Plasma Branched-Chain Amino Acids and Risk of Incident Type 2 Diabetes: Results from the PREVEND Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Baseline Assessment of BCAA
2.3. Clinical and Laboratory Measures
2.4. End Point of the Study
2.5. Statistical Analysis
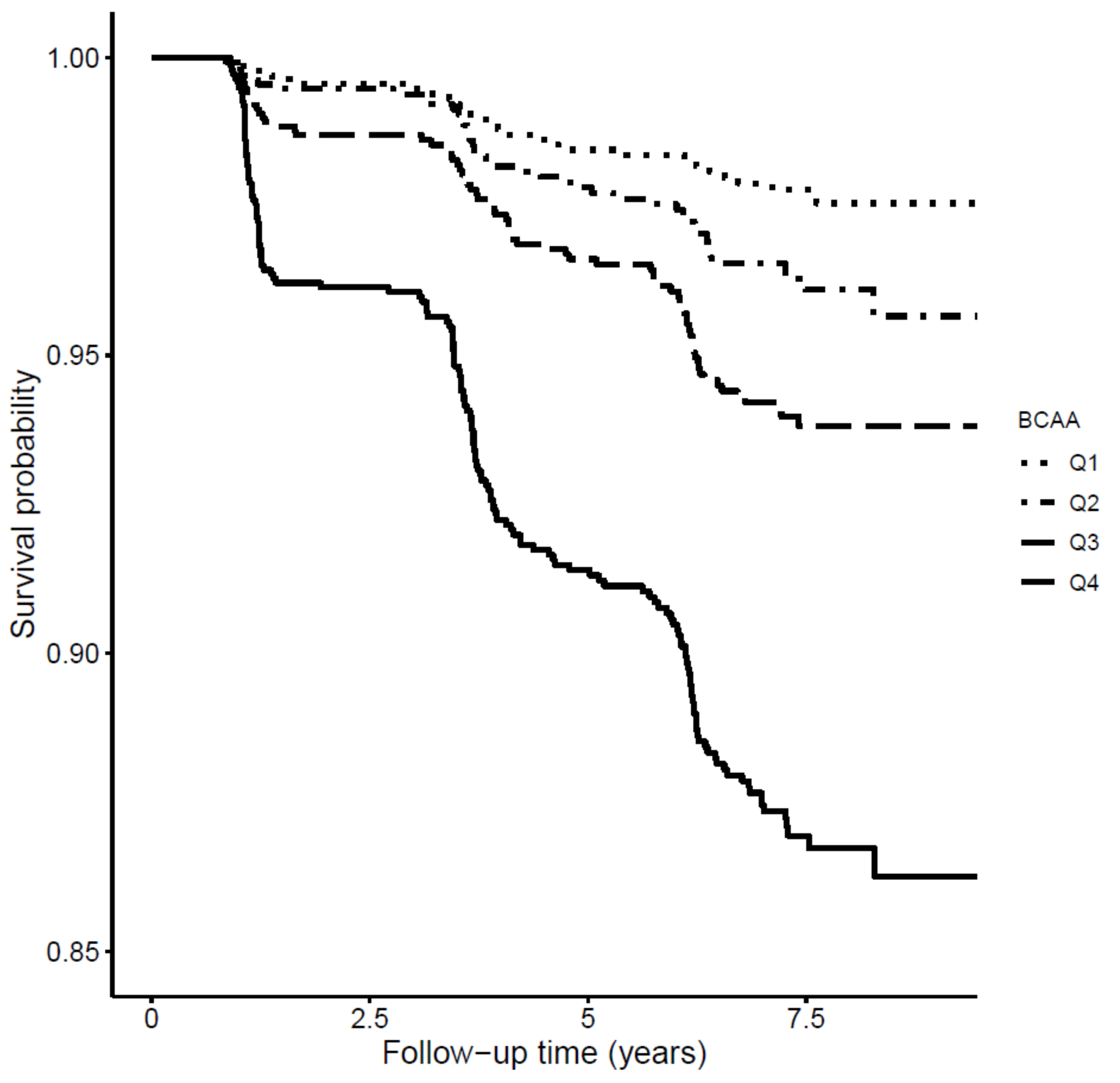
3. Results
3.1. Baseline Characteristics
3.2. Associations at Baseline
3.3. Longitudinal Analysis
3.4. Effect of Inclusion of BCAA on Type 2 Diabetes Risk Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nair, K.S.; Short, K.R. Hormonal and Signaling Role of Branched-Chain Amino Acids. J. Nutr. 2005, 135, 1547S–1552S. [Google Scholar] [CrossRef] [PubMed]
- Platell, C.; Kong, S.E.; McCauley, R.; Hall, J.C. Branched-chain amino acids. J. Gastroenterol. Hepatol. 2000, 15, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Bloomgarden, Z. Diabetes and branched-chain amino acids: What is the link? J. Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef]
- Valerio, A.; D’Antona, G.; Nisoli, E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: An evolutionary perspective. Aging 2011, 3, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Nairizi, A.; She, P.; Vary, T.C.; Lynch, C.J. Leucine Supplementation of Drinking Water Does Not Alter Susceptibility to Diet-Induced Obesity in Mice. J. Nutr. 2009, 139, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Krebs, M.; Dombrowski, L.; Brehm, A.; Bernroider, E.; Roth, E.; Nowotny, P.; Waldhäusl, W.; Marette, A.; Roden, M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005, 54, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Huang, Z.; Li, H.; Yu, J.; Wang, C.; Chen, S.; Meng, Q.; Cheng, Y.; Gao, X.; Li, J.; et al. Leucine Deprivation Increases Hepatic Insulin Sensitivity via GCN2/mTOR/S6K1 and AMPK Pathways. Diabetes 2011, 60, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.; An, J.; Bain, J.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Honda, T.; Kobayashi, Y.; Togashi, K.; Hasegawa, H.; Iwasa, M.; Taguchi, O.; Takei, Y.; Sumida, Y. Associations among circulating branched-chain amino acids and tyrosine with muscle volume and glucose metabolism in individuals without diabetes. Nutrition 2016, 32, 531–538. [Google Scholar] [CrossRef]
- Connelly, M.A.; Wolak-Dinsmore, J.; Dullaart, R.P.F. Branched Chain Amino Acids Are Associated with Insulin Resistance Independent of Leptin and Adiponectin in Subjects with Varying Degrees of Glucose Tolerance. Metab. Syndr. Relat. Disord. 2017, 15, 183–186. [Google Scholar] [CrossRef]
- Wolak-Dinsmore, J.; Gruppen, E.G.; Shalaurova, I.; Matyus, S.P.; Grant, R.P.; Gegen, R.; Bakker, S.J.L.; Otvos, J.D.; Connelly, M.A.; Dullaart, R.P.F. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: Elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin. Biochem. 2018, 54, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, P.; Tiainen, M.; Makinen, V.P.; Kangas, A.J.; Soininen, P.; Saltevo, J.; Keinänen-Kiukaanniemi, S.; Mäntyselkä, P.; Lehtimäki, T.; Laakso, M.; et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care 2012, 35, 1749–1756. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Stancakova, A.; Civelek, M.; Saleem, N.K.; Soininen, P.; Kangas, A.J.; Cederberg, H.; Paananen, J.; Pihlajamäki, J.; Bonnycastle, L.L.; Morken, M.A.; et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes 2012, 61, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A.; Jia, W.; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Guasch-Ferre, M.; Toledo, E.; Clish, C.B.; Razquin, C.; Liang, L.; Wang, D.D.; Corella, D.; Estruch, R.; Hernáez, Á.; et al. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: Case-cohort study within the PREDIMED Trial. Diabetologia 2018, 61, 1560–1571. [Google Scholar] [CrossRef]
- Kulathinal, S.; Karvanen, J.; Saarela, O.; Kuulasmaa, K. Case-cohort design in practice—Experiences from the MORGAM Project. Epidemiol. Perspect. Innov. 2007, 4, 15. [Google Scholar] [CrossRef]
- Ganna, A.; Reilly, M.; de Faire, U.; Pedersen, N.; Magnusson, P.; Ingelsson, E. Risk Prediction Measures for Case-Cohort and Nested Case-Control Designs: An Application to Cardiovascular Disease. Am. J. Epidemiol. 2012, 175, 715–724. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.L.; Kootstra-Ros, J.E.; Gansevoort, R.T.; Dullaart, R.P.F. Circulating gamma glutamyltransferase and prediction of cardiovascular disease. Atherosclerosis 2015, 238, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Kieneker, L.M.; Burgess, S.; Bakker, S.J.L.; Dullaart, R.P.F. Circulating Total Bilirubin and Future Risk of Hypertension in the General Population: The Prevention of Renal and Vascular End-Stage Disease (PREVEND) Prospective Study and a Mendelian Randomization Approach. J. Am. Heart Assoc. 2017, 6, e006503. [Google Scholar] [CrossRef]
- Matyus, S.P.; Braun, P.J.; Wolak-Dinsmore, J.; Jeyarajah, E.J.; Shalaurova, I.; Xu, Y.; Warner, S.M.; Clement, T.S.; Connelly, M.A.; Fischer, T.J.; et al. NMR measurement of LDL particle number using the Vantera® Clinical Analyzer. Clin. Biochem. 2014, 47, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.L.; Kootstra-Ros, J.E.; Blokzijl, H.; Gansevoort, R.T.; Dullaart, R.P.F. Inverse linear associations between liver aminotransferases and incident cardiovascular disease risk: The PREVEND study. Atherosclerosis 2015, 243, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, J.P.; Bakker, S.J.L.; Sparks, C.E.; Dullaart, R.P.F. Apolipoprotein A-II Influences Apolipoprotein E-Linked Cardiovascular Disease Risk in Women with High Levels of HDL Cholesterol and C-Reactive Protein. PLoS ONE 2012, 7, e39110. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.F.; Perton, F.; van der Klauw, M.M.; Hillege, H.L.; Sluiter, W.J.; PREVEND Study Group. High plasma lecithin:cholesterol acyltransferase activity does not predict low incidence of cardiovascular events: Possible attenuation of cardioprotection associated with high HDL cholesterol. Atherosclerosis 2010, 208, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Liu, X.; Song, D.; Zhang, X.; Zhu, B.; Wei, L.; Pei, X.; Wu, J.; Lou, T.; Zhao, W. Estimating glomerular filtration rate by serum creatinine or/and cystatin C equations: An analysis of multi-centre Chinese subjects. Nephrology 2016, 21, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Lyssenko, V.; Jonsson, A.; Almgren, P.; Pulizzi, N.; Isomaa, B.; Tuomi, T.; Berglund, G.; Altshuler, D.; Nilsson, P.; Groop, L. Clinical Risk Factors, DNA Variants, and the Development of Type 2 Diabetes. N. Engl. J. Med. 2008, 359, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Harrel, F.E.; Lee, K.L.; Mark, D.B. Multivariable Prognostic Models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; Meigs, J.B.; Sullivan, L.; Fox, C.S.; Nathan, D.M.; D’Agostino, R.B. Prediction of Incident Diabetes Mellitus in Middle-aged Adults. Arch. Intern. Med. 2007, 167, 1068. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.J.; D’Agostino, R.B.; D’Agostino, R.B.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Cook, N.R. Use and Misuse of the Receiver Operating Characteristic Curve in Risk Prediction. Circulation 2007, 115, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E. Multivariable Modeling Strategies. In Regression Modeling Strategies; Springer: Cham, Switzerland; New York, NY, USA, 2015; pp. 63–102. [Google Scholar]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.G.; Fritsche, A.; Häring, H.U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of Serum Metabolites Associated With Risk of Type 2 Diabetes Using a Targeted Metabolomic Approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Clish, C.; Mora, S.; Li, J.; Liang, L.; Hu, F.B.; Manson, J.E.; Zhang, C. Dietary Intakes and Circulating Concentrations of Branched-Chain Amino Acids in Relation to Incident Type 2 Diabetes Risk Among High-Risk Women with a History of Gestational Diabetes Mellitus. Clin. Chem. 2018, 64, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 2013, 8, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Crosslin, D.R.; Haynes, C.S.; Nelson, S.; Turer, C.B.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Laferrère, B.; et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012, 55, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Natali, A.; Camastra, S.; Nannipieri, M.; Mari, A.; Adam, K.P.; Milburn, M.V.; Kastenmüller, G.; Adamski, J.; Tuomi, T.; et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 2013, 62, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, P.; Makinen, V.P.; Soininen, P.; Kangas, A.J.; Tukiainen, T.; Kettunen, J.; Savolainen, M.J.; Tammelin, T.; Viikari, J.S.; Rönnemaa, T.; et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 2012, 61, 1372–1380. [Google Scholar] [CrossRef]
- Tillin, T.; Hughes, A.D.; Wang, Q.; Würtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.G.; Godsland, I.F.; Eastwood, S.V.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef] [PubMed]
| All Participants | Quartiles of BCAA | p-Value * | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| ♂ < 365.31 | ♂ 365.32–408.34 | ♂ 408.35–454.02 | ♂ > 454.023 | |||
| ♀ < 299.38 | ♀ 299.39–336.23 | ♀ 336.24–377.35 | ♀ > 377.36 | |||
| Participants, n | 6244 | 1562 | 1560 | 1560 | 1562 | |
| Sex, men, % | 49.4 | 49.4 | 49.4 | 49.3 | 49.4 | 0.99 |
| Age, y | 53.1 ± 11.9 | 51.76 ± 13.25 | 52.77 ± 12.31 | 53.75 ± 12.37 | 54.34 ± 11.41 | <0.0001 |
| Race, white, % | 95.4 | 96.3 | 96.2 | 95.8 | 93.2 | <0.0001 |
| Education, high, % | 38.0 | 39.1 | 41.4 | 37.8 | 33.8 | <0.001 |
| BMI, kg/m2 | 26.5 ± 4.2 | 24.7 ± 3.6 | 25.8 ± 3.7 | 26.7 ± 3.9 | 28.6 ± 4.4 | <0.0001 |
| SBP, mm Hg | 125.6 ± 18.5 | 123.0 ± 18.6 | 123.6 ± 17.5 | 125.8 ± 18.5 | 130.3 ± 18.6 | <0.0001 |
| DBP, mm Hg | 73.2 ± 9.0 | 71.9 ± 9.4 | 72.5 ± 8.8 | 73.4 ± 9.0 | 75.0 ± 8.7 | <0.0001 |
| Parental history of CKD, % | 0.5 | 0.6 | 0.3 | 0.8 | 0.3 | 0.18 |
| Parental history of T2D, % | 14.2 | 12.8 | 12.5 | 13.5 | 18.1 | <0.0001 |
| Current smoking status, no % | 70.7 | 65.8 | 70.8 | 73.7 | 72.4 | <0.0001 |
| Alcohol intake, never, % | 24.1 | 23.7 | 22.4 | 24.5 | 25.6 | 0.23 |
| Antihypertensive drugs,% | 18.0 | 14.7 | 14.5 | 18.2 | 24.5 | <0.0001 |
| Lipid-lowering drugs, % | 7.0 | 5.1 | 5.5 | 7.5 | 9.8 | <0.0001 |
| Total BCAA, μM | 370.34 ± 88.63 | <365.31 | 365.32–408.34 | 408.35–454.02 | >454.023 | |
| Valine, μM | 203.08 ± 46.58 | 156.32 ± 50.40 | 194.59 ± 20.71 | 215.14 ± 21.63 | 246.30 ± 31.10 | <0.0001 |
| Leucine, μM | 124.68 ± 32.56 | 92.85 ± 31.17 | 118.95 ± 16.50 | 132.01 ± 18.48 | 154.90 ± 25.31 | <0.0001 |
| Isoleucine, μM | 42.90 ± 16.13 | 30.01 ± 12.73 | 39.45 ± 10.35 | 45.02 ± 11.37 | 56.67 ± 16.43 | <0.0001 |
| TC, mmol/L | 5.43 ± 1.03 | 5.22 ± 0.95 | 5.36 ± 1.02 | 5.51 ± 1.04 | 5.67 ± 1.06 | <0.0001 |
| HDL-C, mmol/L | 1.25 ± 0.32 | 1.131 ± 0.37 | 1.129 ± 0.30 | 1.25 ± 0.29 | 1.17 ± 0.28 | <0.0001 |
| TG, mmol/L | 1.09 (0.79–1.57) | 0.88 (0.65–1.20) | 1.01 (0.75–1.38) | 1.13 (0.84–1.61) | 1.47 (1.06–2.08) | <0.0001 |
| Glucose, mmol/L | 4.84 ± 0.64 | 4.872 ± 0.60 | 4.75 ± 0.59 | 4.85 ± 0.60 | 5.03 ± 0.70 | <0.0001 |
| Insulin, mU/L | 8.00 (5.70–11.80) | 6.50 (4.80–8.72) | 7.20 (5.20–10.25) | 8.30 (6.00–11.70) | 11.845 (7.70–16.62) | <0.0001 |
| Serum creatinine, µmol/L | 84.55 ± 20.64 | 83.43 ± 30.66 | 83.97 ± 16.32 | 85.14 ± 15.35 | 85.64 ± 16.16 | 0.132 |
| eGFR, mL/min/1.73m2 | 92.80 ± 17.00 | 95.45 ± 17.64 | 93.57 ± 16.51 | 91.79 ± 16.33 | 90.38 ± 17.05 | <0.0001 |
| UAE, mg/24h | 8.53 (6.02–15.08) | 8.15 (5.94–13.82) | 8.23 (5.88–13.62) | 8.41 (5.98–14.85) | 9.65 (6.50–18.20) | 0.101 |
| HOMA-IR, (mU mmol/L2)/22.5 | 1.70 (1.17–2.61) | 1.40 (1.02–1.93) | 1.54 (1.10–2.24) | 1.78 (1.25–2.60) | 2.53 (1.66–3.85) | <0.0001 |
| HOMA-β, % | 132.5 (90.6–200.0) | 116.9 (82.5–178.6) | 128.5 (90.0–190.0) | 133.3 (96.0–193.5) | 160.0 (110.5–237.1) | <0.0001 |
| HOMA-β/HOMA-IR | 79.78 (50.90–113.63) | 79.79 (55.1–113.6) | 79.79 (55.1–113.6) | 79.79 (50.9–113.6) | 60.0 (40.9–88.9) | <0.0001 |
| HOMA-IR, (mU mmol/L2)/22.5 | HOMA-β, % | |||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Crude Model | 28.92 (27.16, 30.67) | <0.0001 | 20.46 (18.67, 22.25) | <0.0001 |
| Model 1 | 26.80 (25.18, 28.43) | <0.0001 | 21.01 (19.38, 22.64) | <0.0001 |
| Model 2 | 30.95 (27.83, 34.07) | <0.0001 | −4.73 (−7.77, −1.70) | 0.002 |
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Sex, female vs. male | 69.29 (65.24, 73.33) | <0.0001 | 55.31 (48.32, 62.30) | <0.0001 |
| Age, years/10 | 0.58 (0.39, 0.76) | <0.0001 | −0.04 (−0.38, 0.31) | 0.828 |
| Caucasian, yes vs. no | 7.61 (2.36, 12.86) | 0.0004 | 5.73 (0.01, 11.44) | 0.049 |
| BMI, kg/m2 | 5.17 (4.67, 5.68) | <0.0001 | 2.44 (1.76, 3.13) | <0.0001 |
| High education, yes vs. no | 0.20 (−0.87, 1.28) | 0.7107 | 0.40 (−0.67, 1.48) | 0.463 |
| SBP, mm Hg | 0.81 (0.70, 0.93) | <0.0001 | −0.09 (−0.31, 0.12) | 0.893 |
| DBP, mm Hg | 1.92 (1.69, 2.16) | <0.0001 | 0.15 (−0.27, 0.57) | 0.392 |
| Parental history of CKD, yes vs. no | 9.50 (−20.38, 39.38) | 0.533 | 8.44 (−24.63, 41.52) | 0.616 |
| Parental history of T2D, yes vs. no | 10.22 (3.94, 16.51) | 0.0001 | 7.91 (1.03, 14.80) | 0.024 |
| Current smoking, yes vs. no | −7.94 (−12.85, −3.03) | 0.0001 | −4.76 (−10.52, 1.01) | 0.105 |
| Alcohol consumption, yes vs. no | 11.51 (6.36, 16.66) | <0.0001 | 9.01 (3.06, 14.96) | 0.003 |
| Antihypertensive drugs, yes vs. no | 24.35 (18.55, 30.14) | <0.0001 | 3.78 (−3.19, 10.75) | 0.287 |
| Lipid-lowering drugs, yes vs. no | 24.87 (16.22, 33.52) | <0.0001 | 8.16 (−1.48, 17.80) | 0.097 |
| TC, mmol/L | 7.04 (4.92, 9.15) | <0.0001 | 4.38 (1.58, 7.19) | 0.002 |
| HDL-C, mmol/L | −60.34(−67.05, −53.63) | <0.0001 | −20.54 (−30.64,−10.44) | <0.0001 |
| TG, mmol/L | 25.51 (23.23, 27.78) | <0.0001 | 3.61 (0.61, 6.62) | 0.018 |
| Serum creatinine, µmol/L | 71.16 (61.64, 80.68) | <0.0001 | 12.23 (−6.78, 31.24) | 0.207 |
| eGFR, mL/min/1.73m2 | −0.44 (−0.57, −0.31) | <0.0001 | −0.01 (−0.29, 0.27) | 0.956 |
| UAE, mg/24h | 0.01 (−0.00, 0.03) | 0.095 | −0.02 (−0.04, −0.01) | 0.005 |
| HOMA-IR, (mU mmol/L2)/22.5 | 28.92 (27.16, 30.67) | <0.0001 | 22.21 (17.82, 26.59) | <0.0001 |
| HOMA-β, % | 20.46 (18.67, 22.25) | <0.0001 | −0.89 (−5.01, 3.24) | 0.673 |
| Q1 | Q2 | p-Value | Q3 | p-Value | Q4 | p-Value | BCAA Per 1 SD Increment | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Participants, n | 1561 | 1561 | 1561 | 1561 | 6244 | ||||
| Events, n | 27 | 44 | 72 | 158 | 301 | ||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| Crude Model | (ref) | 1.65 (1.01, 2.66) | 0.042 | 2.67 (1.71, 4.14) | <0.0001 | 6.15 (4.08, 9.24) | <0.0001 | 1.80 (1.64, 1.98) | <0.0001 |
| Model 1 | (ref) | 1.58 (0.97, 2.56) | 0.064 | 2.56 (1.62, 4.05) | <0.0001 | 6.12 (3.92, 9.55) | <0.0001 | 1.76 (1.59, 1.96) | <0.0001 |
| Model 2 | (ref) | 1.41 (0.85, 2.32) | 0.178 | 1.87 (1.17, 3.00) | 0.009 | 3.49 (2.19, 5.55) | <0.0001 | 1.46 (1.29, 1.65) | <0.0001 |
| Model 3 | (ref) | 1.41 (0.85, 2.33) | 0.175 | 1.87 (1.17, 3.01) | 0.008 | 3.56 (2.24, 5.65) | <0.0001 | 1.48 (1.31, 1.68) | <0.0001 |
| Model 4 | (ref) | 1.45 (0.88, 2.41) | 0.142 | 1.84 (1.15, 2.94) | 0.010 | 3.14 (1.99, 4.97) | <0.0001 | 1.39 (1.23, 1.57) | <0.0001 |
| Model 5a | (ref) | 1.50 (0.89, 2.53) | 0.124 | 1.91 (1.17, 3.10) | 0.009 | 2.80 (1.72, 4.53) | <0.0001 | 1.28 (1.13, 1.46) | 0.0001 |
| Model 5b | (ref) | 1.59 (0.94, 2.68) | 0.079 | 2.12 (1.30, 3.44) | 0.002 | 3.64 (2.26, 5.87) | <0.0001 | 1.41 (1.25, 1.60) | <0.0001 |
| Model 5c | (ref) | 1.46 (0.87, 2.46) | 0.149 | 1.70 (1.04, 2.77) | 0.033 | 2.32 (1.42, 3.78) | 0.0007 | 1.19 (1.04, 1.35) | 0.008 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Guerrero, J.L.; Osté, M.C.J.; Kieneker, L.M.; Gruppen, E.G.; Wolak-Dinsmore, J.; Otvos, J.D.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Plasma Branched-Chain Amino Acids and Risk of Incident Type 2 Diabetes: Results from the PREVEND Prospective Cohort Study. J. Clin. Med. 2018, 7, 513. https://doi.org/10.3390/jcm7120513
Flores-Guerrero JL, Osté MCJ, Kieneker LM, Gruppen EG, Wolak-Dinsmore J, Otvos JD, Connelly MA, Bakker SJL, Dullaart RPF. Plasma Branched-Chain Amino Acids and Risk of Incident Type 2 Diabetes: Results from the PREVEND Prospective Cohort Study. Journal of Clinical Medicine. 2018; 7(12):513. https://doi.org/10.3390/jcm7120513
Chicago/Turabian StyleFlores-Guerrero, Jose L., Maryse C. J. Osté, Lyanne M. Kieneker, Eke G. Gruppen, Justyna Wolak-Dinsmore, James D. Otvos, Margery A. Connelly, Stephan J. L. Bakker, and Robin P. F. Dullaart. 2018. "Plasma Branched-Chain Amino Acids and Risk of Incident Type 2 Diabetes: Results from the PREVEND Prospective Cohort Study" Journal of Clinical Medicine 7, no. 12: 513. https://doi.org/10.3390/jcm7120513
APA StyleFlores-Guerrero, J. L., Osté, M. C. J., Kieneker, L. M., Gruppen, E. G., Wolak-Dinsmore, J., Otvos, J. D., Connelly, M. A., Bakker, S. J. L., & Dullaart, R. P. F. (2018). Plasma Branched-Chain Amino Acids and Risk of Incident Type 2 Diabetes: Results from the PREVEND Prospective Cohort Study. Journal of Clinical Medicine, 7(12), 513. https://doi.org/10.3390/jcm7120513







