Perioperative Cumulative Fluid Balance and Its Association with an Increase in Costs after Major Surgery
Abstract
1. Introduction
2. Materials and Methods
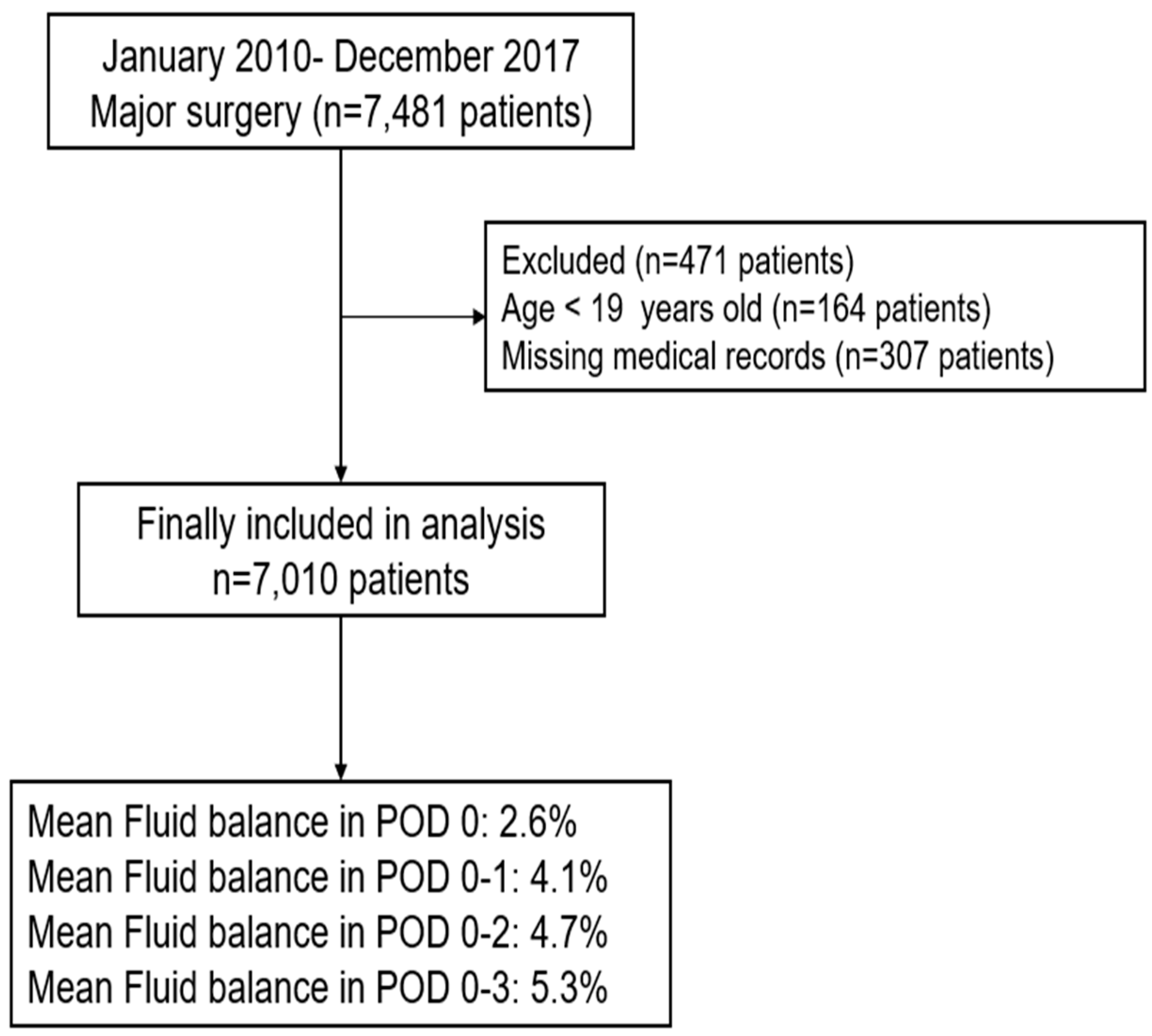
2.1. Patients
2.2. Calculation of FB (%) in Postoperative Day 0–3
2.3. Calculation of Costs for Major Surgical Procedures
2.4. Measurement and Outcome
2.5. Statistical Analysis
3. Results
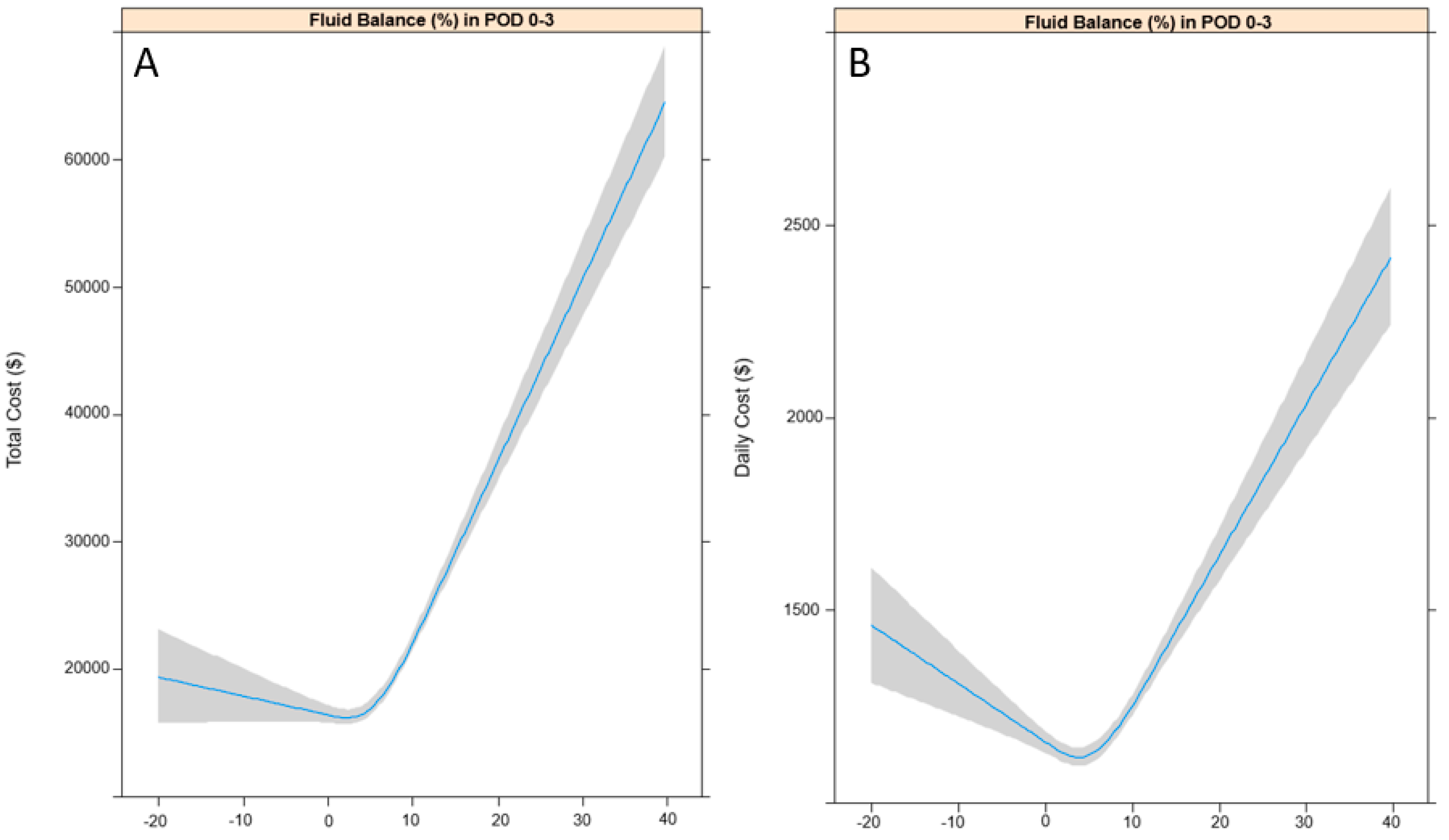
3.1. Total and Daily Costs According to FB (%) in POD 0–3
3.2. Subgroup Analysis According to Type of Surgery
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Jacob, M.; Chappell, D.; Rehm, M. Clinical update: Perioperative fluid management. Lancet 2007, 369, 1984–1986. [Google Scholar] [CrossRef]
- Alexandroni, H.; Bahar, R.; Chill, H.H.; Karavani, G.; Ben-Yossef, O.; Shushan, A. Reducing fluid-related complications during operative hysteroscopy: Use of a new mandatory fluid-balance form. J. Minim. Invasive Gynecol. 2017, 24, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, H.; Liu, N.; Jia, M.; Zhang, H.; Xi, X.; Hou, X. Early negative fluid balance is associated with lower mortality after cardiovascular surgery. Perfusion 2018. [Google Scholar] [CrossRef] [PubMed]
- Voldby, A.W.; Brandstrup, B. Fluid therapy in the perioperative setting—A clinical review. J. Intensive Care 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, T.; Rhodes, J.E.; Clarke, S.; Myles, P.S.; Ho, K.M. Perioperative fluid management strategies in major surgery: A stratified meta-analysis. Anesth. Analg. 2012, 114, 640–651. [Google Scholar] [CrossRef] [PubMed]
- McArdle, G.T.; Price, G.; Lewis, A.; Hood, J.M.; McKinley, A.; Blair, P.H.; Harkin, D.W. Positive fluid balance is associated with complications after elective open infrarenal abdominal aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Winer, L.K.; Dhar, V.K.; Wima, K.; Lee, T.C.; Morris, M.C.; Shah, S.A.; Ahmad, S.A.; Patel, S.H. Perioperative net fluid balance predicts pancreatic fistula after pancreaticoduodenectomy. J. Gastrointest. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.M.; Petit, A.; Chanques, G.; Kwiatkowski, F.; Flamein, R.; Slim, K.; Sapin, V.; Jaber, S.; Bazin, J.E. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: A prospective randomized trial. Arch. Surg. 2010, 145, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Quan, H.; Bugar, J.M.; Lemaire, J.B.; Brant, R.; Ghali, W.A. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J. Gen. Intern. Med. 2006, 21, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.H.; Hall, B.L.; Louie, R.; Ettner, S.L.; Zingmond, D.S.; Han, L.; Rapp, M.; Ko, C.Y. Association between occurrence of a postoperative complication and readmission: Implications for quality improvement and cost savings. Ann. Surg. 2013, 258, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, V.; Murugan, R.; Sileanu, F.E.; Palevsky, P.; Clermont, G.; Kellum, J.A. Both positive and negative fluid balance may be associated with reduced long-term survival in the critically ill. Crit. Care Med. 2017, 45, e749–e757. [Google Scholar] [CrossRef] [PubMed]
- Selewski, D.T.; Cornell, T.T.; Lombel, R.M.; Blatt, N.B.; Han, Y.Y.; Mottes, T.; Kommareddi, M.; Kershaw, D.B.; Shanley, T.P.; Heung, M. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011, 37, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S. Thirty years of national health insurance in south Korea: Lessons for achieving universal health care coverage. Health Policy Plan. 2009, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Birkmeyer, J.D.; Gust, C.; Dimick, J.B.; Birkmeyer, N.J.; Skinner, J.S. Hospital quality and the cost of inpatient surgery in the united states. Ann. Surg. 2012, 255, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced recovery after surgery: A review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.E.; Roche, A.M.; Mythen, M. Fluid management and goal-directed therapy as an adjunct to enhanced recovery after surgery (eras). Can. J. Anaesth. 2015, 62, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Joliat, G.R.; Labgaa, I.; Petermann, D.; Hubner, M.; Griesser, A.C.; Demartines, N.; Schafer, M. Cost-benefit analysis of an enhanced recovery protocol for pancreaticoduodenectomy. Br. J. Surg. 2015, 102, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Roulin, D.; Donadini, A.; Gander, S.; Griesser, A.C.; Blanc, C.; Hubner, M.; Schafer, M.; Demartines, N. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br. J. Surg. 2013, 100, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Soppitt, A.; Maroof, M.; el-Moalem, H.; Robertson, K.M.; Moretti, E.; Dwane, P.; Glass, P.S. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002, 97, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Lietz, K.; Barreiro, C.; Philpott, J.; Flink, C.; McGrath, M.; Herre, J. Impact of surgical bleeding on the cost of hospitalization for left-ventricular assist device implantation. J. Am. Coll. Cardiol. 2017, 69, 868. [Google Scholar] [CrossRef]
- Stephen, A.E.; Berger, D.L. Shortened length of stay and hospital cost reduction with implementation of an accelerated clinical care pathway after elective colon resection. Surgery 2003, 133, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Kelz, R.R.; Gimotty, P.A.; Polsky, D.; Norman, S.; Fraker, D.; DeMichele, A. Morbidity and mortality of colorectal carcinoma surgery differs by insurance status. Cancer 2004, 101, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Kaestner, R. Health insurance and ex ante moral hazard: Evidence from medicare. Int. J. Health Care Financ. Econ. 2009, 9, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, D. Moral hazard in health insurance: Are consumer-directed plans the answer? Ann. Health Law 2006, 15, 251–273. [Google Scholar] [PubMed]
| Variables | Total (n = 7010) | Mean (SD) |
|---|---|---|
| Age, year | 59.2 (14.5) | |
| Body mass index, kg m−2 | 24.2 (3.7) | |
| Sex: male | 3657 (52.2%) | |
| Preoperative comorbidities | ||
| Hypertension | 2493 (35.6%) | |
| Diabete mellitus | 722 (10.2%) | |
| Ischemic heart disease | 168 (2.5%) | |
| Cerebrovascular disease | 187 (2.7%) | |
| Chronic kidney disease | 125 (1.8%) | |
| Liver disease (Hepatitis, LC, HCC) | 448 (6.4%) | |
| Cancer | 482 (6.9%) | |
| Information regarding surgical procedures | ||
| Surgery time, min | 301.7 (137.9) | |
| Estimated blood loss, mL | 1172.2 (1683.7) | |
| Length of hospital stay, da | 19.5 (25.1) | |
| Type of surgery | ||
| General surgery | 2190 (31.3%) | |
| Cardiovascular surgery | 764 (10.9%) | |
| Thoracic surgery | 119 (1.7%) | |
| Obstetrics, Gynecologic, Urologic surgery | 1564 (22.3%) | |
| Plastic, Orthopedic, Neurosurgery | 2373 (33.8%) | |
| Years at surgery | ||
| 2010–2013 | 1222 (17.4%) | |
| 2013–2015 | 1980 (28.2%) | |
| 2015–2017 | 3808 (54.3%) | |
| Fluid balance (%) in postoperative day 0–3 | ||
| Postoperative day 0 (24 h) | 2.6 (3.1) | |
| Postoperative day 0–1 (48 h) | 4.1 (4.3) | |
| Postoperative day 0–2 (72 h) | 4.7 (5.0) | |
| Postoperative day 0–3 (96 h) | 5.3 (6.1) | |
| Total cost ($) | 19,156.9 (22,633.9) | |
| Daily cost ($) | 1198.6 (904.1) | |
| Non-coverage: National Health Insurance Service (%) | 35.3 (15.9) |
| Characteristics | Total Cost ($) | Daily Cost ($) | ||
|---|---|---|---|---|
| Coefficient (95% CI) | p | Coefficient (95% CI) | p | |
| Sex: male (vs. female) | 3889.9 (2832.5, 4947.2) | <0.001 | 85.4 (43.0, 127.8) | <0.001 |
| Age, year | 112.0 (75.6, 148.4) | <0.001 | 1.2 (−0.3, 2.6) | 0.119 |
| Body mass index, kg m−2 | −321.3 (−463.4, −179.3) | <0.001 | 0.1 (−5.6, 5.8) | 0.965 |
| Hypertension | 2129.9 (1024.0, 3235.8) | <0.001 | 66.7 (22.4, 110.9) | 0.003 |
| Diabete mellitus | 9073.8 (6685.3, 11462.4) | <0.001 | 197.9 (102.2, 293.6) | <0.001 |
| Cerebrovascular disease | 5343.8 (2509.5, 8178.0) | <0.001 | 123.9 (−7.8, 255.6) | 0.065 |
| Ischemic heart disease | 10797.3 (7341.4, 14,253.3) | <0.001 | 629.1 (491.0, 767.1) | <0.001 |
| Chronic kidney disease | 14244.9 (10,254.2, 18,235.7) | <0.001 | 312.8 (152.3, 473.2) | <0.001 |
| Liver disease | 13831.4 (11,688.9, 15,973.8) | <0.001 | 397.5 (311.4, 483.5) | <0.001 |
| Cancer | 732.6 (−1361.7, 2827.0) | 0.493 | −33.5 (−117.2, 50.2) | 0.432 |
| Surgery time, min | 34.1 (30.3, 37.8) | <0.001 | 0.5 (0.3, 0.7) | <0.001 |
| Estimated blood loss, mL | 3.6 (3.3, 3.9) | <0.001 | 0.1 (0.1, 0.1) | <0.001 |
| Type of surgery | ||||
| General surgery | 1 | 1 | ||
| Cardiovascular surgery | 14620.4 (12,897.1, 16,343.8) | <0.001 | 1072.6 (1005.1,1140.1 | <0.001 |
| Thoracic surgery | −13734.4 (−15,092.7, −12,376.1) | <0.001 | 199.2 (48.0, 350.3) | 0.010 |
| OG, Urologic surgery | −6502.8 (−7718.1, −5287.5) | <0.001 | −304.5 (−357.7, −251.3) | <0.001 |
| PS, OS, NS | 8411.7 (4551.1, 12,272.3) | <0.001 | −2.6 (−50.2, 45.0) | 0.914 |
| Surgery in 2010–2012 | 1 | 1 | ||
| Surgery in 2013–2015 | 4360.7 (2754.4, 5967.1) | <0.001 | 250.8 (188.2, 313.4) | <0.001 |
| Surgery in 2016–2017 | 6218.5 (4766.8, 7670.2) | <0.001 | 556.7 (500.1, 613.3) | <0.001 |
| FB (%) in POD 0 (24 h) | 1665.9 (1501.6, 1830.3) | <0.001 | 34.0 (27.3, 40.7) | <0.001 |
| FB (%) in POD 0–1 (48 h) | 1224.7 (1104.8, 1344.5) | <0.001 | 30.0 (25.1, 34.8) | <0.001 |
| FB (%) in POD 0–2 (72 h) | 999.1 (895.4, 1102.8) | <0.001 | 22.4 (18.2, 26.6) | <0.001 |
| FB (%) in POD 0–3 (96 h) | 728.0 (642.7, 813.3) | <0.001 | 15.1 (11.6, 18.5) | <0.001 |
| Non–coverage: NHIS (%) | −285.7 (−318.5, −253.0) | <0.001 | −5.1 (−6.4, −3.8) | <0.001 |
| Variables | Multivariable Model (Total Cost, $) | Multivariable Model (Daily Cost, $) | ||
|---|---|---|---|---|
| Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | |
| FB (%) in POD 0 (24 h) a | 967.8 (803.4, 1132.1) | <0.001 | 19.9 (13.5, 26.2) | <0.001 |
| FB (%) in POD 0–1 (48 h) b | 688.8 (566.3, 811.2) | <0.001 | 21.3 (4.5, 7.0) | <0.001 |
| FB (%) in POD 0–2 (72 h) c | 591.1 (485.7, 696.4) | <0.001 | 18.9 (15.0, 22.9) | <0.001 |
| FB (%) in POD 0–3 (96 h) d | 434.2 (349.4, 519.1) | <0.001 | 14.6 (11.4, 17.8) | <0.001 |
| Non-coverage: NHIS (%) d | −66.3 (−97.8, −34.7) | <0.001 | −5.8 (−4.6, −7.0) | <0.001 |
| Variables | Total Costs ($) | p-Value |
|---|---|---|
| Coefficient (95% CI) | ||
| General surgery | ||
| Fluid balance (%) POD 0 (24 h) | 1214.8 (909.9, 1519.6) | <0.001 |
| Fluid balance (%) POD 0–1 (48 h) | 881.5 (651.5, 1111.5) | <0.001 |
| Fluid balance (%) POD 0–2 (72 h) | 828.0 (629.0, 1027.0) | <0.001 |
| Fluid balance (%) POD 0–3 (96 h) | 653.9 (492.3, 815.5) | <0.001 |
| Cardiovascular surgery | ||
| Fluid balance (%) POD 0 (24 h) | 2088.3 (1409.3, 2767.3) | <0.001 |
| Fluid balance (%) POD 0–1 (48 h) | 1524.6 (954.7, 2094.5) | <0.001 |
| Fluid balance (%) POD 0–2 (72 h) | 1099.4 (587.3, 1611.6) | <0.001 |
| Fluid balance (%) POD 0–3 (96 h) | 634.4 (220.7, 1048.1) | 0.003 |
| Thoracic surgery | ||
| Fluid balance (%) POD 0 (24 h) | 9685.0 (5810.9, 13,559.1) | <0.001 |
| Fluid balance (%) POD 0–1 (48 h) | 6194.5 (3407.9, 8981.0) | <0.001 |
| Fluid balance (%) POD 0–2 (72 h) | 3982.7 (1733.6, 6231.7) | 0.001 |
| Fluid balance (%) POD 0–3 (96 h) | 2272.5 (571.3, 3973.6) | 0.009 |
| Obstetrics, Gynecologic, Urologic surgery | ||
| Fluid balance (%) POD 0 (24 h) | 210.9 (106.8, 314.9) | <0.001 |
| Fluid balance (%) POD 0–1 (48 h) | 140.8 (71.4, 210.3) | <0.001 |
| Fluid balance (%) POD 0–2 (72 h) | 112.9 (54.9, 170.9) | <0.001 |
| Fluid balance (%) POD 0–3 (96 h) | 82.3 (35.7, 128.8) | 0.001 |
| Plastic, Orthopedic, Neurosurgery | ||
| Fluid balance (%) POD 0 (24 h) | 314.6 (1.342, 627.9) | 0.049 |
| Fluid balance (%) POD 0–1 (48 h) | 262.9 (11.5, 514.3) | 0.040 |
| Fluid balance (%) POD 0–2 (72 h) | 312.1 (97.7, 526.6) | 0.004 |
| Fluid balance (%) POD 0–3 (96 h) | 255.1 (90.5, 419.7) | 0.002 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, T.K.; Hwang, J.-W.; Jeon, Y.-T.; Do, S.-H. Perioperative Cumulative Fluid Balance and Its Association with an Increase in Costs after Major Surgery. J. Clin. Med. 2018, 7, 227. https://doi.org/10.3390/jcm7090227
Oh TK, Hwang J-W, Jeon Y-T, Do S-H. Perioperative Cumulative Fluid Balance and Its Association with an Increase in Costs after Major Surgery. Journal of Clinical Medicine. 2018; 7(9):227. https://doi.org/10.3390/jcm7090227
Chicago/Turabian StyleOh, Tak Kyu, Jung-Won Hwang, Young-Tae Jeon, and Sang-Hwan Do. 2018. "Perioperative Cumulative Fluid Balance and Its Association with an Increase in Costs after Major Surgery" Journal of Clinical Medicine 7, no. 9: 227. https://doi.org/10.3390/jcm7090227
APA StyleOh, T. K., Hwang, J.-W., Jeon, Y.-T., & Do, S.-H. (2018). Perioperative Cumulative Fluid Balance and Its Association with an Increase in Costs after Major Surgery. Journal of Clinical Medicine, 7(9), 227. https://doi.org/10.3390/jcm7090227




