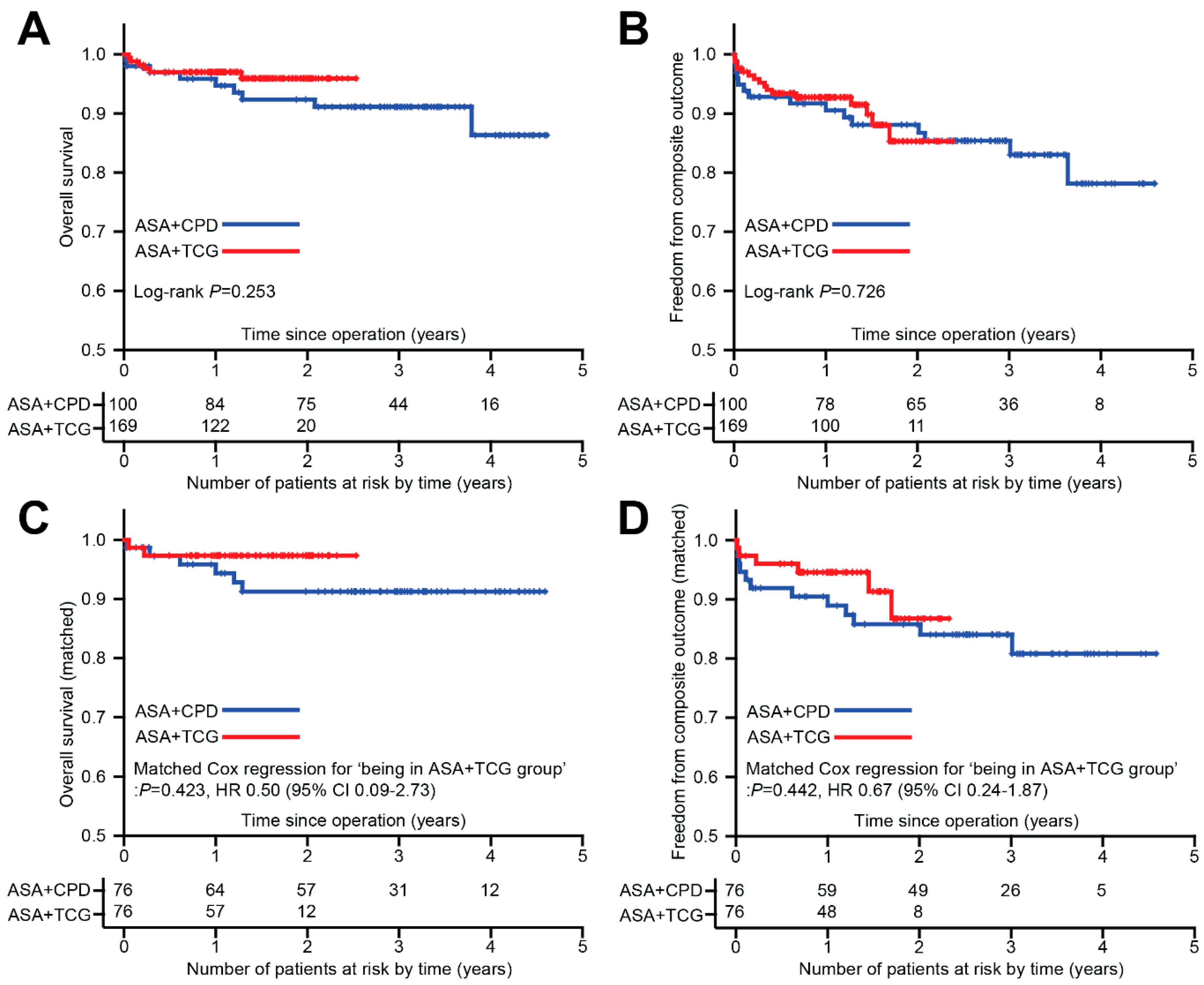
3.3. Overall Outcomes and Multivariable Analysis
3.4. Propensity Score Matched Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 esc guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation of the european society of cardiology (esc). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 acc/aha guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J. Thorac. Cardiovasc. Surg. 2016, 152, 1243–1275. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 esc focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with eacts: The task force for dual antiplatelet therapy in coronary artery disease of the european society of cardiology (esc) and of the european association for cardio-thoracic surgery (eacts). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [PubMed]
- Kulik, A.; Ruel, M.; Jneid, H.; Ferguson, T.B.; Hiratzka, L.F.; Ikonomidis, J.S.; Lopez-Jimenez, F.; McNallan, S.M.; Patel, M.; Roger, V.L.; et al. Secondary prevention after coronary artery bypass graft surgery: A scientific statement from the american heart association. Circulation 2015, 131, 927–964. [Google Scholar] [CrossRef] [PubMed]
- Lytle, B.W.; Blackstone, E.H.; Loop, F.D.; Houghtaling, P.L.; Arnold, J.H.; Akhrass, R.; McCarthy, P.M.; Cosgrove, D.M. Two internal thoracic artery grafts are better than one. J. Thorac. Cardiovasc. Surg. 1999, 117, 855–872. [Google Scholar] [CrossRef]
- Yan, B.P.; Clark, D.J.; Buxton, B.; Ajani, A.E.; Smith, J.A.; Duffy, S.J.; Shardey, G.C.; Skillington, P.D.; Farouque, O.; Yii, M.; et al. Clinical characteristics and early mortality of patients undergoing coronary artery bypass grafting compared to percutaneous coronary intervention: Insights from the australasian society of cardiac and thoracic surgeons (ascts) and the melbourne interventional group (mig) registries. Heart Lung Circ. 2009, 18, 184–190. [Google Scholar] [PubMed]
- ElBardissi, A.W.; Aranki, S.F.; Sheng, S.; O’Brien, S.M.; Greenberg, C.C.; Gammie, J.S. Trends in isolated coronary artery bypass grafting: An analysis of the society of thoracic surgeons adult cardiac surgery database. J. Thorac. Cardiovasc. Surg. 2012, 143, 273–281. [Google Scholar] [CrossRef]
- Gurbuz, A.T.; Zia, A.A.; Vuran, A.C.; Cui, H.; Aytac, A. Postoperative clopidogrel improves mid-term outcome after off-pump coronary artery bypass graft surgery: A prospective study. Eur. J. Cardio-Thorac. Surg. 2006, 29, 190–195. [Google Scholar] [CrossRef]
- Kim, D.H.; Daskalakis, C.; Silvestry, S.C.; Sheth, M.P.; Lee, A.N.; Adams, S.; Hohmann, S.; Medvedev, S.; Whellan, D.J. Aspirin and clopidogrel use in the early postoperative period following on-pump and off-pump coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2009, 138, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zheng, Z.; Pi, Y.; Lu, B.; Lu, J.; Hu, S. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery a single-center, randomized, controlled trial. J. Am. Coll. Cardiol. 2010, 56, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, R.; Abildstrom, S.Z.; Hansen, P.R.; Hvelplund, A.; Andersson, C.; Charlot, M.; Fosbol, E.L.; Kober, L.; Madsen, J.K.; Gislason, G.H.; et al. Efficacy of post-operative clopidogrel treatment in patients revascularized with coronary artery bypass grafting after myocardial infarction. J. Am. Coll. Cardiol. 2011, 57, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Mannacio, V.A.; Di Tommaso, L.; Antignan, A.; De Amicis, V.; Vosa, C. Aspirin plus clopidogrel for optimal platelet inhibition following off-pump coronary artery bypass surgery: Results from the cryssa (prevention of coronary artery bypass occlusion after off-pump procedures) randomised study. Heart 2012, 98, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Kurlansky, P.A. Is there a hypercoagulable state after off-pump coronary artery bypass surgery? What do we know and what can we do? J. Thorac. Cardiovasc. Surg. 2003, 126, 7–10. [Google Scholar] [CrossRef]
- Puskas, J.D.; Williams, W.H.; Mahoney, E.M.; Huber, P.R.; Block, P.C.; Duke, P.G.; Staples, J.R.; Glas, K.E.; Marshall, J.J.; Leimbach, M.E.; et al. Off-pump vs conventional coronary artery bypass grafting: Early and 1-year graft patency, cost, and quality-of-life outcomes: A randomized trial. JAMA 2004, 291, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Hillis, L.D.; Smith, P.K.; Anderson, J.L.; Bittl, J.A.; Bridges, C.R.; Byrne, J.G.; Cigarroa, J.E.; DiSesa, V.J.; Hiratzka, L.F.; Hutter, A.M., Jr.; et al. 2011 accf/aha guideline for coronary artery bypass graft surgery: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J. Thorac. Cardiovasc. Surg. 2012, 143, 4–34. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Clare, R.M.; Gao, R.; Held, C.; Himmelmann, A.; James, S.K.; Lim, S.T.; Santoso, A.; Yu, C.M.; Wallentin, L.; et al. Ticagrelor versus clopidogrel in asian patients with acute coronary syndrome: A retrospective analysis from the platelet inhibition and patient outcomes (plato) trial. Am. Heart J. 2015, 169, 899–905. [Google Scholar] [CrossRef] [PubMed]
| Variables | ASA + CPD (n = 100) | ASA + TCG (n = 169) | P Value |
|---|---|---|---|
| Age, years | 64 ± 10 | 64 ± 10 | 0.865 |
| Male sex, n (%) | 80 (80%) | 121 (72%) | 0.147 |
| Diabetes, (%) | 52 (52%) | 88 (51%) | 0.900 |
| HbA1c, (%) | 6.6 ± 1.3 | 6.5 ± 1.3 | 0.540 |
| Hypertension, n (%) | 66 (66%) | 89 (53%) | 0.041 a |
| Dyslipidaemia, n (%) | 100 (100.0%) | 168 (99%) | >0.999 |
| CVA (<1 year), n (%) | 1 (1%) | 7 (4%) | 0.265 |
| Old MI (>1 year), n (%) | 13 (13%) | 13 (8%) | 0.200 |
| Serum creatinine (mg/dL) | 1.9 ± 2.7 | 1.0 ± 0.5 | 0.001 a |
| Hemodialysis, n (%) | 10 (10%) | 1 (1%) | <0.001 a |
| Poor mobility, n (%) | 2 (2%) | 2 (1%) | 0.632 |
| Recent PCI (<3 months), n (%) | 5 (5%) | 13 (8%) | 0.458 |
| Abnormal brain CT, n (%) | 17/58(29%) | 34/115 (30%) | >0.999 |
| Abnormal carotid CT, n (%) | 25/83 (30%) | 47/122 (39%) | 0.236 |
| PAOD, n (%) | 6 (6%) | 4 (2%) | 0.181 |
| COPD, n (%) | 7 (7%) | 0 (0%) | 0.001 a |
| Current smoker, n (%) | 31 (31%) | 47 (28%) | 0.581 |
| Atrial fibrillation, n (%) | 11 (11%) | 7 (4%) | 0.042 a |
| EuroSCORE II, (%) | 10 ± 9 | 8 ± 7 | 0.093 |
| LV ejection fraction, (%) | 52 ± 14 | 50 ± 14 | 0.136 |
| LV dysfunction, (<35%) | 13 (13%) | 25 (15%) | 0.856 |
| PASP (mmHg) | 27 ± 6 | 27 ± 8 | 0.787 |
| Unstable angina, n (%) | 51 (51%) | 55 (32%) | 0.002 a |
| NSTEMI, n (%) | 49 (49%) | 114 (68%) | 0.002 a |
| Left main disease, n (%) | 33 (33%) | 46 (27%) | 0.334 |
| 1-vessel disease, n (%) | 0 | 8 (5%) | 0.028 a |
| 2-vessel disease, n (%) | 25 (25%) | 33 (20%) | 0.357 |
| 3-vessel disease, n (%) | 72 (72%) | 129 (76%) | 0.469 |
| Variables | ASA + CPD (n = 100) | ASA + TCG (n = 169) | P Value |
|---|---|---|---|
| Urgent operation, n (%) | 4 (4%) | 6 (4%) | >0.999 |
| Number of anastomoses | 3.6 ± 0.9 | 3.7 ± 1.0 | 0.206 |
| Only bilateral ITA for grafting, n (%) | 100 (100%) | 168 (99%) | >0.999 |
| Perioperative IABP support, n (%) | 14 (14%) | 14 (8%) | 0.152 |
| Postoperative ECMO support, n (%) | 1 (1%) | 0 | 0.372 |
| Re-exploration for bleeding, n (%) | 5 (5%) | 4 (2%) | 0.299 |
| Early mortality (<30 days), n (%) | 2 (2%) | 2 (1%) | 0.630 |
| ASA + CPD (16 Outcomes in 100 Patients) | ASA + TCG (16 Outcomes in 169 Patients) | ||||
|---|---|---|---|---|---|
| Sex/Age | Time since Surgery (Years) | Explanation | Sex/Age | Time since Surgery (Years) | Explanation |
| M/61 | 0.00 | Stroke | M/50 | 0.01 | Stroke |
| M/60 | 0.01 | Stroke | F/75 | 0.01 | Stroke |
| M/61 | 0.02 | Death | F/75 | 0.03 | Upper GI bleeding |
| M/52 | 0.02 | Cerebral hemorrhage | M/56 | 0.03 | Upper GI bleeding |
| M/74 | 0.03 | Death | F/78 | 0.08 | Upper GI bleeding |
| F/62 | 0.04 | Upper GI bleeding | F/77 | 0.16 | Death |
| F/74 | 0.11 | Upper GI bleeding | M/70 | 0.22 | Death |
| M/67 | 0.16 | Upper GI bleeding | M/64 | 0.28 | Death |
| M/67 | 0.61 | Death | M/80 | 0.33 | Upper GI bleeding |
| M/62 | 1.00 | Death | M/48 | 0.36 | Upper GI bleeding |
| M/86 | 1.20 | Death | F/75 | 0.41 | New MI during follow-up |
| M/73 | 1.29 | Death | M/67 | 0.68 | Stroke |
| M/68 | 2.01 | Upper GI bleeding | M/79 | 1.28 | Death |
| M/79 | 2.08 | Death | F/72 | 1.45 | Lower GI bleeding |
| M/61 | 3.01 | Cerebellar hemorrhage | M/61 | 1.51 | TVR (redo CABG) |
| M/58 | 3.64 | Upper GI bleeding | M/60 | 1.69 | Upper GI bleeding |
| Variables | Univariate Analysis P Value | Multivariable Analysis P Value | HR (95% CI) |
|---|---|---|---|
| Age | 0.114 | 0.566 | 1.01 (0.97–1.06) |
| Sex | 0.613 | ||
| Use of ticagrelor | 0.727 | 0.972 | 0.99 (0.43–2.28) |
| Body surface area | 0.044 | 0.406 | 0.35 (0.03–4.19) |
| Diabetes | 0.157 | 0.473 | 1.32 (0.62–2.85) |
| Hypertension | 0.245 | ||
| Hemodialysis | 0.032 | 0.046 a | 3.96 (1.02–15.33) |
| Atrial fibrillation | 0.076 | 0.183 | 1.97 (0.73–5.33) |
| PAOD | 0.596 | ||
| Current smoker | 0.906 | ||
| EuroSCORE II | <0.001 | 0.107 | 1.03 (0.99–1.06) |
| NSTEMI | 0.056 | 0.176 | 1.81 (0.77–4.29) |
| Left main disease | 0.182 | 0.203 | 0.54 (0.21–1.39) |
| Number of anastomoses | 0.387 |
| ASA + CPD (n = 100) | ASA + TCG (n = 169) | SMD (Before Matching) | ASA + CPD (n = 76) | ASA + TCG (n = 76) | SMD (After Matching) | |
|---|---|---|---|---|---|---|
| Age (years) | 64 ± 10 | 64 ± 10 | 2% | 64 ± 10 | 64 ± 9 | 3% |
| Sex (male) | 80 (80%) | 121 (72%) | 19% | 59 (78%) | 56 (74%) | 9% |
| BSA (m2) | 1.7 ± 0.2 | 1.7 ± 0.2 | 9% | 1.7 ± 0.2 | 1.7 ± 0.2 | 11% |
| Diabetes | 52 (52%) | 86 (51%) | 2% | 40 (53%) | 36 (47%) | 11% |
| Hypertension a | 66 (66%) | 89 (53%) | 27% | 45 (59%) | 44 (58%) | 3% |
| Creatinine clearance (mL/min) a | 66 ± 33 | 76 ± 30 | 32% | 69 ± 34 | 73 ± 30 | 14% |
| Atrial fibrillation a | 11 (11%) | 7 (4%) | 34% | 6 (8%) | 6 (8%) | 0% |
| Old MI | 13 (13%) | 13 (8%) | 20% | 6 (8%) | 9 (12%) | 15% |
| Recent PCI | 5 (5%) | 13 (8%) | 10% | 3 (4%) | 4 (5%) | 5% |
| Current smoker | 31 (31%) | 47 (28%) | 7% | 26 (34%) | 23 (30%) | 9% |
| EuroSCORE II (%) a | 9.5 ± 8.7 | 7.7 ± 7.1 | 25% | 8.1 ± 6.3 | 7.8 ± 7.4 | 4% |
| NSTEMI a | 49 (49%) | 114 (68%) | 39% | 42 (55%) | 39 (51%) | 8% |
| Left main disease | 33 (33%) | 46 (27%) | 13% | 22 (29%) | 23 (30%) | 3% |
| Number of anastomoses | 3.6 ± 0.9 | 3.7 ± 1.0 | 15% | 3.5 ± 0.9 | 3.5 ± 1.1 | 1% |
| Urgent operation | 4 (4%) | 6 (3.6%) | 2% | 3 (4%) | 3 (4%) | 0% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.W.; Kim, H.J.; Yoo, J.S.; Kim, D.J.; Cho, K.R. Clopidogrel versus Ticagrelor for Secondary Prevention after Coronary Artery Bypass Grafting. J. Clin. Med. 2019, 8, 104. https://doi.org/10.3390/jcm8010104
Chang HW, Kim HJ, Yoo JS, Kim DJ, Cho KR. Clopidogrel versus Ticagrelor for Secondary Prevention after Coronary Artery Bypass Grafting. Journal of Clinical Medicine. 2019; 8(1):104. https://doi.org/10.3390/jcm8010104
Chicago/Turabian StyleChang, Hyoung Woo, Hee Jung Kim, Jae Suk Yoo, Dong Jin Kim, and Kwang Ree Cho. 2019. "Clopidogrel versus Ticagrelor for Secondary Prevention after Coronary Artery Bypass Grafting" Journal of Clinical Medicine 8, no. 1: 104. https://doi.org/10.3390/jcm8010104
APA StyleChang, H. W., Kim, H. J., Yoo, J. S., Kim, D. J., & Cho, K. R. (2019). Clopidogrel versus Ticagrelor for Secondary Prevention after Coronary Artery Bypass Grafting. Journal of Clinical Medicine, 8(1), 104. https://doi.org/10.3390/jcm8010104





