Intravenous Colistin Monotherapy versus Combination Therapy against Carbapenem-Resistant Gram-Negative Bacteria Infections: Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
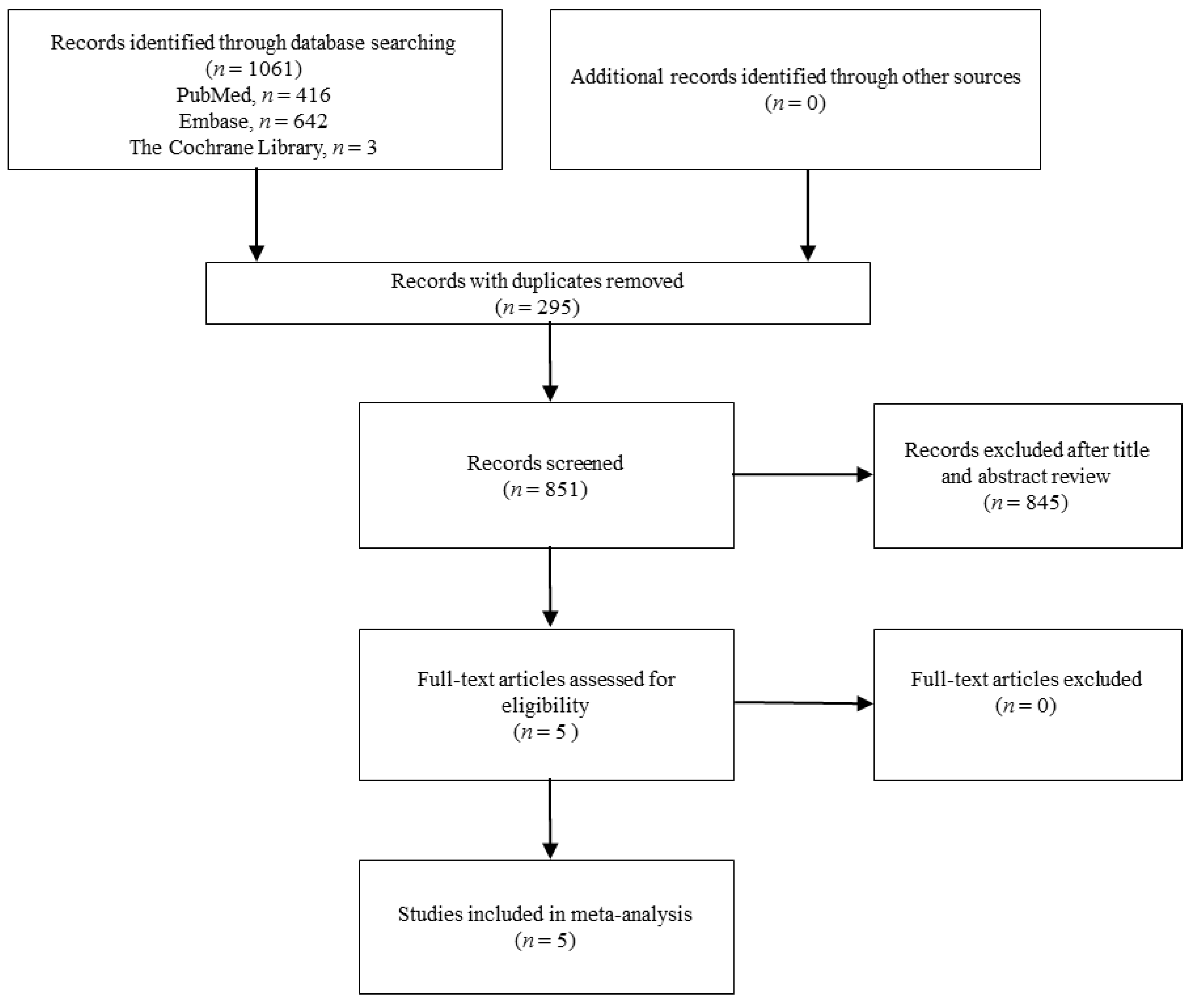
2.1. Study Search and Selection
2.2. Definitions and Outcome
2.3. Data Analysis
3. Results
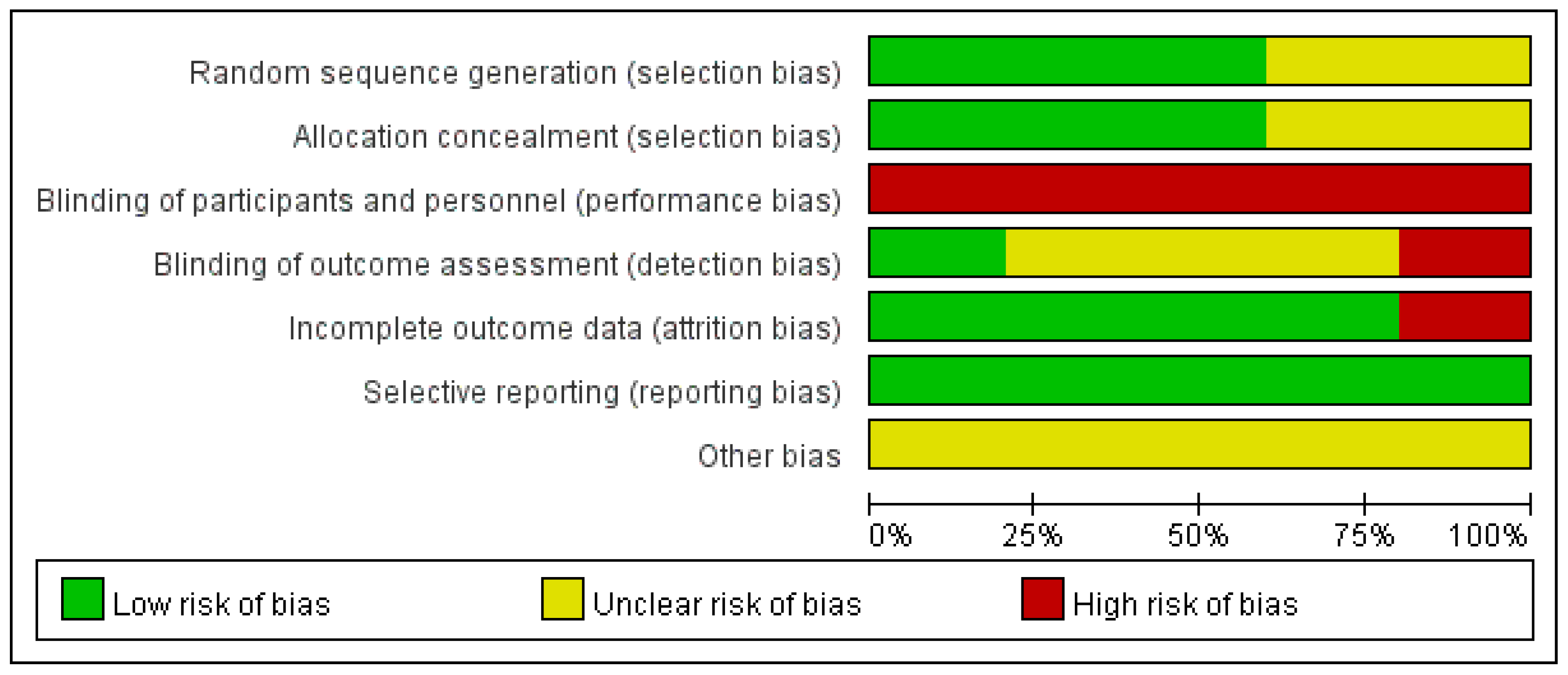
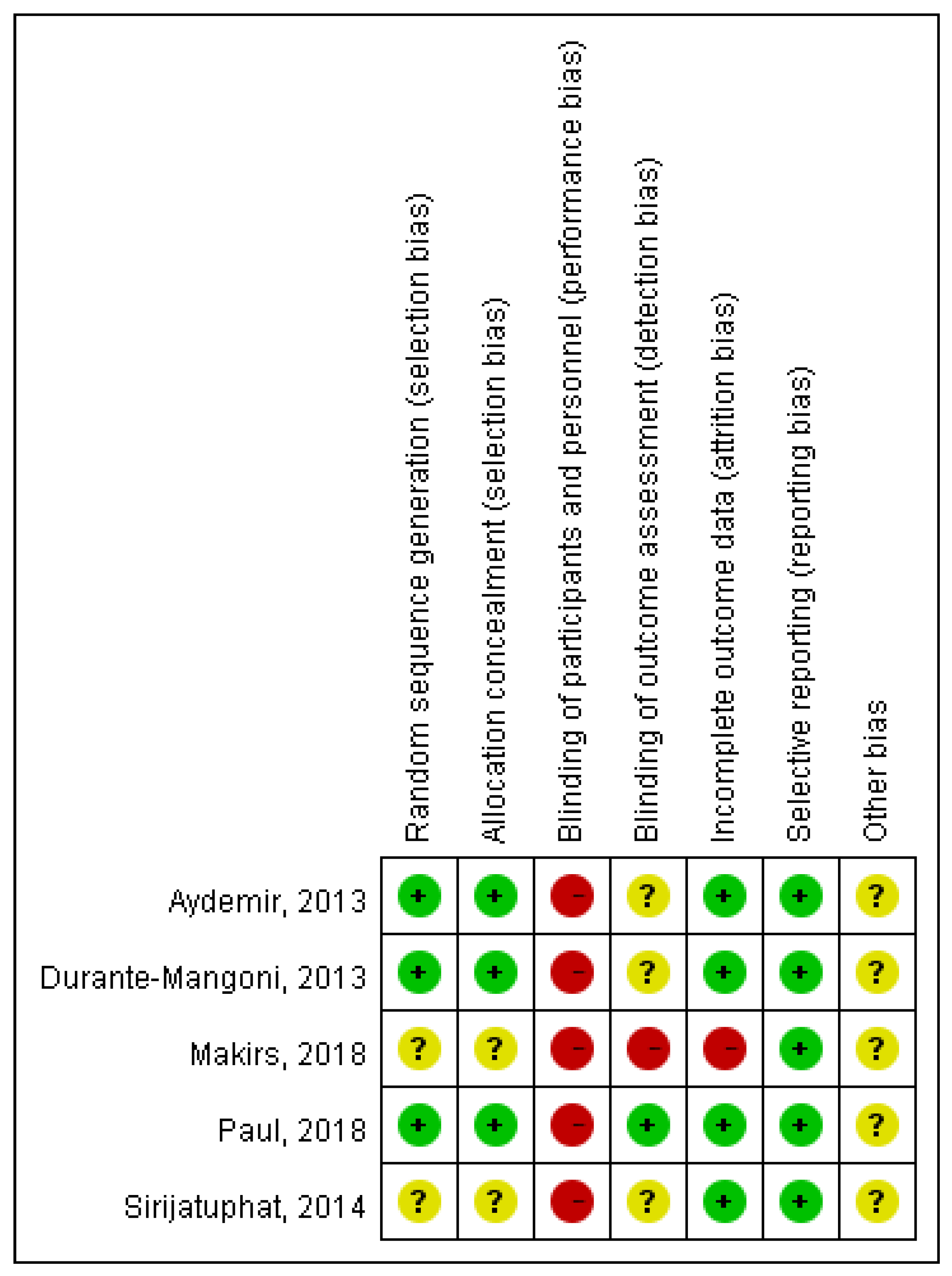
3.1. Study Selection and Characteristics
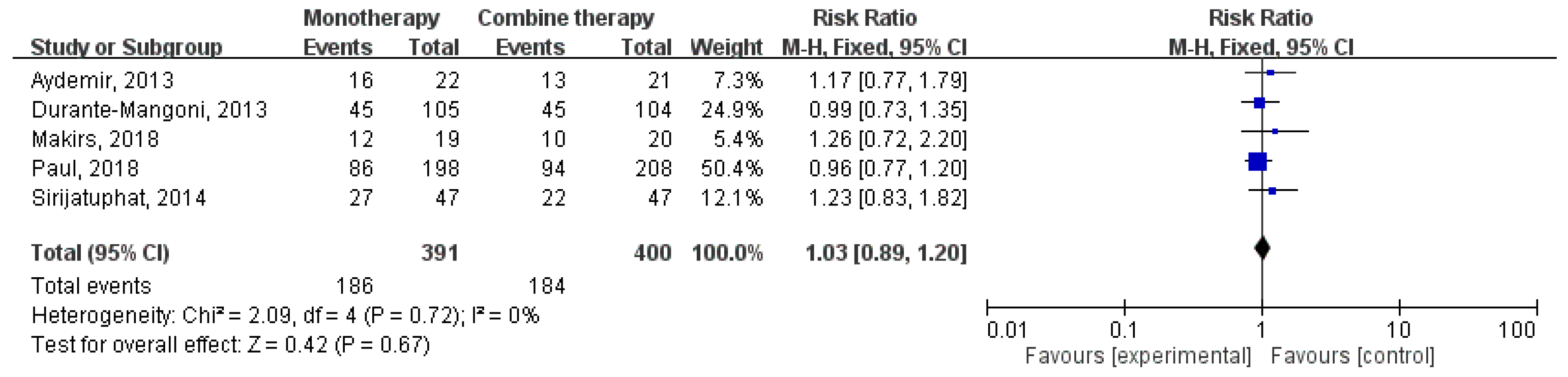
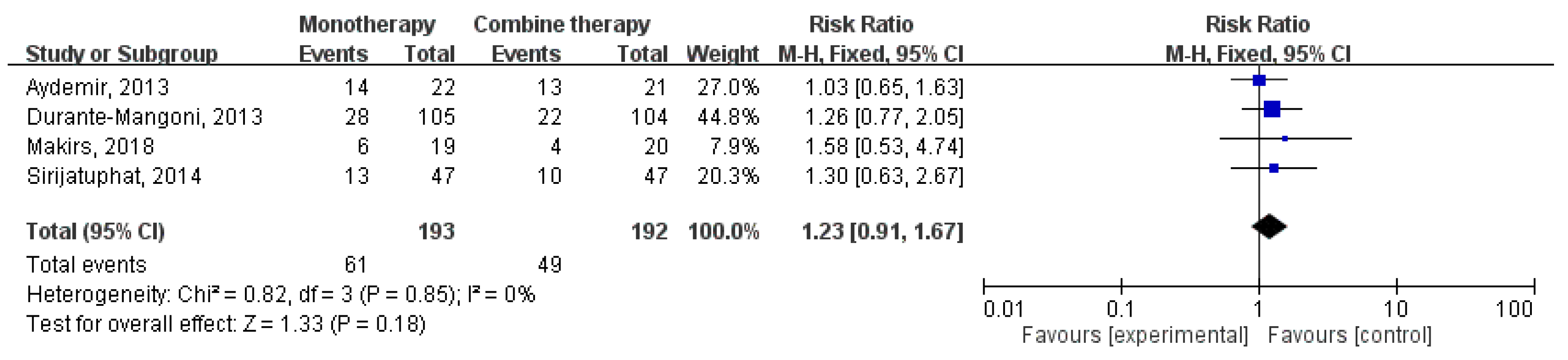
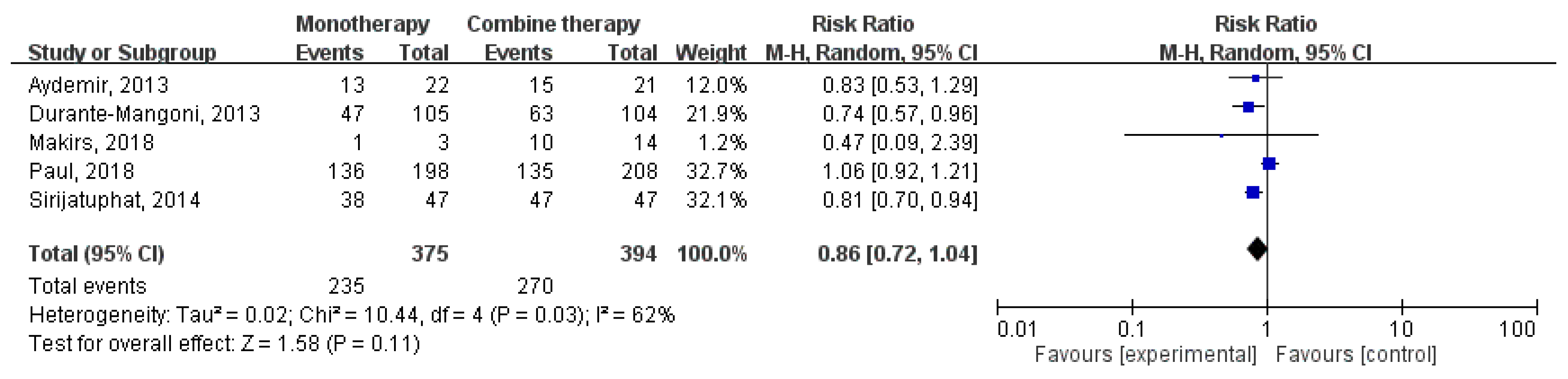
3.2. Clinical Outcomes and Microbiological Response
3.3. Subgroup Analysis
3.4. Nephrotoxicity
4. Discussion
Author Contributions
Conflicts of Interest
Appendix A. List of Terms of the Search Strategy
- “colistin” [Mesh]
- “colistin” [All Fields]
- “polymyxins“ [All Fields]
- 1 OR 2 OR 3
- “gram-negative bacteria“ [MeSH]
- “acinetobacter baumannii“ [All Fields]
- “klebsiella pneumoniae“ [MeSH Terms]
- “klebs a pneumoniae“ [All Fields]
- “pseudomonas aeruginosa“ [MeSH Terms]
- “pseudomonas aeruginosa“ [All Fields]
- “enterobacteriaceae“ [MeSH Terms]
- 5 OR 6 OR 7 OR 8 OR9 OR 10 OR 11
- “randomized“ [All Fields]
- “randomised“ [All Fields]
- “longitudinal studies“ [MeSH Terms]
- “longitudinal studies“ [All Fields]
- “prospective“ [All Fields]
- 13 OR 14 OR 15 OR 16 OR 17
- 4 AND 12 AND 18
- colistin
- polymyxin
- 1 OR 2
- gram negative bacteria
- acinetobacter baumannii
- klebsiella pneumonia
- pseudomonas aeruginosa
- enterobacteriaceae
- 4 OR 5 OR 6 OR 7 OR 8
- randomized
- prospective
- 13 OR 14
- 3 AND 9 AND 12
- MeSH descriptor colistin explode all trees
- MeSH descriptor polymyxin explode all trees
- 1 OR 2
References
- Zhang, Y.; Wang, Q.; Yin, Y.; Chen, H.; Jin, L.; Gu, B.; Xie, L.; Yang, C.; Ma, X.; Li, H.; et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: Report from the China CRE network. Antimicrob. Agents Chemother. 2018, 62, e01882-17. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.; Ye, M.J.; Zhao, Q. Prevalence of invasive infections due to carbapenem-resistant Enterobacteriaceae among adult patients in US hospitals. Antimicrob. Agents Chemother. 2017, 61, e00228-17. [Google Scholar] [CrossRef] [PubMed]
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, M.; Saigal, S.; Baronia, A.K.; Rao, B.P.; Azim, A.; Poddar, B.; Singh, R.K. Carbapenem-resistant acinetobacter ventilator-associated pneumonia: Clinical characteristics and outcome. Indian J. Crit. Care Med. 2013, 17, 129–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Righi, E.; Peri, A.M.; Harris, P.N.; Wailan, A.M.; Liborio, M.; Lane, S.W.; Paterson, D.L. Global prevalence of carbapenem resistance in neutropenic patients and association with mortality and carbapenem use: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.G.; Shah, S.R. Treatment and outcome of carbapenem-resistant Gram-negative bacilli blood-stream infections in a tertiary care hospital. J. Assoc. Physicians India 2015, 63, 14–18. [Google Scholar] [PubMed]
- Katip, W.; Meechoui, M.; Thawornwittayakom, P.; Chinwong, D.; Oberdorfer, P. Efficacy and safety of high loading dose of colistin in multidrug-resistant Acinetobacter baumannii: A prospective cohort study. J. Intensive Care Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Trifi, A.; Abdellatif, S.; Daly, F.; Mahjoub, K.; Nasri, R.; Oueslati, M.; Mannai, R.; Bouzidi, M.; Lakhal, S.B. Efficacy and toxicity of high-dose colistin in multidrug-resistant Gram-negative bacilli infections: A comparative study of a matched series. Chemotherapy 2016, 61, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Valachis, A.; Samonis, G.; Kofteridis, D.P. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: A systematic review and meta-analysis. Crit. Care Med. 2015, 43, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Leelasupasri, S.; Santimaleeworagun, W. Antimicrobial susceptibility among colistin, sulbactam, and fosfomycin and a synergism study of colistin in combination with sulbactam or fosfomycin against clinical isolates of carbapenem-resistant Acinetobacter baumannii. J. Pathog. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.H.; Chen, C.C.; Lee, M.F.; Chuang, Y.C.; Tang, H.J.; Yu, W.L. Comparison of synergism between colistin, fosfomycin and tigecycline against extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates or with carbapenem resistance. J. Microbiol. Immunol. Infect. 2017, 50, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Soudeiha, M.A.H.; Dahdouh, E.A.; Azar, E.; Sarkis, D.K.; Daoud, Z. In vitro evaluation of the colistin-carbapenem combination in clinical isolates of A. baumannii using the checkerboard, Etest, and Time-Kill Curve Techniques. Front. Cell. Infect. Microbiol. 2017, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Zusman, O.; Altunin, S.; Koppel, F.; Benattar, Y.D.; Gedik, H.; Paul, M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Mavroudis, A.D.; Georgiou, M.; Falagas, M.E. Intravenous colistin combination antimicrobial treatment vs. monotherapy: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2018, 51, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, H.; Akduman, D.; Piskin, N.; Comert, F.; Horuz, E.; Comert, F.; Horuz, E.; Terzi, A.; Kokturk, F.; Ornek, T.; et al. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol. Infect. 2013, 141, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Sirijatuphat, R.; Thamlikitkul, V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2014, 58, 5598–5601. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Signoriello, G.; Andini, R.; Mattei, A.; De Cristoforo, M.; Murino, P.; Bassetti, M.; Malacarne, P.; Petrosillo, N.; Galdieri, N.; et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant. Acinetobacter baumannii: A multicenter, randomized clinical trial. Clin. Infect. Dis. 2013, 57, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.; Petinaki, E.; Tsolaki, V.; Manoulakas, E.; Mantzarlis, K.; Apostolopoulou, O.; Sfyras, D.; Zakynthinos, E. Colistin versus colistin combined with ampicillin-sulbactam for multiresistant Acinetobacter baumannii ventilator-associated pneumonia treatment: An open-label prospective study. Indian J. Crit. Care Med. 2018, 22, 67–77. [Google Scholar] [PubMed]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Amaya-Villar, R.; Ferrándiz-Millón, C.; Díaz-Martín, A.; López-Sánchez, J.M.; Gutiérrez-Pizarraya, A. Optimum treatment strategies for carbapenem-resistant Acinetobacter baumannii bacteremia. Expert Rev. Anti. Infect. Ther. 2015, 13, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Viehman, J.A.; Nguyen, M.H.; Doi, Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs 2014, 74, 1315–1333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Y.; Fang, Y.; Wang, X.; Chen, Y.; Qi, Q.; Huang, F.; Xiao, X. Meta-analysis of colistin for the treatment of Acinetobacter baumannii infection. Sci. Rep. 2015, 5, 17091. [Google Scholar] [CrossRef] [PubMed]
| Author/Publication Year | Study Year | Study Site | Bacteria | Polymicrobial | Setting | Infection Type (%) | Usage of IV Colistin Dose | No. of Polymyxin | No. of Combination with |
|---|---|---|---|---|---|---|---|---|---|
| Aydemir, 2013 | 2011–2012 | Turkey | Carbapenem-resistant A. baumannii | No | ICU | VAP (100) | 300 mg colistin based activity/day, t.id. (9 MIU per day) | Colistin (22) | Rifampicin (21) |
| Durante-Mangoni, 2013 | 2010–2011 | Italy | Extensive-drug resistant A. baumannii | Yes | ICU | VAP (69), BSI (20), HAP (9), cIAI (2) | 2 MIU every 8 h | Colistin (105) | Rifampicin (104) |
| Sirijatuphat, 2014 | 2010–2011 | Thailand | Carbapenem-resistant A. baumannii | Yes | ICU and ward | Pneumonia (76.6), BSI (5.4), UTI (5.4), IAI (6.4), SSTI (3.2), CNSI (1.0), other (2.1) | 5 mg colistin based activity/kg/day (9 MIU per day) | Colistin (47) | Fosfomycin (47) |
| Paul, 2018 | 2013–2016 | Israel, Greece, Italy | Carbapenem-resistant gram-negative bacteria, including A. baumannii, Enterobacteriaceae, Pseudomonas, and others | No | ICU and ward | VAP/HAP (44.8), BSI (42.6), UTI (6.4), pVAP (6.2) | 9 MIU loading, followed by 4.5 MIU every 12 h | Colistin (198) | Meropenem (208) |
| Makirs, 2018 | - | Greece | Carbapenem-resistant A. baumannii | No | ICU | VAP (100) | 3 MIU t.i.d. | Colistin (19) | Ampicillin-sulbactam (20) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, I.-L.; Chen, Y.-H.; Lai, C.-C.; Tang, H.-J. Intravenous Colistin Monotherapy versus Combination Therapy against Carbapenem-Resistant Gram-Negative Bacteria Infections: Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2018, 7, 208. https://doi.org/10.3390/jcm7080208
Cheng I-L, Chen Y-H, Lai C-C, Tang H-J. Intravenous Colistin Monotherapy versus Combination Therapy against Carbapenem-Resistant Gram-Negative Bacteria Infections: Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2018; 7(8):208. https://doi.org/10.3390/jcm7080208
Chicago/Turabian StyleCheng, I-Ling, Yu-Hung Chen, Chih-Cheng Lai, and Hung-Jen Tang. 2018. "Intravenous Colistin Monotherapy versus Combination Therapy against Carbapenem-Resistant Gram-Negative Bacteria Infections: Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 7, no. 8: 208. https://doi.org/10.3390/jcm7080208
APA StyleCheng, I.-L., Chen, Y.-H., Lai, C.-C., & Tang, H.-J. (2018). Intravenous Colistin Monotherapy versus Combination Therapy against Carbapenem-Resistant Gram-Negative Bacteria Infections: Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 7(8), 208. https://doi.org/10.3390/jcm7080208





