Safety, Efficacy and Evidence Base for Use of the Subcutaneous Implantable Cardioverter Defibrillator
Abstract
1. Introduction
2. The S-ICD System
3. S-ICD Implantation
4. Screening and Eligibility
5. Safety
5.1. Infections
5.2. Implant Site Complications
5.3. Lead/Pulse Generator Related Complications
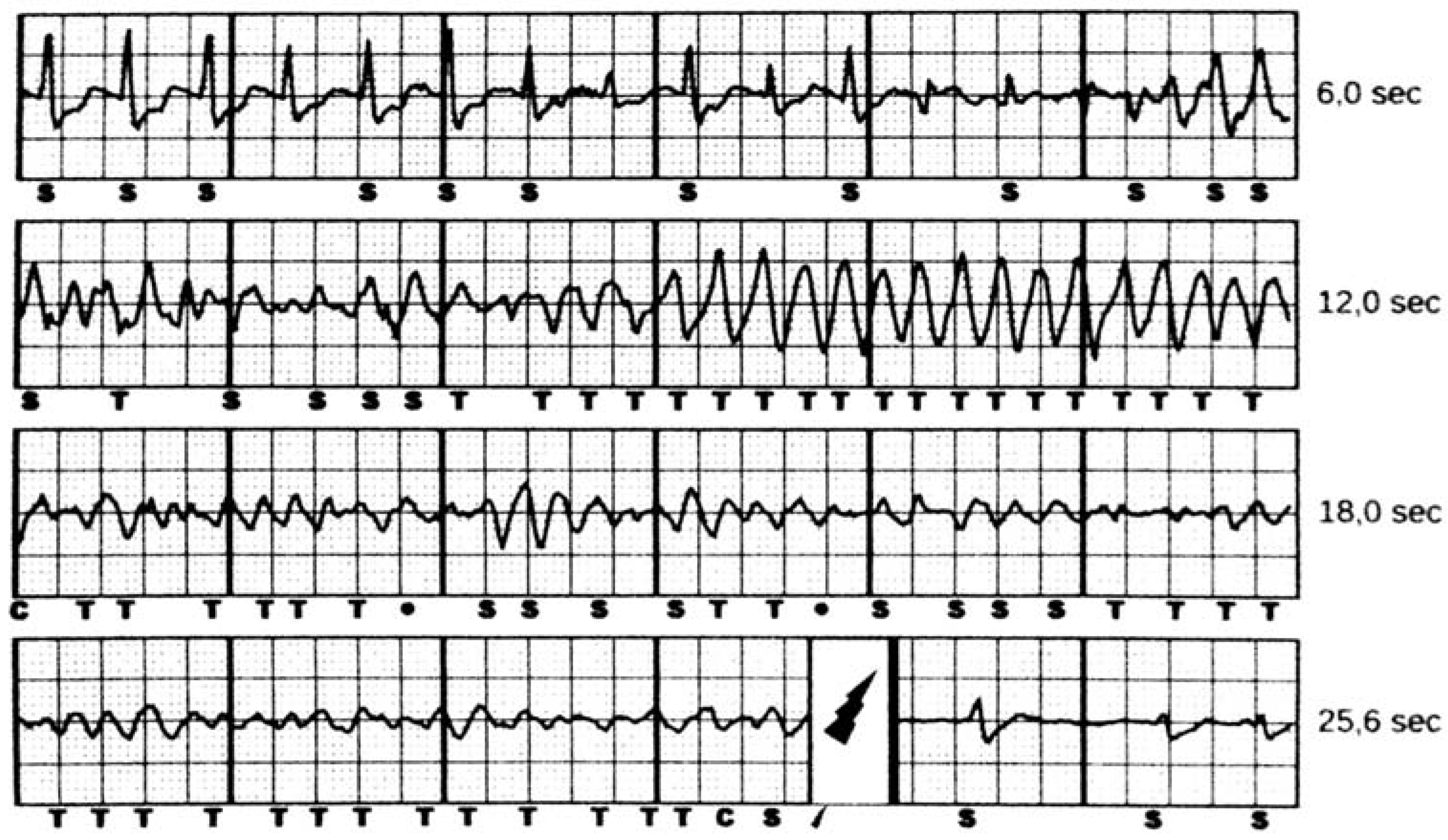
5.4. Inappropriate Shocks
6. Efficacy
6.1. Acute Defibrillation Test
6.2. Spontaneous VT/VF Events
7. Patient Selection
8. Conclusions and Perspectives
Author Contributions
Conflicts of Interest
References
- Buxton, A.E.; Lee, K.L.; Fisher, J.D.; Josephson, M.E.; Prystowsky, E.N.; Hafley, G. A randomized study of the prevention of sudden death in patients with coronary artery disease. N. Engl. J. Med. 1999, 341, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Klein, H.; Levine, J.H.; Saksena, S.; Waldo, A.L.; Wilber, D.; et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N. Engl. J. Med. 1996, 335, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L.; et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Van Rees, J.B.; de Bie, M.K.; Thijssen, J.; Borleffs, C.J.; Schalij, M.J.; van Erven, L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: A systematic review of randomized clinical trials. J. Am. Coll. Cardiol. 2011, 58, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, T.; Becker, T.; Doenges, K.; Vater, M.; Senges, J.; Schneider, S.; Saggau, W.; Weisse, U.; Seidl, K. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation 2007, 115, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, V.A.; Lee, V.; Ahsan, S.; Chow, A.W.; Segal, O.; Rowland, E.; Lowe, M.D.; Lambiase, P.D. A systematic review of icd complications in randomised controlled trials versus registries: Is our ‘real-world’ data an underestimation? Open Heart 2015, 2, e000198. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.R.; Theuns, D.A.; Knight, B.P.; Sturdivant, J.L.; Sanghera, R.; Ellenbogen, K.A.; Wood, M.A.; Burke, M.C. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: The start study. J. Cardiovasc. Electrophysiol. 2012, 23, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Knops, R.E.; Olde Nordkamp, L.R.; de Groot, J.R.; Wilde, A.A. Two-incision technique for implantation of the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2013, 10, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Giofre, F.; De Filippo, P. Intermuscular pocket for subcutaneous implantable cardioverter defibrillator: Single-center experience. J. Arrhythm. 2016, 32, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.C.; Gold, M.R.; Knight, B.P.; Barr, C.S.; Theuns, D.; Boersma, L.V.A.; Knops, R.E.; Weiss, R.; Leon, A.R.; Herre, J.M.; et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the ide study and effortless registry. J. Am. Coll. Cardiol. 2015, 65, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Boersma, L.; Barr, C.; Knops, R.; Theuns, D.; Eckardt, L.; Neuzil, P.; Scholten, M.; Hood, M.; Kuschyk, J.; Jones, P.; et al. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: The effortless study. J. Am. Coll. Cardiol. 2017, 70, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.R.; Aasbo, J.D.; El-Chami, M.F.; Niebauer, M.; Herre, J.; Prutkin, J.M.; Knight, B.P.; Kutalek, S.; Hsu, K.; Weiss, R.; et al. Subcutaneous implantable cardioverter-defibrillator post-approval study: Clinical characteristics and perioperative results. Heart Rhythm 2017, 14, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Parzynski, C.S.; Varosy, P.D.; Prutkin, J.M.; Patton, K.K.; Mithani, A.; Russo, A.M.; Curtis, J.P.; Al-Khatib, S.M. Trends and in-hospital outcomes associated with adoption of the subcutaneous implantable cardioverter defibrillator in the united states. JAMA Cardiol. 2016, 1, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Knops, R.E.; Brouwer, T.F.; Barr, C.S.; Theuns, D.A.; Boersma, L.; Weiss, R.; Neuzil, P.; Scholten, M.; Lambiase, P.D.; Leon, A.R.; et al. The learning curve associated with the introduction of the subcutaneous implantable defibrillator. EP Europace 2016, 18, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Le, K.Y.; Sohail, M.R.; Friedman, P.A.; Uslan, D.Z.; Cha, S.S.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Baddour, L.M. Clinical predictors of cardiovascular implantable electronic device-related infective endocarditis. Pacing Clin. Electrophysiol. PACE 2011, 34, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L. How to treat and identify device infections. Heart Rhythm 2007, 4, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, K.A.; Konstantelias, A.A.; Falagas, M.E. Risk factors for cardiac implantable electronic device infection: A systematic review and meta-analysis. EP Europace 2015, 17, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Epstein, A.E.; Erickson, C.C.; Knight, B.P.; Levison, M.E.; Lockhart, P.B.; Masoudi, F.A.; Okum, E.J.; Wilson, W.R.; Beerman, L.B.; et al. Update on cardiovascular implantable electronic device infections and their management: A scientific statement from the american heart association. Circulation 2010, 121, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Athan, E.; Chu, V.H.; Tattevin, P.; Selton-Suty, C.; Jones, P.; Naber, C.; Miro, J.M.; Ninot, S.; Fernandez-Hidalgo, N.; Durante-Mangoni, E.; et al. Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. JAMA 2012, 307, 1727–1735. [Google Scholar] [CrossRef]
- Wilkoff, B.L. Lead-induced venous thrombosis:Consequences? J. Cardiovasc. Electrophysiol. 2004, 15, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.V.; Wang, Y.; Curtis, J.P.; Heidenreich, P.A.; Hlatky, M.A. Physician procedure volume and complications of cardioverter-defibrillator implantation. Circulation 2012, 125, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, T.F.; Yilmaz, D.; Lindeboom, R.; Buiten, M.S.; Olde Nordkamp, L.R.; Schalij, M.J.; Wilde, A.A.; van Erven, L.; Knops, R.E. Long-term clinical outcomes of subcutaneous versus transvenous implantable defibrillator therapy. J. Am. Coll. Cardiol. 2016, 68, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Van Rees, J.B.; Borleffs, C.J.; de Bie, M.K.; Stijnen, T.; van Erven, L.; Bax, J.J.; Schalij, M.J. Inappropriate implantable cardioverter-defibrillator shocks: Incidence, predictors, and impact on mortality. J. Am. Coll. Cardiol. 2011, 57, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, P.D.; Barr, C.; Theuns, D.A.; Knops, R.; Neuzil, P.; Johansen, J.B.; Hood, M.; Pedersen, S.; Kaab, S.; Murgatroyd, F.; et al. Worldwide experience with a totally subcutaneous implantable defibrillator: Early results from the effortless S-ICD registry. Eur. Heart J. 2014, 35, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Knight, B.P.; Gold, M.R.; Leon, A.R.; Herre, J.M.; Hood, M.; Rashtian, M.; Kremers, M.; Crozier, I.; Lee, K.L.; et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013, 128, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.; Patel, N.; Noseworthy, P.A.; Patel, A.A.; Patel, N.; Arora, S.; Kapa, S.; Noheria, A.; Mulpuru, S.; Badheka, A.; et al. Trends in use and adverse outcomes associated with transvenous lead removal in the United States. Circulation 2015, 132, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Theuns, D.A.; Crozier, I.G.; Barr, C.S.; Hood, M.A.; Cappato, R.; Knops, R.E.; Maass, A.H.; Boersma, L.V.; Jordaens, L. Longevity of the subcutaneous implantable defibrillator: Long-term follow-up of the European regulatory trial cohort. Circ. Arrhythm. Electrophysiol. 2015, 8, 1159–1163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Groh, C.A.; Sharma, S.; Pelchovitz, D.J.; Bhave, P.D.; Rhyner, J.; Verma, N.; Arora, R.; Chicos, A.B.; Kim, S.S.; Lin, A.C.; et al. Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2014, 11, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Kooiman, K.M.; Knops, R.E.; Olde Nordkamp, L.; Wilde, A.A.; de Groot, J.R. Inappropriate subcutaneous implantable cardioverter-defibrillator shocks due to T-wave oversensing can be prevented: Implications for management. Heart Rhythm 2014, 11, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Francia, P.; Adduci, C.; Palano, F.; Semprini, L.; Serdoz, A.; Montesanti, D.; Santini, D.; Musumeci, B.; Salvati, A.; Volpe, M.; et al. Eligibility for the subcutaneous implantable cardioverter-defibrillator in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2015, 26, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.T.; Patel, K.H.; Qamar, K.; Taylor, A.; Baca, M.; Providencia, R.; Tome-Esteban, M.; Elliott, P.M.; Lambiase, P.D. Disease severity and exercise testing reduce subcutaneous implantable cardioverter-defibrillator left sternal ECG screening success in hypertrophic cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2017, 10, e004801. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.R.; Evenson, C.; Badin, A.; Patel, D.; Godara, H.; Essandoh, M.; Okabe, T.; Tyler, J.; Houmsse, M.; Augostini, R.; et al. Role of exercise electrocardiogram to screen for t-wave oversensing after implantation of subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2017, 14, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Brisben, A.J.; Burke, M.C.; Knight, B.P.; Hahn, S.J.; Herrmann, K.L.; Allavatam, V.; Mahajan, D.; Sanghera, R.; Gold, M.R. A new algorithm to reduce inappropriate therapy in the S-ICD system. J. Cardiovasc. Electrophysiol. 2015, 26, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Bardy, G.H.; Smith, W.M.; Hood, M.A.; Crozier, I.G.; Melton, I.C.; Jordaens, L.; Theuns, D.; Park, R.E.; Wright, D.J.; Connelly, D.T.; et al. An entirely subcutaneous implantable cardioverter-defibrillator. N. Engl. J. Med. 2010, 363, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Dabiri Abkenari, L.; Theuns, D.A.; Valk, S.D.; Van Belle, Y.; de Groot, N.M.; Haitsma, D.; Muskens-Heemskerk, A.; Szili-Torok, T.; Jordaens, L. Clinical experience with a novel subcutaneous implantable defibrillator system in a single center. Clin. Res. Cardiol. 2011, 100, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Hohnloser, S.H.; Glikson, M.; Neuzner, J.; Mabo, P.; Vinolas, X.; Kautzner, J.; O’Hara, G.; VanErven, L.; Gadler, F.; et al. Cardioverter defibrillator implantation without induction of ventricular fibrillation: A single-blind, non-inferiority, randomised controlled trial (simple). Lancet 2015, 385, 785–791. [Google Scholar] [CrossRef]
- Bansch, D.; Bonnemeier, H.; Brandt, J.; Bode, F.; Svendsen, J.H.; Felk, A.; Hauser, T.; Wegscheider, K.; Investigators, N.I.T. The no regular defibrillation testing in cardioverter defibrillator implantation (nordic ICD) trial: Concept and design of a randomized, controlled trial of intra-operative defibrillation testing during de novo defibrillator implantation. EP Europace 2015, 17, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Olde Nordkamp, L.R.; Postema, P.G.; Knops, R.E.; van Dijk, N.; Limpens, J.; Wilde, A.A.; de Groot, J.R. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: A systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 2016, 13, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.F.; Vriesendorp, P.A.; Sijbrands, E.J.; Jordaens, L.J.; ten Cate, F.J.; Michels, M. Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: Systematic review and meta-analysis. Circ. Heart Fail. 2012, 5, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, P.D.; Gold, M.R.; Hood, M.; Boersma, L.; Theuns, D.A.; Burke, M.C.; Weiss, R.; Russo, A.M.; Kaab, S.; Knight, B.P. Evaluation of subcutaneous icd early performance in hypertrophic cardiomyopathy from the pooled effortless and ide cohorts. Heart Rhythm 2016, 13, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Rudic, B.; Tulumen, E.; Berlin, V.; Roger, S.; Stach, K.; Liebe, V.; El-Battrawy, I.; Dosch, C.; Papavassiliu, T.; Akin, I.; et al. Low prevalence of inappropriate shocks in patients with inherited arrhythmia syndromes with the subcutaneous implantable defibrillator single center experience and long-term follow-up. J. Am. Heart Assoc. 2017, 6, e006265. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Manero, M.; Sacher, F.; de Asmundis, C.; Maury, P.; Lambiase, P.D.; Sarkozy, A.; Probst, V.; Gandjbakhch, E.; Castro-Hevia, J.; Saenen, J.; et al. Monomorphic ventricular tachycardia in patients with brugada syndrome: A multicenter retrospective study. Heart Rhythm 2016, 13, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, L.L. Monomorphic ventricular tachycardia in brugada syndrome: True-true but related? Heart Rhythm 2016, 13, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American college of cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2017. [Google Scholar] [CrossRef]
- Olde Nordkamp, L.R.; Knops, R.E.; Bardy, G.H.; Blaauw, Y.; Boersma, L.V.; Bos, J.S.; Delnoy, P.P.; van Dessel, P.F.; Driessen, A.H.; de Groot, J.R.; et al. Rationale and design of the praetorian trial: A prospective, randomized comparison of subcutaneous and transvenous implantable cardioverter-defibrillator therapy. Am. Heart J. 2012, 163, 753.e2–760.e2. [Google Scholar] [CrossRef] [PubMed]
| EFFORTLESS | S-ICD Post Approval Study | US S-ICD Trends | |
|---|---|---|---|
| N | 985 | 1637 | 3717 |
| Males | 72% | 69% | 69% |
| Age (years) | 48 ± 17 | 53 ± 15 | 53 ± 15 |
| CAD (previous MI) | 29% | 33% | 40% |
| EF (mean) | 43 ± 18 | 32 ± 14 | 32 ± 14 |
| Hypertrophic Cardiomiopathy | 11% | NA | 5% |
| Channelopathies | 20% | 4% | 8% |
| Diabetes | 11% | 34% | 38.5% |
| Atrial Fibrillation | 16% | 16% | 20% |
| CKD | 8% | 26% (dyalisis 13%) | 41% (dyalisis 20%) |
| Complications | SICD | TV-ICD | Reference |
|---|---|---|---|
| Infections | |||
| Infection rate (per year) | 2% | 1.6% | [16,17,18] |
| 1.7% | >50% | [19] |
| 0% | 22–54% | [20,21] |
| Implant Site Complications | |||
| Haematoma | 4% | 0.86–2.4% | [5,7,22] |
| Device erosion | 1.2–3% | 1.5% | [12,23] |
| Lead or Pulse Generator Complications | |||
| Inappropriate shocks (per year) | 1.6% | 7–10% (first year) | [12,24] |
| 18% (5 year follow-up) | |||
| Electrode dislodgement | 0.6% | 1.8% (single/dual ICD) 5.9% (CRT) | [11,12,24] |
| Pooled Analysis IDE + EFFORTLESS | EFFORTLESS Midterm | S-ICD Post Approval Study | US S-ICD Trends | |
|---|---|---|---|---|
| Infection requiring removal/revision | 1.7% | 2.4% | 1.2% | 0.05% |
| Erosion | 1.2% | 1.7% | ||
| Hematoma | 0.4% | 0.9% | 0.4% | 0.3% |
| Discomfort | 0.9% | 0.8% | 0.1% | |
| Lead dislodgment | 0.6% | 0.7% | 0.1% | |
| Superficial Infection | 0.3% | 0.5% | 0.1% | |
| Suboptimal PG or/and lead position | 1.4% | 1.6% | 0.5% | |
| Inappropriate shocks: oversensing | 4.6% | 5.1% | 0.2% | |
| Inappropriate shocks: SVTs | 2.8% | 2.3% | ||
| Total complications | 9.6% | 11.7% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adduci, C.; Palano, F.; Francia, P. Safety, Efficacy and Evidence Base for Use of the Subcutaneous Implantable Cardioverter Defibrillator. J. Clin. Med. 2018, 7, 53. https://doi.org/10.3390/jcm7030053
Adduci C, Palano F, Francia P. Safety, Efficacy and Evidence Base for Use of the Subcutaneous Implantable Cardioverter Defibrillator. Journal of Clinical Medicine. 2018; 7(3):53. https://doi.org/10.3390/jcm7030053
Chicago/Turabian StyleAdduci, Carmen, Francesca Palano, and Pietro Francia. 2018. "Safety, Efficacy and Evidence Base for Use of the Subcutaneous Implantable Cardioverter Defibrillator" Journal of Clinical Medicine 7, no. 3: 53. https://doi.org/10.3390/jcm7030053
APA StyleAdduci, C., Palano, F., & Francia, P. (2018). Safety, Efficacy and Evidence Base for Use of the Subcutaneous Implantable Cardioverter Defibrillator. Journal of Clinical Medicine, 7(3), 53. https://doi.org/10.3390/jcm7030053





