Risks of Pneumonia in COPD Patients with New-Onset Atrial Fibrillation
Abstract
1. Introduction
2. Methods
2.1. Data Source
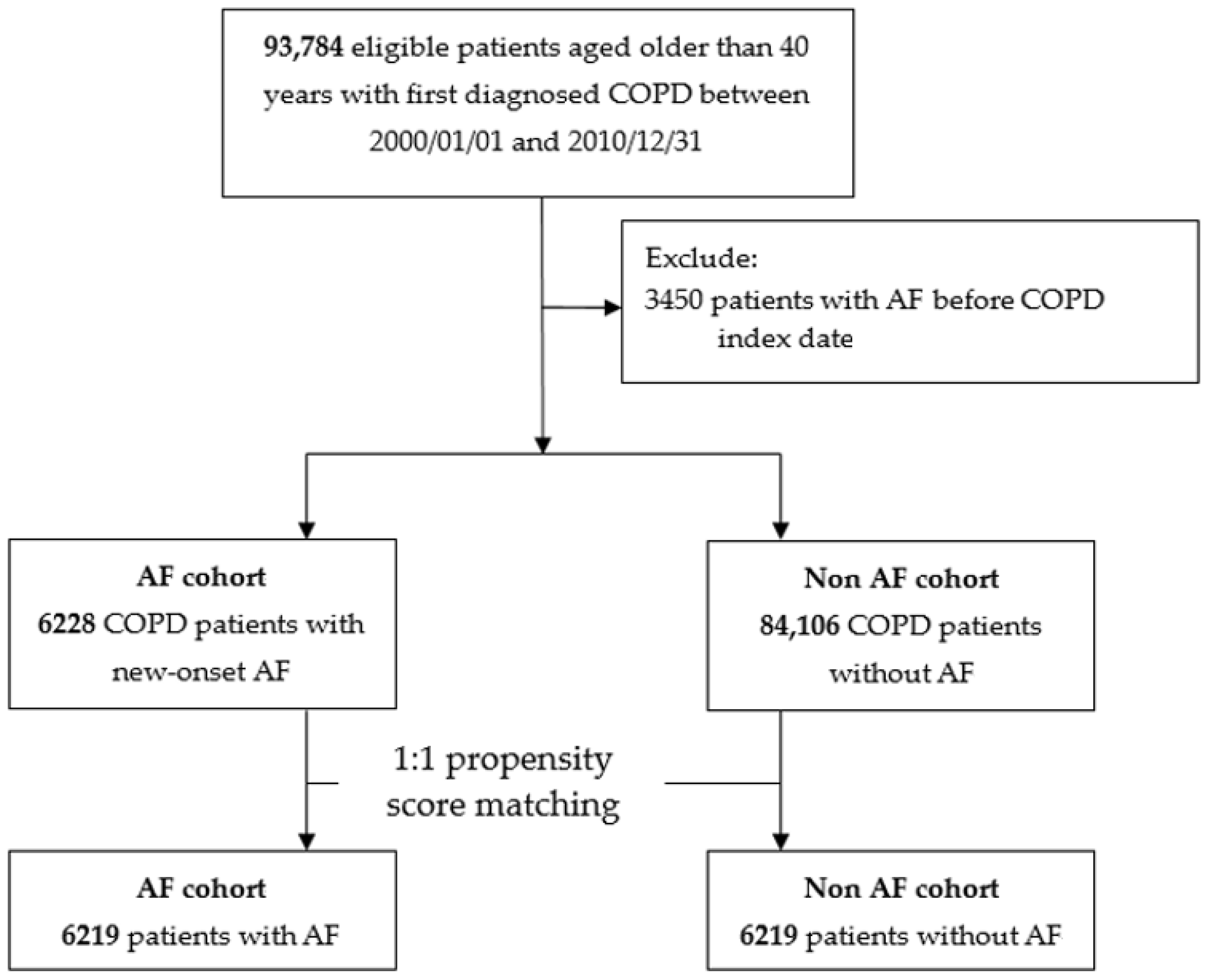
2.2. Study Cohort
2.3. Demographic Characteristics and Comorbidities
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Risk of Death and Pneumonia
3.3. Association between CHA2DS2-VASc/CHADS2/CCI Scores and Risk of Pneumonia in COPD Patients with AF
3.4. Association between COPD Medications and Risk of Pneumonia in COPD Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takir, H.B.; Esquinas, A.M. Community-acquired pneumonia and survival of critically ill acute exacerbation of COPD patients in respiratory intensive care units. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 783–784. [Google Scholar] [CrossRef] [PubMed]
- Koulenti, D.; Blot, S.; Dulhunty, J.M.; Papazian, L.; Martin-Loeches, I.; Dimopoulos, G.; Brun-Buisson, C.; Nauwynck, M.; Putensen, C.; Sole-Violan, J.; et al. COPD patients with ventilator-associated pneumonia: Implications for management. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Chen, H.X.; Liu, W.; Fan, T.; Liu, G.J.; Mao, B. Is COPD associated with increased mortality and morbidity in hospitalized pneumonia. A systematic review and meta-analysis. Respirology 2015, 20, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Brechin, C.M.; Lane, D.A. The global burden of atrial fibrillation and stroke: A systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest 2012, 142, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.; Carrington, M.J.; McMurray, J.J.; Stewart, S. Atrial fibrillation: Profile and burden of an evolving epidemic in the 21st century. Int. J. Cardiol. 2013, 167, 1807–1824. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Lainscak, M.; Dagres, N.; Filippatos, G.S.; Anker, S.D.; Kremastinos, D.T. Atrial fibrillation in chronic non-cardiac disease: Where do we stand. Int. J. Cardiol. 2008, 128, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Providencia, R.; Ferreira, M.J.; Goncalves, L.M. Atrial fibrillation and non-cardiovascular diseases: A systematic review. Arq. Bras. Cardiol. 2015, 105, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Liu, C.J.; Chang, P.M.; Tsao, H.M.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Tuan, T.C.; Li, C.H.; Chao, T.F.; et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int. J. Cardiol. 2013, 165, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, S.; Klein Klouwenberg, P.M.; Cremer, O.L. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: A systematic review. Crit. Care 2014, 18, 688. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Bajwa, A.; Gajic, O.; Afessa, B. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J. Intensive Care Med. 2008, 23, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, R.; Steinhilber, E.; Eggermann, C.; Weiss, M.; Voglic, S.; Bogelein, D.; Gauss, A.; Georgieff, M.; Stahl, W. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: A prospective observational study. Crit. Care 2010, 14, R108. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Pressman, G.; Caples, S.M.; Kanagala, R.; Gard, J.J.; Davison, D.E.; Malouf, J.F.; Ammash, N.M.; Friedman, P.A.; Somers, V.K. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004, 110, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Oza, N.; Baveja, S.; Khayat, R.; Houmsse, M. Obstructive sleep apnea and atrial fibrillation: Understanding the connection. Expert Rev. Cardiovasc. Ther. 2014, 12, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Lopez, F.L.; Matsushita, K.; Loehr, L.R.; Agarwal, S.K.; Chen, L.Y.; Soliman, E.Z.; Astor, B.C.; Coresh, J. Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011, 123, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Watanabe, T.; Sasaki, S.; Nagai, K.; Roden, D.M.; Aizawa, Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: The Niigata preventive medicine study. Am. Heart J. 2009, 158, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Neuberger, H.R. Chronic kidney disease and atrial fibrillation. Heart Rhythm 2012, 9, 2032–2033. [Google Scholar] [CrossRef] [PubMed]
- Sidney, S.; Sorel, M.; Quesenberry, C.P., Jr.; DeLuise, C.; Lanes, S.; Eisner, M.D. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest 2005, 128, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.M.; House-Fancher, M.A. Management of atrial fibrillation in patients with chronic obstructive pulmonary disease. J. Cardiovasc. Nurs. 2005, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Steer, J.; Gibson, J.; Bourke, S.C. The DECAF Score: Predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax 2012, 67, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Terzano, C.; Conti, V.; Di Stefano, F.; Petroianni, A.; Ceccarelli, D.; Graziani, E.; Mariotta, S.; Ricci, A.; Vitarelli, A.; Puglisi, G.; et al. Comorbidity, hospitalization, and mortality in COPD: Results from a longitudinal study. Lung 2010, 188, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Mapel, D.W.; Dedrick, D.; Davis, K. Trends and cardiovascular co-morbidities of COPD patients in the Veterans Administration Medical System, 1991–1999. COPD 2005, 2, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yang, Y. Radiofrequency catheter ablation of atrial fibrillation in patients with chronic obstructive pulmonary disease: Opportunity and challenge: response to Dr. Kumar’s comment. J. Am. Med. Dir. Assoc. 2015, 16, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, X.; Shi, G.; Yi, K.; Tan, X. Atrial fibrillation is an independent risk factor for hospital-acquired pneumonia. PLoS ONE 2015, 10, e0131782. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.F.; Liu, C.J.; Tuan, T.C.; Chen, S.J.; Wang, K.L.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chen, T.J.; et al. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: Which scoring system should be used for Asians? Heart Rhythm 2016, 13, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Al-Zaiti, S.S. Inflammation-induced atrial fibrillation: Pathophysiological perspectives and clinical implications. Heart Lung 2015, 44, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Sin, D.D. Lung inflammation in COPD: Why does it matter? F1000 Med. Rep. 2012, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lip, G.Y.; Apostolakis, S. Inflammation in atrial fibrillation. J. Am. Coll. Cardiol. 2012, 60, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Galea, R.; Cardillo, M.T.; Caroli, A.; Marini, M.G.; Sonnino, C.; Narducci, M.L.; Biasucci, L.M. Inflammation and C-reactive protein in atrial fibrillation: Cause or effect? Tex. Heart Inst. J. 2014, 41, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.C.; Du, Y.M.; Wu, S.L.; Chen, Q.X.; Wu, H.L.; Zhou, S.F. Activated nuclear factor-kappaB and increased tumor necrosis factor-alpha in atrial tissue of atrial fibrillation. Scand. Cardiovasc. J. 2009, 43, 292–297. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Franceschi, C.; Monti, D.; Ginaldi, L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005, 579, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, L.M.; Rabe, K.F. From COPD to chronic systemic inflammatory syndrome? Lancet 2007, 370, 797–799. [Google Scholar] [CrossRef]
- Gan, W.Q.; Man, S.F.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A. Systemic effects of chronic obstructive pulmonary disease: What we know and what we don’t know (but should). Proc. Am. Thorac. Soc. 2007, 4, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Sze, M.A.; Dimitriu, P.A.; Hayashi, S.; Elliott, W.M.; McDonough, J.E.; Gosselink, J.V.; Cooper, J.; Sin, D.D.; Mohn, W.W.; Hogg, J.C. The lung tissue microbiome in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; Hindricks, G.; Prendergast, B.; et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Beddhu, S.; Bruns, F.J.; Saul, M.; Seddon, P.; Zeidel, M.L. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am. J. Med. 2000, 108, 609–613. [Google Scholar] [CrossRef]
- Chang, C.H.; Lee, Y.C.; Tsai, C.T.; Chang, S.N.; Chung, Y.H.; Lin, M.S.; Lin, J.W.; Lai, M.S. Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis 2014, 232, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Cheng, M.H.; Lee, C.H.; Wung, D.C.; Cheng, C.L.; Kao Yang, Y.H. Compliance with antithrombotic prescribing guidelines for patients with atrial fibrillation—A nationwide descriptive study in Taiwan. Clin. Ther. 2008, 30, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
| Variables | Non-AF Cohort | AF Cohort | ||
|---|---|---|---|---|
| Patient (no.) | 6219 | 6219 | ||
| Years from COPD diagnosis to index date | 3.61 ± 3.05 | 3.65 ± 3.10 | ||
| Age (year) | 71.16 ± 9.66 | 71.15 ± 9.47 | ||
| Male gender | 4560 | 73.32% | 4518 | 72.65% |
| Index year of AF | ||||
| 2000 | 223 | 3.59% | 196 | 3.15% |
| 2001 | 374 | 6.01% | 379 | 6.09% |
| 2002 | 410 | 6.59% | 415 | 6.67% |
| 2003 | 418 | 6.72% | 410 | 6.59% |
| 2004 | 550 | 8.84% | 555 | 8.92% |
| 2005 | 534 | 8.59% | 598 | 9.62% |
| 2006 | 569 | 9.15% | 500 | 8.04% |
| 2007 | 582 | 9.36% | 610 | 9.81% |
| 2008 | 670 | 10.77% | 627 | 10.08% |
| 2009 | 544 | 8.75% | 591 | 9.50% |
| 2010 | 733 | 11.79% | 696 | 11.19% |
| 2011 | 612 | 9.84% | 642 | 10.32% |
| Monthly income, n (%) | ||||
| <19,100 | 2489 | 40.02% | 2462 | 39.59% |
| 19,100–41,999 | 3047 | 49.00% | 3063 | 49.25% |
| ≥42,000 | 683 | 10.98% | 694 | 11.16% |
| Hospital level, n (%) | ||||
| Level 1 | 2171 | 34.91% | 2113 | 33.98% |
| Level 2 | 2339 | 37.61% | 2416 | 38.85% |
| Level 3 | 1383 | 22.24% | 1375 | 22.11% |
| Level 4 (rural area) | 326 | 5.24% | 315 | 5.07% |
| COPD severe AE | ||||
| 0 | 3036 | 48.82% | 2996 | 48.17% |
| 1 | 1060 | 17.04% | 1090 | 17.53% |
| ≥2 | 2123 | 34.14% | 2133 | 34.30% |
| Medication for COPD | ||||
| LABA | 132 | 2.12% | 129 | 2.07% |
| SABA | 1046 | 16.82% | 1017 | 16.35% |
| LAMA | 395 | 6.35% | 387 | 6.22% |
| ICS | 1228 | 19.75% | 1220 | 19.62% |
| Medication for hypertension | ||||
| Alpha-Blocker | 877 | 14.10% | 908 | 14.60% |
| Beta-Blocker | 2663 | 42.82% | 2645 | 42.53% |
| Calcium-Channel Blocker | 3889 | 62.53% | 3839 | 61.73% |
| Diuretic | 3629 | 58.35% | 3606 | 57.98% |
| ACEi or ARB | 3130 | 50.33% | 3142 | 50.52% |
| Other medication | ||||
| Aspirin | 1252 | 20.13% | 1332 | 21.42% |
| Clopidogrel | 518 | 8.33% | 532 | 8.55% |
| Ticlopidine | 221 | 3.55% | 248 | 3.99% |
| Dipyridamole | 1505 | 24.20% | 1498 | 24.09% |
| Nitrate | 153 | 2.46% | 166 | 2.67% |
| Statin | 733 | 11.79% | 772 | 12.41% |
| NSAID | 4903 | 78.84% | 4913 | 79.00% |
| Anti-hyperglycemic drugs | 1310 | 21.06% | 1305 | 20.98% |
| Proton-pump inhibitor | 926 | 14.89% | 911 | 14.65% |
| Baseline comorbidities | ||||
| Charlson score | 1.98 ± 1.24 | 1.99 ± 1.21 | ||
| Sleep apnea | 14 | 0.23% | 26 | 0.42% |
| Old myocardial infarction | 194 | 3.12% | 179 | 2.88% |
| Congestive heart failure | 1251 | 20.12% | 1322 | 21.26% |
| Peripheral vascular disease | 58 | 0.93% | 63 | 1.01% |
| Cerebrovascular disease | 553 | 8.89% | 562 | 9.04% |
| Dementia | 206 | 3.31% | 202 | 3.25% |
| Rheumatologic disease | 47 | 0.76% | 41 | 0.66% |
| Peptic ulcer disease | 1100 | 17.69% | 1069 | 17.19% |
| Hemiplegia or paraplegia | 8 | 0.13% | 8 | 0.13% |
| Renal disease | 263 | 4.23% | 297 | 4.78% |
| Moderate/Severe liver disease | 232 | 3.73% | 207 | 3.33% |
| Tumor | 273 | 4.39% | 284 | 4.57% |
| Diabetes | 902 | 14.50% | 896 | 14.41% |
| Outcome | Crude | Adjusted | Competing Risk |
|---|---|---|---|
| HR (95% CI) | HR a (95% CI) | HR a (95% CI) | |
| Mortality | 1.28 (1.19, 1.37) | 1.24 (1.15, 1.34) | - |
| Pneumonia | 1.65 (1.54, 1.76) | 1.17 (1.07, 1.27) | 1.12 (1.03, 1.22) |
| Pneumonia with MV | 1.78 (1.62, 1.97) | 1.33 (1.18, 1.50) | 1.15 (1.03, 1.28) |
| Scores | Pneumonia | Crude | Adjusted | Competing Risk | ||
|---|---|---|---|---|---|---|
| #Event | PY a | IR b | HR (95% CI) | HR c (95% CI) | HR c (95% CI) | |
| CHA2DS2-VASc | ||||||
| 0 | 52 | 559 | 9.31 | Reference | Reference | Reference |
| 1 | 192 | 1587 | 12.10 | 1.26 (0.93, 1.72) | 1.28 (0.89, 1.86) | 1.25 (0.87, 1.81) |
| 2 | 395 | 2815 | 14.03 | 1.41 (1.06, 1.88) | 1.54 (1.09, 2.18) | 1.46 (1.03, 2.07) |
| 3 | 533 | 3095 | 17.22 | 1.64 (1.23, 2.18) | 1.84 (1.31, 2.59) | 1.68 (1.20, 2.37) |
| 4 | 595 | 2864 | 20.78 | 1.86 (1.40, 2.47) | 1.93 (1.37, 2.72) | 1.68 (1.19, 2.36) |
| 5 | 515 | 2356 | 21.86 | 1.90 (1.43, 2.53) | 2.13 (1.51, 3.00) | 1.79 (1.27, 2.52) |
| 6 | 397 | 1410 | 28.15 | 2.31 (1.73, 3.09) | 2.82 (1.99, 3.99) | 2.34 (1.65, 3.31) |
| 7 | 239 | 786 | 30.41 | 2.36 (1.75, 3.19) | 2.65 (1.85, 3.81) | 2.11 (1.47, 3.04) |
| 8 | 85 | 191 | 44.53 | 3.00 (2.12, 4.23) | 3.63 (2.39, 5.51) | 2.69 (1.77, 4.08) |
| 9 | 11 | 21 | 51.31 | 2.98 (1.55, 5.71) | 5.41 (2.68, 10.91) | 3.51 (1.74, 7.08) |
| p for trend test | <0.0001 | |||||
| CHADS2 Score | ||||||
| 0 | 146 | 1195 | 12.21 | Reference | Reference | Reference |
| 1 | 447 | 3472 | 12.88 | 1.05 (0.87, 1.26) | 1.12 (0.90, 1.41) | 1.10 (0.88, 1.38) |
| 2 | 714 | 4096 | 17.43 | 1.30 (1.09, 1.56) | 1.43 (1.15, 1.77) | 1.34 (1.08, 1.66) |
| 3 | 656 | 3039 | 21.58 | 1.49 (1.25, 1.78) | 1.64 (1.32, 2.04) | 1.42 (1.14, 1.76) |
| 4 | 506 | 2113 | 23.94 | 1.61 (1.34, 1.94) | 1.79 (1.43, 2.24) | 1.58 (1.27, 1.98) |
| 5 | 427 | 1516 | 28.17 | 1.82 (1.50, 2.19) | 2.11 (1.68, 2.64) | 1.78 (1.42, 2.24) |
| 6 | 118 | 254 | 46.49 | 2.40 (1.88, 3.06) | 3.18 (2.37, 4.25) | 2.44 (1.82, 3.26) |
| p for trend test | <0.0001 | |||||
| CCI score | ||||||
| 1 | 1216 | 7865 | 15.46 | Reference | Reference | Reference |
| 2–3 | 1437 | 6712 | 21.41 | 1.28 (1.19, 1.38) | 1.29 (1.18, 1.42) | 1.23 (1.12, 1.34) |
| ≥4 | 361 | 1108 | 32.57 | 1.67 (1.48, 1.88) | 1.80 (1.56, 2.08) | 1.47 (1.28, 1.70) |
| p for trend test | <0.0001 | |||||
| Subgroups | Crude | p Value | Adjusted | p Value | pinteractiona |
|---|---|---|---|---|---|
| HR (95% CI) | HR a (95% CI) | ||||
| Risk of pneumonia | |||||
| LABA | 0.558 | ||||
| No | 1.44 (1.37, 1.52) | <0.001 | 1.14 (1.07, 1.21) | <0.001 | |
| Yes | 1.76 (1.26, 2.45) | 0.001 | 1.3 1(0.90, 1.90) | 0.161 | |
| SABA | 0.358 | ||||
| No | 1.43 (1.34, 1.52) | <0.001 | 1.13 (1.05, 1.21) | 0.001 | |
| Yes | 1.57 (1.39, 1.76) | <0.001 | 1.23 (1.07, 1.40) | 0.003 | |
| LAMA | 0.920 | ||||
| No | 1.43 (1.35, 1.51) | <0.001 | 1.14 (1.07, 1.21) | <0.001 | |
| Yes | 1.88 (1.54, 2.30) | <0.001 | 1.28 (1.01, 1.61) | 0.042 | |
| ICS | 0.262 | ||||
| No | 1.42 (1.34, 1.51) | <0.001 | 1.12 (1.05, 1.20) | 0.001 | |
| Yes | 1.56 (1.40, 1.75) | <0.001 | 1.24 (1.09, 1.41) | 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-H.; Lai, C.-C.; Wang, C.-Y.; Wang, H.-C.; Yu, C.-J.; Chen, L.; On Behalf of the Taiwan Clinical Trial Consortium for Respiratory Diseases. Risks of Pneumonia in COPD Patients with New-Onset Atrial Fibrillation. J. Clin. Med. 2018, 7, 229. https://doi.org/10.3390/jcm7090229
Wang Y-H, Lai C-C, Wang C-Y, Wang H-C, Yu C-J, Chen L, On Behalf of the Taiwan Clinical Trial Consortium for Respiratory Diseases. Risks of Pneumonia in COPD Patients with New-Onset Atrial Fibrillation. Journal of Clinical Medicine. 2018; 7(9):229. https://doi.org/10.3390/jcm7090229
Chicago/Turabian StyleWang, Ya-Hui, Chih-Cheng Lai, Cheng-Yi Wang, Hao-Chien Wang, Chong-Jen Yu, Likwang Chen, and On Behalf of the Taiwan Clinical Trial Consortium for Respiratory Diseases. 2018. "Risks of Pneumonia in COPD Patients with New-Onset Atrial Fibrillation" Journal of Clinical Medicine 7, no. 9: 229. https://doi.org/10.3390/jcm7090229
APA StyleWang, Y.-H., Lai, C.-C., Wang, C.-Y., Wang, H.-C., Yu, C.-J., Chen, L., & On Behalf of the Taiwan Clinical Trial Consortium for Respiratory Diseases. (2018). Risks of Pneumonia in COPD Patients with New-Onset Atrial Fibrillation. Journal of Clinical Medicine, 7(9), 229. https://doi.org/10.3390/jcm7090229





