Impact of PSP Technique on Clinical Outcomes Following Bioresorbable Scaffolds Implantation
Abstract
:1. Introduction
2. PSP Technique Concept
- Pre-dilation: using an NC balloon 1:1 ratio with reference vessel diameter (RVD) is recommended. If the balloon is not completely expanded, alternative scoring or cutting of balloon should be considered. Eventually, if an optimal lesion preparation is not obtained, a metallic stent should be implanted.
- Scaffold sizing: due to the expansion limits and limited BRS sizes available, to perform an accurate scaffold sizing is critical. The manufacturer has published the recommendations to select the scaffold diameter according to the RVD [16].
- ○
- A 2.5 mm diameter scaffold in a vessel with a proximal/distal RVD ≥2.5 mm and <2.75 mm;
- ○
- A 3.0 mm diameter scaffold in a vessel with a proximal/distal RVD ≥2.75 mm and <3.25 mm;
- ○
- A 3.5 mm diameter scaffold in a vessel with a proximal/distal RVD ≥3.25 mm and ≤3.75 mm;
- ○
- If the proximal and distal RVD differed, the mean value is used.
- Post-dilation: using an NC balloon >1:1 ratio with reference vessel diameter up to 0.5 mm (avoid over-expansion) at ≥16 atmosphere is recommended.
3. PSP Technique and Intravascular Imaging
4. PSP Technique and Clinical Outcomes
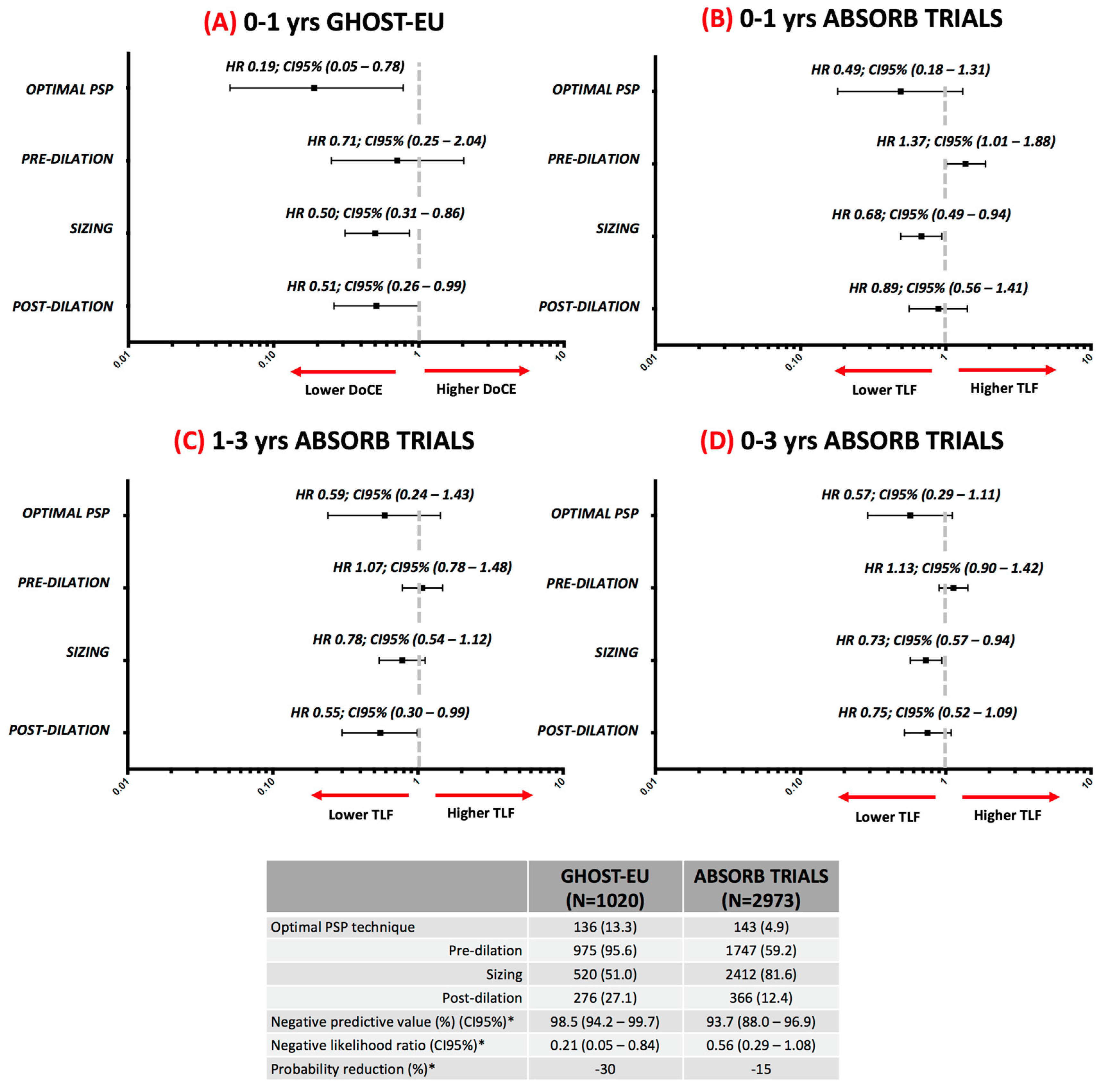
4.1. One-Year Follow-up Data
4.2. Three-Year Follow-Up Data
4.3. Evidence in Context
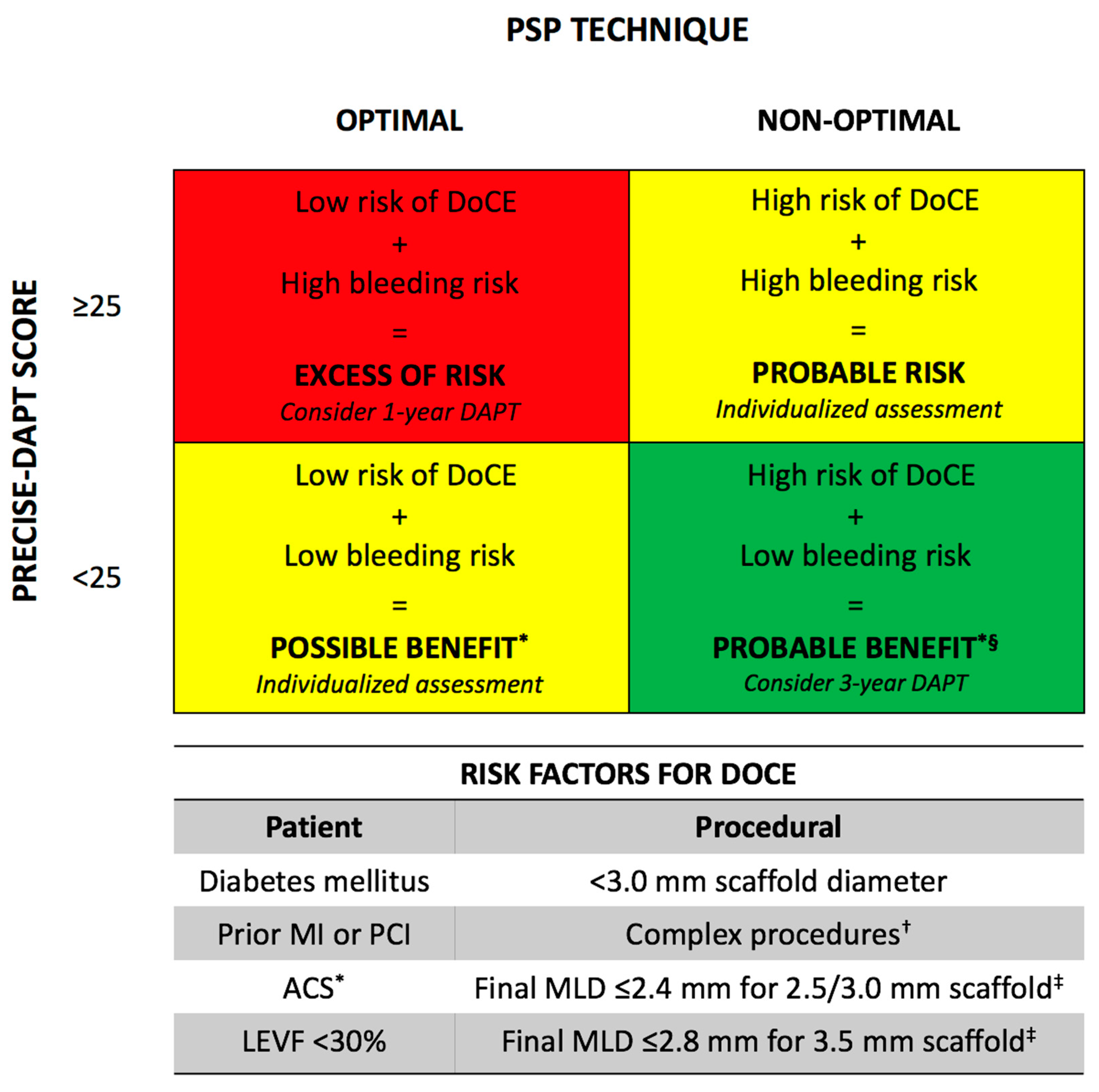
5. DAPT Regimen and PSP Technique
6. Future Perspective
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Giacchi, G.; Ortega-Paz, L.; Brugaletta, S.; Ishida, K.; Sabate, M. Bioresorbable vascular scaffold implantation in acute coronary syndromes: Clinical evidence, tips and tricks. Postepy Kardiol. Interwencyjnej 2015, 11, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lara, J.; Brugaletta, S.; Jacobi, F.; Ortega-Paz, L.; Nato, M.; Roura, G.; Romaguera, R.; Ferreiro, J.L.; Teruel, L.; Gracida, M.; et al. Five-year optical coherence tomography in patients with st-segment-elevation myocardial infarction treated with bare-metal versus everolimus-eluting stents. Circ. Cardiovasc. Interv. 2016, 9, e003670. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Chevalier, B.; Sotomi, Y.; Cequier, A.; Carrie, D.; Piek, J.J.; Van Boven, A.J.; Dominici, M.; Dudek, D.; McClean, D.; et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (absorb ii): A 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016, 388, 2479–2491. [Google Scholar] [CrossRef]
- Wykrzykowska, J.J.; Kraak, R.P.; Hofma, S.H.; van der Schaaf, R.J.; Arkenbout, E.K.; AJ, I.J.; Elias, J.; van Dongen, I.M.; Tijssen, R.Y.G.; Koch, K.T.; et al. Bioresorbable scaffolds versus metallic stents in routine pci. N. Engl. J. Med. 2017, 376, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Ellis, S.G.; Metzger, C.; Caputo, R.P.; Rizik, D.G.; Teirstein, P.S.; Litt, M.R.; Kini, A.; Kabour, A.; Marx, S.O.; et al. 3-year clinical outcomes with everolimus-eluting bioresorbable coronary scaffolds: The absorb iii trial. J. Am. Coll. Cardiol. 2017, 70, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Serruys, P.W.; Kimura, T.; Gao, R.; Ellis, S.G.; Kereiakes, D.J.; Onuma, Y.; Simonton, C.; Zhang, Z.; Stone, G.W. 2-year outcomes with the absorb bioresorbable scaffold for treatment of coronary artery disease: A systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet 2017, 390, 760–772. [Google Scholar] [CrossRef]
- Puricel, S.; Cuculi, F.; Weissner, M.; Schmermund, A.; Jamshidi, P.; Nyffenegger, T.; Binder, H.; Eggebrecht, H.; Munzel, T.; Cook, S.; et al. Bioresorbable coronary scaffold thrombosis: Multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J. Am. Coll. Cardiol. 2016, 67, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L.; Capodanno, D.; Gori, T.; Nef, H.; Latib, A.; Caramanno, G.; Di Mario, C.; Naber, C.; Lesiak, M.; Capranzano, P.; et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events: Development and internal validation of the psp score. EuroIntervention 2017, 12, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Latib, A.; Kawamoto, H.; Jabbour, R.J.; Sato, K.; Miyazaki, T.; Naganuma, T.; Mangieri, A.; Pagnesi, M.; Montalto, C.; et al. Clinical outcomes of a real-world cohort following bioresorbable vascular scaffold implantation utilising an optimised implantation strategy. EuroIntervention 2017, 12, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Abizaid, A.; Onuma, Y.; Seth, A.; Gao, R.; Ormiston, J.; Kimura, T.; Chevalier, B.; Ben-Yehuda, O.; Dressler, O.; et al. Effect of technique on outcomes following bioresorbable vascular scaffold implantation: Analysis from the absorb trials. J. Am. Coll. Cardiol. 2017, 70, 2863–2874. [Google Scholar] [CrossRef] [PubMed]
- Gori, T.; Weissner, M.; Gonner, S.; Wendling, F.; Ullrich, H.; Ellis, S.; Anadol, R.; Polimeni, A.; Munzel, T. Characteristics, predictors, and mechanisms of thrombosis in coronary bioresorbable scaffolds: Differences between early and late events. JACC Cardiovasc. Interv. 2017, 10, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, C.; Latib, A.; van Geuns, R.J.; Sabate, M.; Mehilli, J.; Gori, T.; Achenbach, S.; Alvarez, M.P.; Nef, H.; Lesiak, M.; et al. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: A european perspective. EuroIntervention 2015, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.G.; Steffenino, G.; Kereiakes, D.J.; Stone, G.W.; van Geuns, R.J.; Abizaid, A.; Nef, H.; Cortese, B.; Testa, L.; Menichelli, M.; et al. Clinical, angiographic, and procedural correlates of acute, subacute, and late absorb scaffold thrombosis. JACC Cardiovasc. Interv. 2017, 10, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Angiolillo, D.J. Antiplatelet therapy after implantation of bioresorbable vascular scaffolds: A review of the published data, practical recommendations, and future directions. JACC Cardiovasc. Interv. 2017, 10, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 esc focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with eacts: The task force for dual antiplatelet therapy in coronary artery disease of the european society of cardiology (esc) and of the european association for cardio-thoracic surgery (eacts). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [PubMed]
- Abbott Vascular. Simple PSP Implant Strategy. Available online: https://www.absorb.com/technique-why-absorb (accessed on 28 December 2017).
- Everaert, B.; Felix, C.; Koolen, J.; den Heijer, P.; Henriques, J.; Wykrzykowska, J.; van der Schaaf, R.; de Smet, B.; Hofma, S.; Diletti, R.; et al. Appropriate use of bioresorbable vascular scaffolds in percutaneous coronary interventions: A recommendation from experienced users: A position statement on the use of bioresorbable vascular scaffolds in the netherlands. Neth. Heart J. 2015, 23, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L. Pre-dilation, sizing and post-dilation (psp) score for evaluating adverse cardiac events in patients undergoing everolimus-eluting brs implantation: External validation of the psp score. In EuroPCR; Clarivate Analytics: Paris, France, 2017. [Google Scholar]
- Stamper, D.; Weissman, N.J.; Brezinski, M. Plaque characterization with optical coherence tomography. J. Am. Coll. Cardiol. 2006, 47, C69–C79. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L.; Capodanno, D.; Giacchi, G.; Gori, T.; Nef, H.; Latib, A.; Caramanno, G.; Di Mario, C.; Naber, C.; Lesiak, M.; et al. Impact of overlapping on 1-year clinical outcomes in patients undergoing everolimus-eluting bioresorbable scaffolds implantation in routine clinical practice: Insights from the european multicenter ghost-eu registry. Catheter. Cardiovasc. Interv. 2017, 89, 812–818. [Google Scholar] [CrossRef] [PubMed]
- De Jaegere, P.; Mudra, H.; Figulla, H.; Almagor, Y.; Doucet, S.; Penn, I.; Colombo, A.; Hamm, C.; Bartorelli, A.; Rothman, M.; et al. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the multicenter ultrasound stenting in coronaries study (music study). Eur. Heart J. 1998, 19, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Suwannasom, P.; Sotomi, Y.; Ishibashi, Y.; Cavalcante, R.; Albuquerque, F.N.; Macaya, C.; Ormiston, J.A.; Hill, J.; Lang, I.M.; Egred, M.; et al. The impact of post-procedural asymmetry, expansion, and eccentricity of bioresorbable everolimus-eluting scaffold and metallic everolimus-eluting stent on clinical outcomes in the absorb ii trial. JACC Cardiovasc. Interv. 2016, 9, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.; Radu, M.D.; Zaugg, S.; Amabile, N.; Garcia-Garcia, H.M.; Yamaji, K.; Jorgensen, E.; Kelbaek, H.; Pilgrim, T.; Caussin, C.; et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation 2016, 133, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Soeda, T.; Uemura, S.; Park, S.J.; Jang, Y.; Lee, S.; Cho, J.M.; Kim, S.J.; Vergallo, R.; Minami, Y.; Ong, D.S.; et al. Incidence and clinical significance of poststent optical coherence tomography findings: One-year follow-up study from a multicenter registry. Circulation 2015, 132, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Mattesini, A.; Secco, G.G.; Dall’Ara, G.; Ghione, M.; Rama-Merchan, J.C.; Lupi, A.; Viceconte, N.; Lindsay, A.C.; De Silva, R.; Foin, N.; et al. Absorb biodegradable stents versus second-generation metal stents: A comparison study of 100 complex lesions treated under oct guidance. JACC Cardiovasc. Interv. 2014, 7, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Latib, A.; Caussin, C.; Presbitero, P.; Galli, S.; Menozzi, A.; Varbella, F.; Mauri, F.; Valgimigli, M.; Arampatzis, C.; et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: The avio trial. Am. Heart J. 2013, 165, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Asano, T.; Sotomi, Y.; Cavalcante, R.; Miyazaki, Y.; Zeng, Y.; Tummala, K.; Stanetic, B.; Tijssen, J.; DE Winter, R.; et al. Early, late and very late incidence of bioresorbable scaffold thrombosis: A systematic review and meta-analysis of randomized clinical trials and observational studies. Minerva Cardioangiol. 2017, 65, 32–51. [Google Scholar] [CrossRef]
- Sotomi, Y.; Suwannasom, P.; Serruys, P.W.; Onuma, Y. Possible mechanical causes of scaffold thrombosis: Insights from case reports with intracoronary imaging. EuroIntervention 2017, 12, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Ueki, Y.; Souteyrand, G.; Daemen, J.; Wiebe, J.; Nef, H.; Adriaenssens, T.; Loh, J.P.; Lattuca, B.; Wykrzykowska, J.J.; et al. Mechanisms of very late bioresorbable scaffold thrombosis: The invest registry. J. Am. Coll. Cardiol. 2017, 70, 2330–2344. [Google Scholar] [CrossRef] [PubMed]
- Raber, L.; Brugaletta, S.; Yamaji, K.; O’Sullivan, C.J.; Otsuki, S.; Koppara, T.; Taniwaki, M.; Onuma, Y.; Freixa, X.; Eberli, F.R.; et al. Very late scaffold thrombosis: Intracoronary imaging and histopathological and spectroscopic findings. J. Am. Coll. Cardiol. 2015, 66, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Brugaletta, S.; Ortega-Paz, L.; Onuma, Y. Data from real-world registries: Can it guide development of the bioresorbable scaffolds of tomorrow? EuroIntervention 2017, 13, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Felix, C.M.; Vlachojannis, G.J.; AJJ, I.J.; Fam, J.M.; Smits, P.C.; Lansink, W.J.; Diletti, R.; Zijlstra, F.; Regar, E.S.; Boersma, E.; et al. Potentially increased incidence of scaffold thrombosis in patients treated with absorb bvs who terminated dapt before 18 months. EuroIntervention 2017, 13, e177–e184. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.G.; Kereiakes, D.J.; Metzger, D.C.; Caputo, R.P.; Rizik, D.G.; Teirstein, P.S.; Litt, M.R.; Kini, A.; Kabour, A.; Marx, S.O.; et al. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N. Engl. J. Med. 2015, 373, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Raber, L.; Feres, F.; Pilgrim, T.; Hong, M.K.; Kim, H.S.; Colombo, A.; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (precise-dapt) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef]
- Stone, G.W. A clinical evaluation of absorb™ bvs, the everolimus eluting bioresorbable vascular scaffold in the treatment of subjects with de novo native coronary artery lesions. In Proceedings of the TCT 2017, Denver, CO, USA, 29 October–2 November 2017. [Google Scholar]
- Giacchi, G.; Ortega-Paz, L.; Brugaletta, S.; Ishida, K.; Sabate, M. Bioresorbable vascular scaffolds technology: Current use and future developments. Med. Devices (Auckl.) 2016, 9, 185–198. [Google Scholar] [PubMed]
- Rapoza, R.J. Progress with a second generation absorb: Falcon design and development plans. In Proceedings of the TCT 2017, Denver, CO, USA, 29 October–2 November 2017. [Google Scholar]
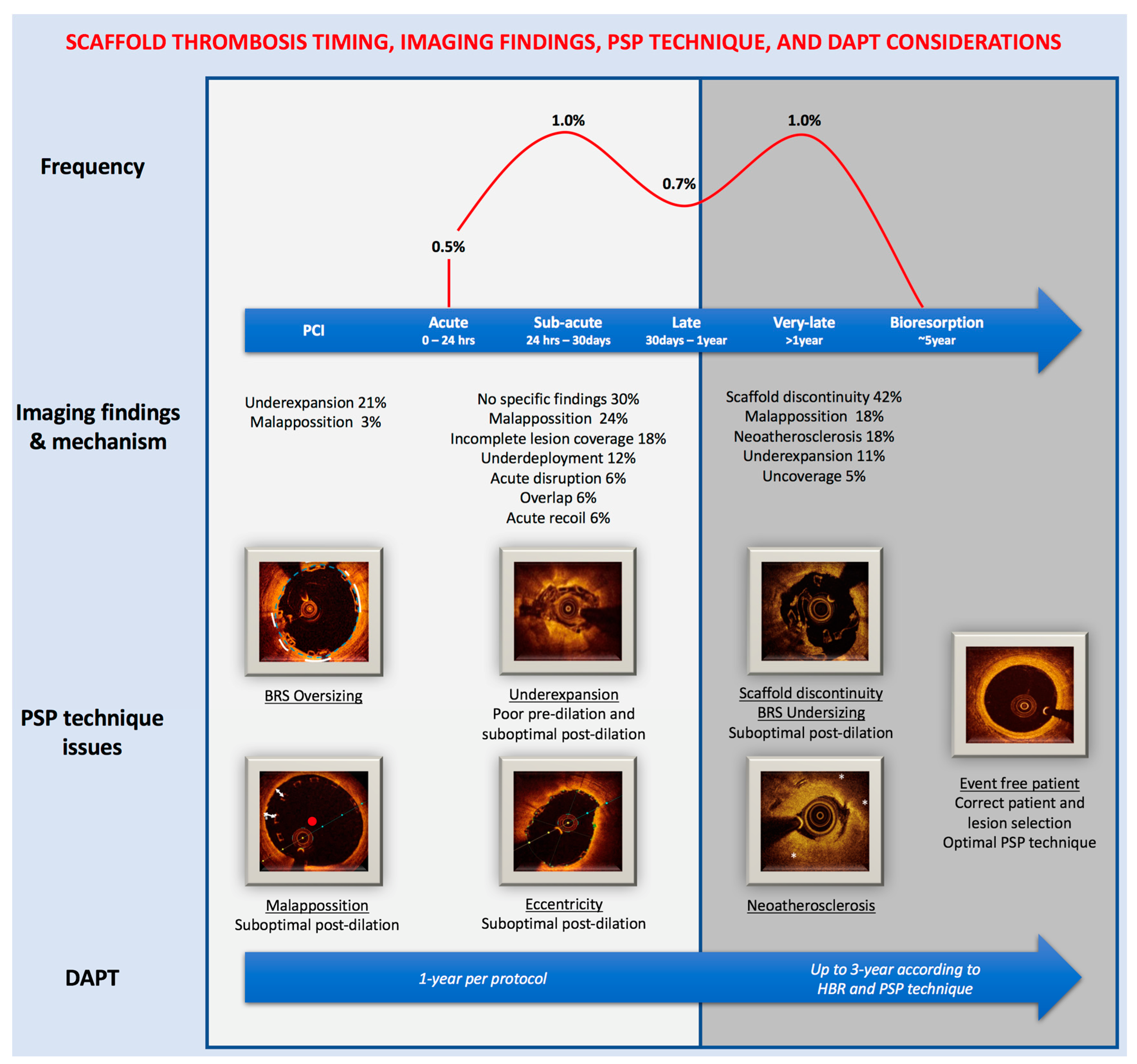
| PSP Step | Angiography—QCA Guided | Intravascular Imaging Guided |
|---|---|---|
| Pre-dilation |
| To assess plaque composition [19]:
|
| Scaffold sizing |
|
|
| Post-dilation |
| The following should be assessed:
|
| Trial Characteristic | GHOST-EU Registry | ABSORB TRIALS |
|---|---|---|
| Studies designs (publication date) | Retrospective registry of consecutive cases (February 2015) | ABSORB II RCT (January 2015), ABSORB III RCT (November 2015), ABSORB CHINA RCT (December 2015), ABSORB JAPAN RCT (December 2015), ABSORB EXTEND registry (April 2015) |
| Post-hoc analysis | Yes | Yes |
| Patients | 1020 | 2973 |
| Clinical settings | CAD, ACS (including STEMI), CTO, Ostial, Bifurcations, LMCA and ISR. | CAD and ACS |
| Scaffold overlap | Yes | Only ABSORB II and EXTEND |
| Lesion characteristics | No lesion length restriction Up to four lesions | De novo Lesion length <28 mm (except ABSORB II) Up to two lesions |
| Intravascular imaging | Not mandatory, performed in a minority | Not mandatory, performed in a minority |
| Endpoint | DoCE: Cardiac death, target-vessel myocardial infarction, or clinically-driven target lesion revascularization | TLF: Cardiac death, target-vessel myocardial infarction, or ischemia-driven target lesion revascularization |
| Core lab analysis and event adjudication | No | Yes (different from each study) |
| Follow-up | Up to 1-year | Up to 3-year |
| Optimal PSP technique | All steps performed correctly in all lesions. Angiography guided. Offline QCA analysis. | All steps performed correctly in all lesions. Angiography guided. Offline QCA analysis. |
| Pre-dilation | NC balloon ≥1:1 ratio with RVD | NC balloon ≥1:1 ratio with RVD |
| Sizing | According to manufacturer recommendations * | QCA-RVD ≥2.25 mm and ≤3.75 mm |
| Post-dilation | NC balloon >1:1 ratio with RVD up to 0.5 mm at ≥16 atmosphere | NC balloon at ≥18 atm and with nominal diameter larger than the nominal scaffold diameter, but not >0.5 mm larger |
| Improvement | Comment [37] |
|---|---|
| Strut thickness | Reduction from 157 to 99 µm May reduce acute thrombogenicity and achieve full endothelialization earlier Increase deliverability |
| Increase size matrix | From 14 to 40 sizes Longer scaffold to avoid overlap New diameters for optimal sizing |
| Delivery balloon system | Reduce compliance of the delivery balloon Optimized for PSP technique More accurate deployment diameters |
| Intravascular imaging | Optical Coherence Tomography guidance to ensure optimal implantation |
| Radial strength resorption | Will maintain poly-l-lactic acid structure Will maintain poly-dl-lactic acid/Everolimus Gradual loss of radial strength after complete coverage |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Paz, L.; Brugaletta, S.; Sabaté, M. Impact of PSP Technique on Clinical Outcomes Following Bioresorbable Scaffolds Implantation. J. Clin. Med. 2018, 7, 27. https://doi.org/10.3390/jcm7020027
Ortega-Paz L, Brugaletta S, Sabaté M. Impact of PSP Technique on Clinical Outcomes Following Bioresorbable Scaffolds Implantation. Journal of Clinical Medicine. 2018; 7(2):27. https://doi.org/10.3390/jcm7020027
Chicago/Turabian StyleOrtega-Paz, Luis, Salvatore Brugaletta, and Manel Sabaté. 2018. "Impact of PSP Technique on Clinical Outcomes Following Bioresorbable Scaffolds Implantation" Journal of Clinical Medicine 7, no. 2: 27. https://doi.org/10.3390/jcm7020027
APA StyleOrtega-Paz, L., Brugaletta, S., & Sabaté, M. (2018). Impact of PSP Technique on Clinical Outcomes Following Bioresorbable Scaffolds Implantation. Journal of Clinical Medicine, 7(2), 27. https://doi.org/10.3390/jcm7020027





