Adverse Outcomes after Major Surgeries in Patients with Diabetes: A Multicenter Matched Study
Abstract
1. Introduction
2. Methods
2.1. Source of Data
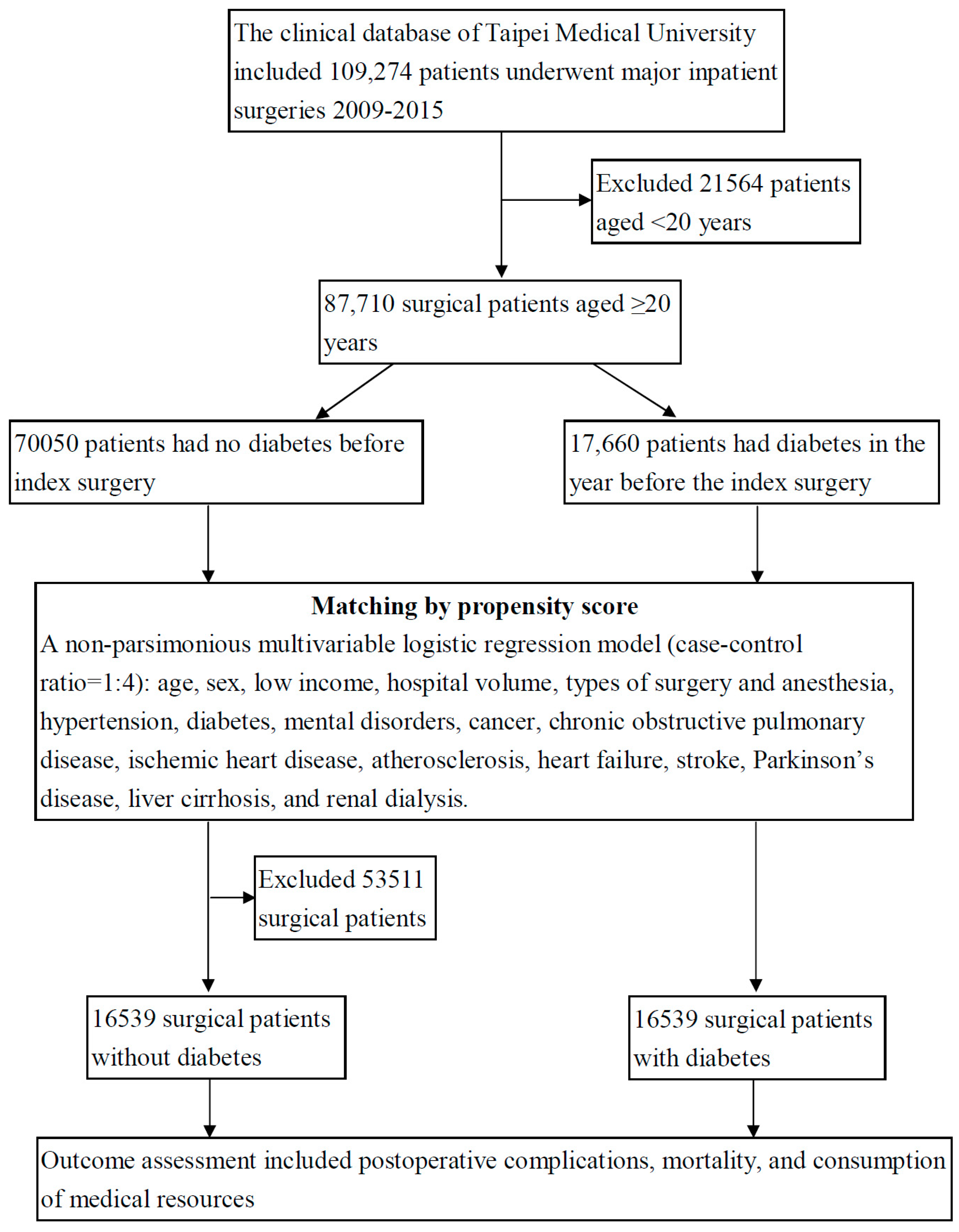
2.2. Study Design
2.3. Criteria and Definition
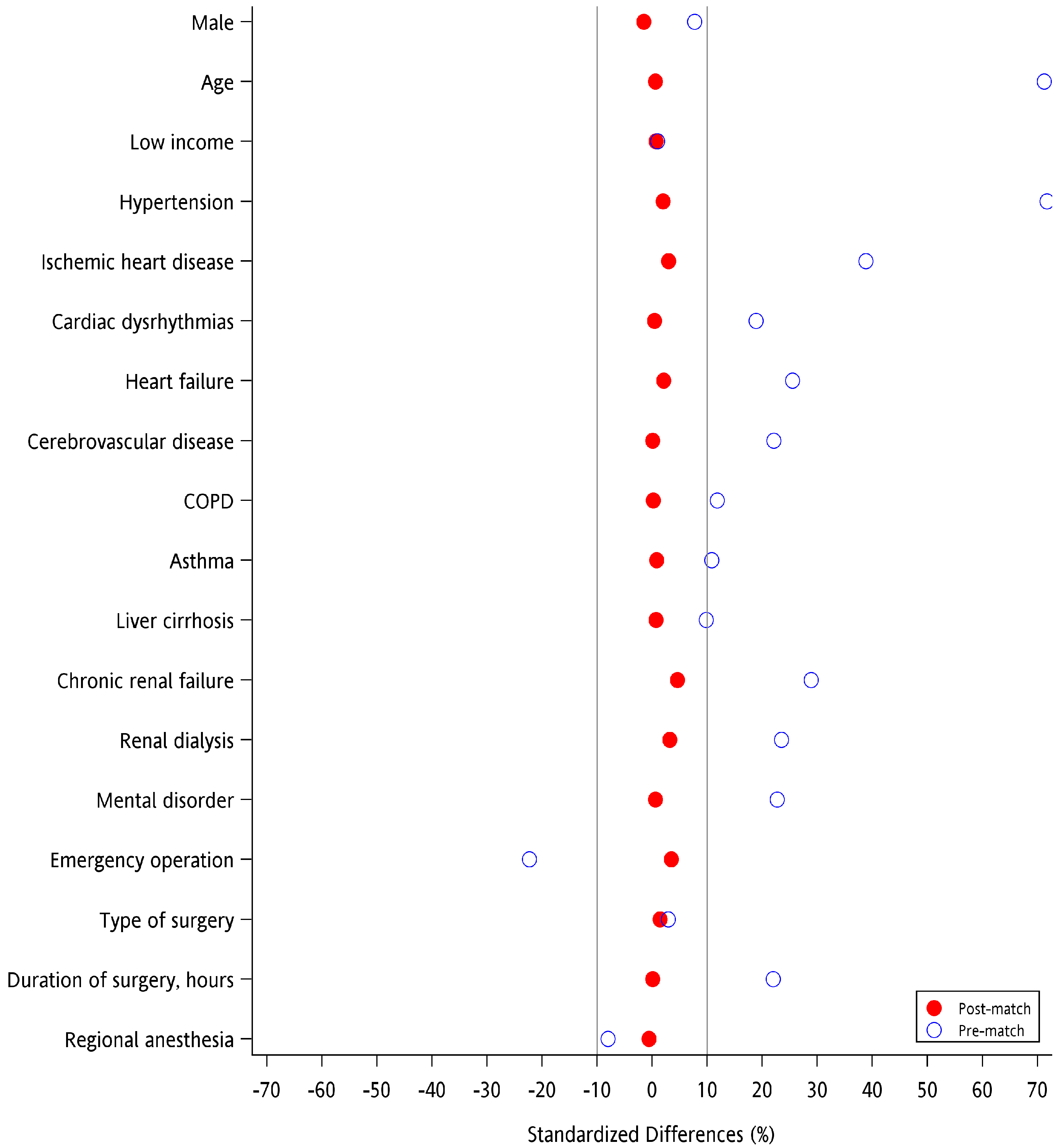
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4·4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Claessen, H.; Kvitkina, T.; Narres, M.; Trautner, C.; Zöllner, I.; Bertram, B.; Icks, A. Markedly decreasing incidence of blindness in people with and without diabetes in southern Germany. Diabetes Care 2018, 41, 478–484. [Google Scholar] [CrossRef]
- Helve, J.; Sund, R.; Arffman, M.; Harjutsalo, V.; Groop, P.H.; Grönhagen-Riska, C.; Finne, P. Incidence of end-stage renal disease in patients with Type 1 diabetes. Diabetes Care 2018, 41, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalak, B.B.; Knittel, J.; Abdelmalak, J.B.; Dalton, J.E.; Christiansen, E.; Foss, J.; Argalious, M.; Zimmerman, R.; Van den Berghe, G. Preoperative blood glucose concentrations and postoperative outcomes after elective non-cardiac surgery: An observational study. Br. J. Anaesth. 2014, 112, 79–88. [Google Scholar] [CrossRef]
- Greco, G.; Ferket, B.S.; D’Alessandro, D.A.; Shi, W.; Horvath, K.A.; Rosen, A.; Welsh, S.; Bagiella, E.; Neill, A.E.; Williams, D.L.; et al. Diabetes and the association of postoperative hyperglycemia with clinical and economic outcomes in cardiac surgery. Diabetes Care 2016, 39, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huedo, M.A.; Jiménez-García, R.; Jiménez-Trujillo, I.; Hernández-Barrera, V.; Del Rio Lopez, B.; López-de-Andrés, A. Effect of type 2 diabetes on in-hospital postoperative complications and mortality after primary total hip and knee arthroplasty. J. Arthroplasty 2017, 32, 3729–3734. [Google Scholar] [CrossRef]
- Toor, A.S.; Jiang, J.J.; Shi, L.L.; Koh, J.L. Comparison of perioperative complications after total elbow arthroplasty in patients with and without diabetes. J. Shoulder Elbow Surg. 2014, 23, 1599–1606. [Google Scholar] [CrossRef]
- Debing, E.; Aerden, D.; Van den Brande, P. Diabetes mellitus is a predictor for early adverse outcome after carotid endarterectomy. Vasc. Endovasc. Surg. 2011, 45, 28–32. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Thorell, A.; Soop, M.; Ljungqvist, O.; Nygren, J. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br. J. Surg. 2009, 96, 1358–1364. [Google Scholar] [CrossRef]
- Goodenough, C.J.; Liang, M.K.; Nguyen, M.T.; Nguyen, D.H.; Holihan, J.L.; Alawadi, Z.M.; Roth, J.S.; Wray, C.J.; Ko, T.C.; Kao, L.S. Preoperative glycosylated hemoglobin and postoperative glucose together predict major complications after abdominal surgery. J. Am. Coll. Surg. 2015, 221, 854–861. [Google Scholar] [CrossRef]
- Yeh, C.C.; Liao, C.C.; Chang, Y.C.; Jeng, L.B.; Yang, H.R.; Shih, C.C.; Chen, T.L. Adverse outcomes after noncardiac surgery in patients with diabetes: A nationwide population-based retrospective cohort study. Diabetes Care 2013, 36, 3216–3221. [Google Scholar] [CrossRef] [PubMed]
- Kotagal, M.; Symons, R.G.; Hirsch, I.B.; Umpierrez, G.E.; Dellinger, E.P.; Farrokhi, E.T.; Flum, D.R. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann. Surg. 2015, 261, 97–103. [Google Scholar] [CrossRef] [PubMed]
- King, J.T., Jr.; Goulet, J.L.; Perkal, M.F.; Rosenthal, R.A. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann. Surg. 2011, 253, 158–165. [Google Scholar] [CrossRef]
- Pugely, A.J.; Martin, C.T.; Gao, Y.; Klocke, N.F.; Callaghan, J.J.; Marsh, J.L. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J. Orthop. Trauma 2014, 28, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.D.; Saltzberg, S.S.; Sheahan, M.; Froelich, J.; Akbari, C.M.; Campbell, D.R.; LoGerfo, F.W.; Pomposelli, F.B., Jr. Lack of association of diabetes with increased postoperative mortality and cardiac morbidity: Results of 6565 major vascular operations. Arch. Surg. 2002, 137, 417–421. [Google Scholar] [CrossRef]
- Buchleitner, A.M.; Martínez-Alonso, M.; Hernández, M.; Solà, I.; Mauricio, D. Perioperative glycaemic control for diabetic patients undergoing surgery. Cochrane Database Syst. Rev. 2012, 9, CD007315. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat. Med. 2010, 29, 2137–2148. [Google Scholar] [CrossRef]
- Lin, P.J.; Kent, D.M.; Winn, A.; Cohen, J.T.; Neumann, P.J. Multiple chronic conditions in type 2 diabetes mellitus: Prevalence and consequences. Am. J. Manag. Care 2015, 21, e23–e34. [Google Scholar]
- Liao, C.C.; Lin, C.S.; Shih, C.C.; Yeh, C.C.; Chang, Y.C.; Lee, Y.W.; Chen, T.L. Increased risk of fracture and postfracture adverse events in patients with diabetes: Two nationwide population-based retrospective cohort studies. Diabetes Care 2014, 37, 2246–2252. [Google Scholar] [CrossRef]
- Martinez-Millana, A.; Bayo-Monton, J.L.; Argente-Pla, M.; Fernandez-Llatas, C.; Merino-Torres, J.F.; Traver-Salcedo, V. Integration of distributed services and hybrid models based on process choreography to predict and detect type 2 diabetes. Sensors 2018, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Hux, J.E. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 2003, 26, 510–513. [Google Scholar] [CrossRef]
- Brophy, R.H.; Wright, R.W.; Huston, L.J.; Nwosu, S.K.; Spindler, K.P. Factors associated with infection following anterior cruciate ligament reconstruction. J. Bone Jt. Surg. Am. 2015, 97, 450–454. [Google Scholar] [CrossRef]
- Dryden, M.; Baguneid, M.; Eckmann, C.; Corman, S.; Stephens, J.; Solem, C.; Li, J.; Charbonneau, C.; Baillon-Plot, N.; Haider, S. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: Focus on skin and soft-tissue infections. Clin. Microbiol. Infect. 2015, 21 (Suppl. S2), S27–S32. [Google Scholar] [CrossRef]
- Bagdade, J.D.; Root, R.K.; Bulger, R.J. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes 1974, 23, 9–15. [Google Scholar] [CrossRef]
- Nielson, C.P.; Hindson, D.A. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes 1989, 38, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- Krogh-Madsen, R.; Moller, K.; Dela, F.; Kronborg, G.; Jauffred, S.; Pedersen, B.K. Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-alpha, and FFAs to low-dose endotoxemia in humans. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E766–E772. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, K.W.; Kim, Y.S.; Oh, S.; Chae, I.H.; Kim, H.S.; Kim, C.H. Effects of acute hyperglycemia on endothelium-dependent vasodilation in patients with diabetes mellitus or impaired glucose metabolism. Endothelium 2003, 10, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Black, C.T.; Hennessey, P.J.; Andrassy, R.J. Short-term hyperglycemia depresses immunity through nonenzymatic glycosylation of circulating immunoglobulin. J. Trauma 1990, 30, 830–832. [Google Scholar] [CrossRef]
- Hennessey, P.J.; Black, C.T.; Andrassy, R.J. Nonenzymatic glycosylation of immunoglobulin G impairs complement fixation. JPEN J. Parenter Enteral Nutr. 1991, 15, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Stegenga, M.E.; van der Crabben, S.N.; Levi, M.; de Vos, A.F.; Tanck, M.W.; Sauerwein, H.P.; van der Poll, T. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes 2006, 55, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Casagrande, S.; Geiss, L.; Cowie, C.C. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015, 314, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Wang, D.; Lee, A.K.; Bergenstal, R.M.; Coresh, J. Identifying trends in undiagnosed diabetes in U.S. adults by using a confirmatory definition: A cross-sectional study. Ann. Intern. Med. 2017, 167, 769–776. [Google Scholar] [CrossRef]
- Meisinger, C.; Strassburger, K.; Heier, M.; Thorand, B.; Baumeister, S.E.; Giani, G.; Rathmann, W. Prevalence of undiagnosed diabetes and impaired glucose regulation in 35–59-year-old individuals in Southern Germany: The KORA F4 Study. Diabet. Med. 2010, 27, 360–362. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study Group. UK Prospective Diabetes Study 6. Complications in newly diagnosed Type 2 diabetic patients and their association with different clinical and biochemical risk factors. Diabetes Res. 1990, 13, 1–11. [Google Scholar]
- Mykkanen, L.; Haffner, S.M.; Kuusisto, J.; Pyorala, K.; Laakso, M. Microalbuminuria precedes the development of NIDDM. Diabetes 1994, 43, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.I.; Eastman, R.C. Early detection of undiagnosed diabetes mellitus: A US perspective. Diabetes Metab. Res. Rev. 2000, 16, 230–236. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Wilmer, A.; Hermans, G.; Meersseman, W.; Wouters, P.J.; Milants, I.; Van Wijngaerden, E.; Bobbaers, H.; Bouillon, R. Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 2006, 354, 449–461. [Google Scholar] [CrossRef]
| No DM (N = 70,050) | DM (N = 17,660) | p * | |||
|---|---|---|---|---|---|
| Sex | n | (%) | n | (%) | <0.0001 |
| Male | 31,653 | (45.2) | 8658 | (49.0) | |
| Female | 38,397 | (54.8) | 9002 | (51.0) | |
| Age, years | <0.0001 | ||||
| 20–29 | 8726 | (12.5) | 366 | (2.1) | |
| 30–39 | 15,193 | (21.7) | 1277 | (7.2) | |
| 40–49 | 13,278 | (19.0) | 2430 | (13.8) | |
| 50–59 | 13,303 | (19.0) | 3903 | (22.1) | |
| 60–69 | 9711 | (13.9) | 4491 | (25.4) | |
| 70–79 | 5880 | (8.4) | 3276 | (18.6) | |
| ≥80 | 3959 | (5.7) | 1917 | (10.9) | |
| Mean ± standard deviation | 49.3 ± 17.1 | 60.7 ± 14.9 | <0.0001 | ||
| Low income | 905 | (1.3) | 245 | (1.4) | 0.3193 |
| Comorbidities | |||||
| Hypertension | 6479 | (9.2) | 6686 | (37.9) | <0.0001 |
| Ischemic heart disease | 2625 | (3.7) | 2610 | (14.8) | <0.0001 |
| Mental disorder | 3410 | (4.9) | 1930 | (10.9) | <0.0001 |
| Cardiac dysrhythmias | 1759 | (2.5) | 1126 | (6.4) | <0.0001 |
| Heart failure | 953 | (1.4) | 1090 | (6.2) | <0.0001 |
| Chronic renal failure | 578 | (0.8) | 1065 | (6.0) | <0.0001 |
| Cerebrovascular disease | 1258 | (1.8) | 1071 | (6.1) | <0.0001 |
| Renal dialysis | 337 | (0.5) | 689 | (3.9) | <0.0001 |
| COPD | 859 | (1.2) | 511 | (2.9) | <0.0001 |
| Asthma | 591 | (0.8) | 380 | (2.2) | <0.0001 |
| Liver cirrhosis | 264 | (0.4) | 220 | (1.2) | <0.0001 |
| Emergency operation | 7831 | (11.2) | 907 | (5.1) | <0.0001 |
| Types of surgery | <0.0001 | ||||
| Skin | 696 | (1.0) | 206 | (1.2) | |
| Breast | 1568 | (2.2) | 291 | (1.6) | |
| Musculoskeletal | 14,847 | (21.2) | 3433 | (19.4) | |
| Respiratory | 3598 | (5.1) | 977 | (5.5) | |
| Cardiovascular | 954 | (1.4) | 605 | (3.4) | |
| Digestive | 13,944 | (19.9) | 3970 | (22.5) | |
| Kidney, ureter, bladder | 4047 | (5.8) | 1226 | (6.9) | |
| Delivery, caesarean section | 3856 | (5.5) | 106 | (0.6) | |
| Neurosurgery | 6035 | (8.6) | 2286 | (12.9) | |
| Other | 20,505 | (29.3) | 4560 | (25.8) | |
| Duration of surgery, hours | <0.0001 | ||||
| <2 | 47,339 | (67.6) | 10,225 | (57.9) | |
| 2–4 | 19,684 | (28.1) | 5936 | (33.6) | |
| ≥5 | 3027 | (4.3) | 1499 | (8.5) | |
| Mean ± standard deviation | 1.84 ± 1.55 | 2.23 ± 1.99 | <0.0001 | ||
| Types of anesthesia | <0.0001 | ||||
| General | 49,367 | (70.5) | 13,085 | (74.1) | |
| Regional | 20,683 | (29.5) | 4575 | (25.9) | |
| No DM (N = 16,539) | DM (N = 16,539) | p-Value * | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Sex | 0.1082 | ||||
| Male | 8196 | (49.6) | 8068 | (48.8) | |
| Female | 8343 | (50.4) | 8471 | (51.2) | |
| Age, years | 0.4130 | ||||
| 20–29 | 304 | (1.8) | 364 | (2.2) | |
| 30–39 | 1135 | (6.9) | 1266 | (7.7) | |
| 40–49 | 2369 | (14.3) | 2385 | (14.4) | |
| 50–59 | 3870 | (23.4) | 3754 | (22.7) | |
| 60–69 | 4239 | (25.6) | 4120 | (24.9) | |
| 70–79 | 2876 | (17.4) | 2895 | (17.5) | |
| ≥80 | 1746 | (10.6) | 1755 | (10.6) | |
| Mean ± standard deviation | 60.3 ± 14.9 | 60.2 ± 15.0 | 0.7082 | ||
| Low income | 213 | (1.3) | 226 | (1.4) | 0.5312 |
| Coexisting medical conditions | |||||
| Hypertension | 5515 | (33.3) | 5668 | (34.3) | 0.7629 |
| Ischemic heart disease | 1900 | (11.5) | 2059 | (12.4) | 0.2263 |
| Mental disorder | 1607 | (9.7) | 1638 | (9.9) | 0.5536 |
| Cardiac dysrhythmias | 966 | (5.8) | 982 | (5.9) | 0.6993 |
| Cerebrovascular disease | 876 | (5.3) | 880 | (5.3) | 0.9184 |
| Heart failure | 725 | (4.4) | 798 | (4.8) | 0.0964 |
| Chronic renal failure | 499 | (3.0) | 636 | (3.8) | 0.2915 |
| COPD | 449 | (2.7) | 455 | (2.8) | 0.8384 |
| Renal dialysis | 299 | (1.8) | 373 | (2.3) | 0.2134 |
| Asthma | 299 | (1.8) | 316 | (1.9) | 0.4814 |
| Liver cirrhosis | 167 | (1.0) | 178 | (1.1) | 0.5478 |
| Emergency surgery | 754 | (4.6) | 880 | (5.3) | 0.1376 |
| Types of surgery | 0.5863 | ||||
| Skin | 182 | (1.1) | 189 | (1.1) | |
| Breast | 254 | (1.5) | 287 | (1.7) | |
| Musculoskeletal | 3245 | (19.6) | 3270 | (19.8) | |
| Respiratory | 923 | (5.6) | 920 | (5.6) | |
| Cardiovascular | 403 | (2.4) | 455 | (2.8) | |
| Digestive | 3759 | (22.7) | 3736 | (22.6) | |
| Kidney, ureter, bladder | 1171 | (7.1) | 1157 | (7.0) | |
| Delivery, caesarean section | 90 | (0.5) | 106 | (0.6) | |
| Neurosurgery | 2097 | (12.7) | 2087 | (12.6) | |
| Other | 4415 | (26.7) | 4332 | (26.2) | |
| Duration of surgery, hours | 0.9565 | ||||
| <2 | 9697 | (58.6) | 9687 | (58.6) | |
| 2–4 | 5558 | (33.6) | 5544 | (33.5) | |
| ≥5 | 1284 | (7.8) | 1308 | (7.9) | |
| Mean ± standard deviation | 2.19 ± 1.90 | 2.19 ± 1.92 | 0.1930 | ||
| Types of anesthesia | 0.4890 | ||||
| General | 12,146 | (73.4) | 12,193 | (73.7) | |
| Regional | 4393 | (26.6) | 4346 | (26.3) | |
| Post-Operative Outcomes | No DM (N = 16,539) | DM (N = 16,539) | Risk of Outcomes | |||
|---|---|---|---|---|---|---|
| Events | Rate, % | Events | Rate, % | OR | (95%CI) * | |
| In-hospital mortality | 61 | (0.4) | 89 | (0.5) | 1.51 | (1.07–2.13) |
| Non-infectious complications | 56 | (0.3) | 54 | (0.3) | 1.01 | (0.69–1.48) |
| Acute myocardial infarction | 4 | (0.0) | 5 | (0.0) | 1.32 | (0.29–5.99) |
| Stroke | 19 | (0.1) | 11 | (0.1) | 0.60 | (0.29–1.28) |
| Acute kidney injury | 24 | (0.1) | 27 | (0.2) | 1.25 | (0.71–2.20) |
| Post-operative bleeding | 6 | (0.0) | 6 | (0.0) | 1.04 | (0.33–3.28) |
| Pulmonary embolism | 3 | (0.0) | 2 | (0.0) | 0.51 | (0.08–3.39) |
| Deep vein thrombosis | 4 | (0.0) | 4 | (0.0) | 0.94 | (0.22–4.01) |
| Infectious complications | 430 | (2.6) | 551 | (3.3) | 1.26 | (1.10–1.43) |
| Pneumonia | 104 | (0.6) | 88 | (0.5) | 0.81 | (0.61–1.09) |
| Septicemia | 96 | (0.6) | 132 | (0.8) | 1.33 | (1.01–1.74) |
| Urinary tract infection | 181 | (1.1) | 211 | (1.3) | 1.14 | (0.93–1.40) |
| Surgical site infection | 58 | (0.4) | 80 | (0.5) | 1.32 | (0.94–1.86) |
| Fungal infection | 20 | (0.1) | 18 | (0.1) | 0.88 | (0.46–1.69) |
| Necrotizing fasciitis | 3 | (0.0) | 15 | (0.1) | 3.98 | (1.12–14.2) |
| Cellulitis | 43 | (0.3) | 96 | (0.6) | 2.10 | (1.46–3.03) |
| Acute pyelonephritis | 16 | (0.1) | 32 | (0.2) | 1.86 | (1.01–3.41) |
| Infectious arthritis | 4 | (0.0) | 13 | (0.1) | 3.89 | (1.19–12.7) |
| Osteomyelitis | 9 | (0.1) | 16 | (0.1) | 1.68 | (0.73–3.85) |
| Post-operative adverse events † | 895 | (5.4) | 1234 | (7.5) | 1.45 | (1.32–1.60) |
| Admitted to intensive care unit | 1587 | (9.6) | 1901 | (11.5) | 1.24 | (1.14–1.34) |
| Length of stay, days (mean ± SD) ‡ | 7.1 ± 10.1 | 9.1 ± 14.5 | p-value < 0.0001 | |||
| Medical expenditure, (mean ± SD) ‡ | 4221 ± 4693 | 5167 ± 5720 | p-value < 0.0001 | |||
| n * | Post-Operative Adverse Events | ||||
|---|---|---|---|---|---|
| Events | Rate, % | OR | (95% CI) | ||
| No diabetes | 16,539 | 1145 | (6.9) | 1.00 | (references) |
| Patients with diabetes had | |||||
| Undiagnosed diabetes | 9908 | 757 | (7.6) | 1.40 | (1.26–1.56) |
| Diabetes-related complications | 2231 | 350 | (15.7) | 1.68 | (1.45–1.94) |
| Type 1 diabetes | 99 | 11 | (11.1) | 1.90 | (0.96–3.75) |
| Types 2 diabetes | 6503 | 736 | (11.3) | 1.39 | (1.25–1.55) |
| Admission due to diabetes | 725 | 128 | (17.7) | 2.33 | (1.85–2.93) |
| HbA1c, 6.5–8% | 2314 | 255 | (11.0) | 1.43 | (1.22–1.67) |
| HbA1c, >8% | 1639 | 194 | (11.8) | 1.96 | (1.64–2.33) |
| Fasting glucose 126–180 mg/dL | 8578 | 545 | (6.4) | 1.13 | (1.01–1.26) |
| Fasting glucose >180 mg/dL | 3747 | 457 | (12.2) | 1.90 | (1.68–2.16) |
| Use of biguanides | 5532 | 537 | (9.7) | 1.32 | (1.03–1.70) |
| Use of sulfonylureas | 3912 | 475 | (12.1) | 1.84 | (1.39–2.42) |
| Use of meglitinides | 1051 | 237 | (22.5) | 2.71 | (1.81–4.07) |
| Use of dipeptidyl peptidase 4 inhibitors | 2522 | 337 | (13.4) | 1.68 | (1.22–2.30) |
| Use of α-glucosidase inhibitors | 1233 | 178 | (14.4) | 1.82 | (1.22–2.73 |
| Use of glucagon-like peptide-1 agonist | 325 | 70 | (21.5) | 1.98 | (0.59–6.57) |
| Use of thiazolidinediones | 571 | 67 | (11.7) | 1.43 | (0.73–2.81) |
| Use of insulin | 7797 | 1116 | (14.3) | 1.80 | (1.45–2.24) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-S.; Chang, C.-C.; Lee, Y.-W.; Liu, C.-C.; Yeh, C.-C.; Chang, Y.-C.; Chuang, M.-T.; Chang, T.-H.; Chen, T.-L.; Liao, C.-C. Adverse Outcomes after Major Surgeries in Patients with Diabetes: A Multicenter Matched Study. J. Clin. Med. 2019, 8, 100. https://doi.org/10.3390/jcm8010100
Lin C-S, Chang C-C, Lee Y-W, Liu C-C, Yeh C-C, Chang Y-C, Chuang M-T, Chang T-H, Chen T-L, Liao C-C. Adverse Outcomes after Major Surgeries in Patients with Diabetes: A Multicenter Matched Study. Journal of Clinical Medicine. 2019; 8(1):100. https://doi.org/10.3390/jcm8010100
Chicago/Turabian StyleLin, Chao-Shun, Chuen-Chau Chang, Yuan-Wen Lee, Chih-Chung Liu, Chun-Chieh Yeh, Yi-Cheng Chang, Ming-Tsang Chuang, Tzu-Hao Chang, Ta-Liang Chen, and Chien-Chang Liao. 2019. "Adverse Outcomes after Major Surgeries in Patients with Diabetes: A Multicenter Matched Study" Journal of Clinical Medicine 8, no. 1: 100. https://doi.org/10.3390/jcm8010100
APA StyleLin, C.-S., Chang, C.-C., Lee, Y.-W., Liu, C.-C., Yeh, C.-C., Chang, Y.-C., Chuang, M.-T., Chang, T.-H., Chen, T.-L., & Liao, C.-C. (2019). Adverse Outcomes after Major Surgeries in Patients with Diabetes: A Multicenter Matched Study. Journal of Clinical Medicine, 8(1), 100. https://doi.org/10.3390/jcm8010100





