Efficacy and Safety of Denosumab Therapy for Osteogenesis Imperfecta Patients with Osteoporosis—Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of BMD
2.2. Analysis of Laboratory Data
2.3. Ethical Approval
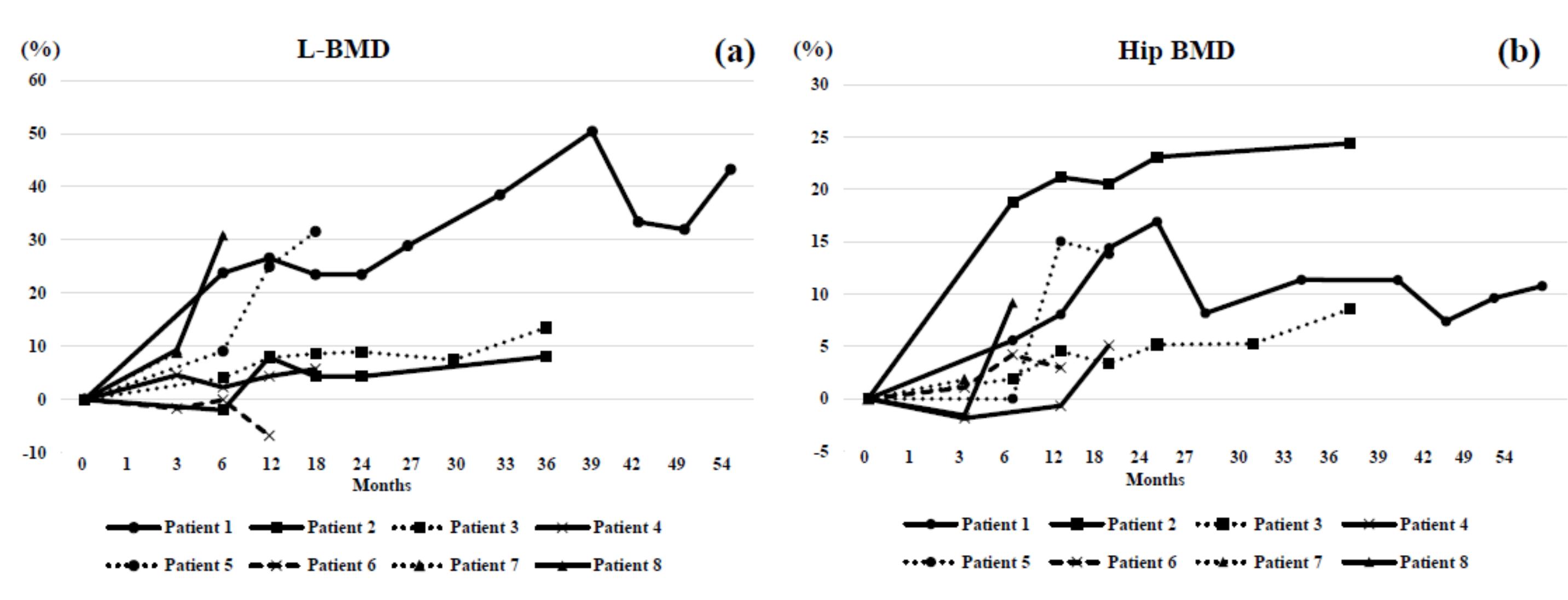
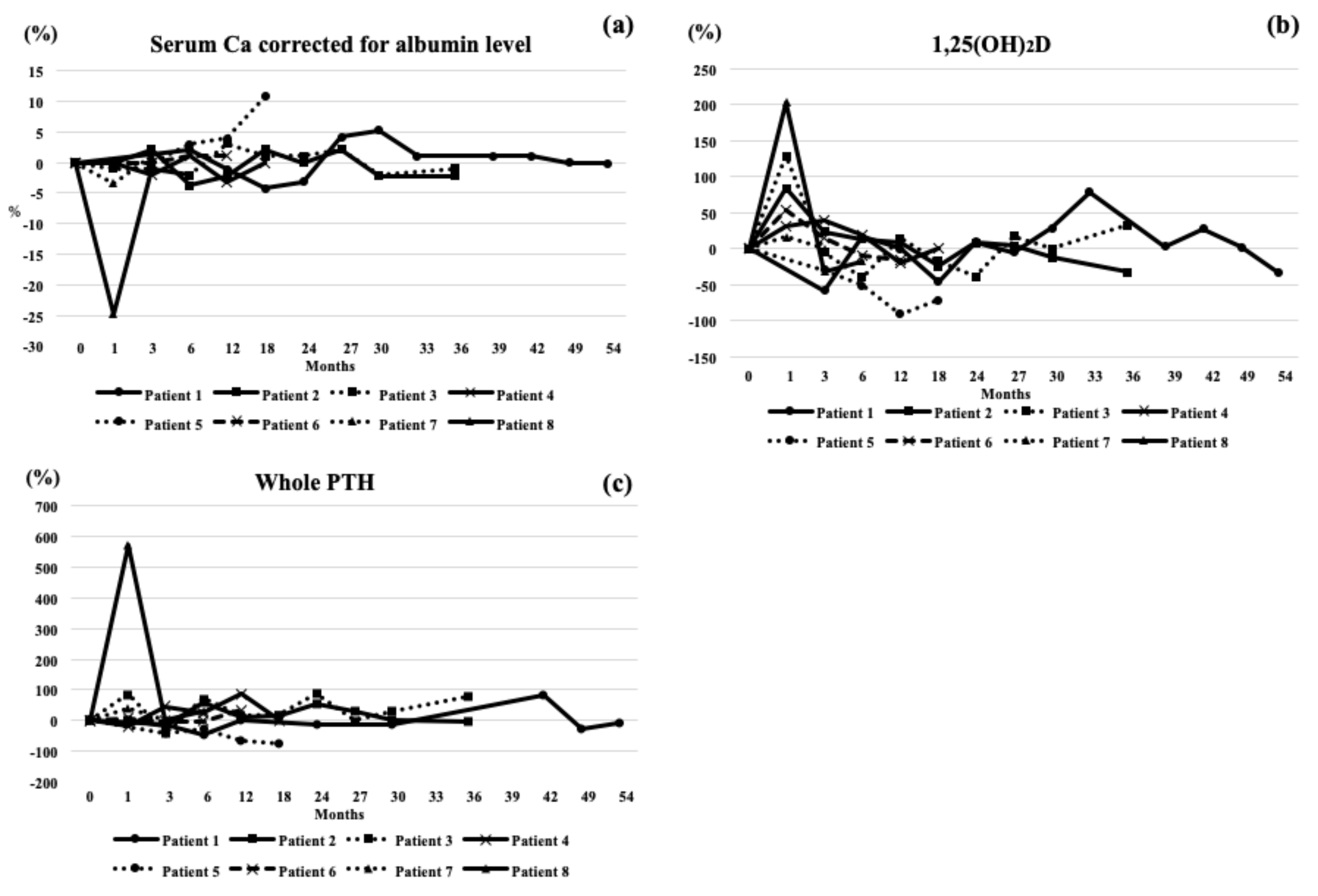
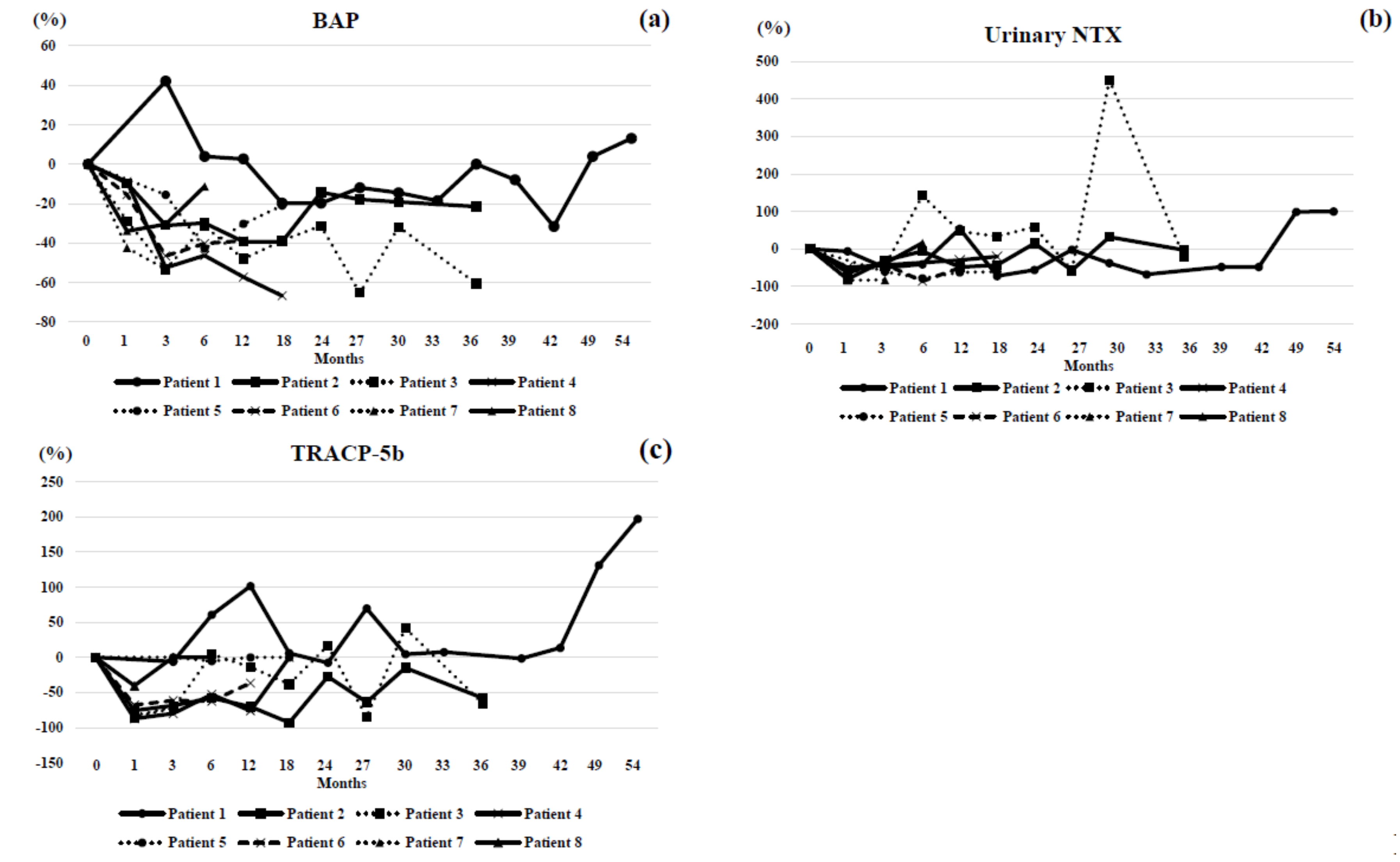
3. Results
4. Case Highlights
5. Discussion
Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rauch, F.; Glorieux, F.H. Osteogenesis imperfecta. Lancet 2004, 363, 1377–1385. [Google Scholar] [CrossRef]
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef]
- Sillence, D.O.; Senn, A.; Danks, D.M. Genetic heterogeneity in osteogenesis imperfecta. J. Med. Genet. 1979, 16, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, F.S.; Sillence, D.O. Osteogenesis imperfecta: Clinical diagnosis, nomenclature and severity assessment. Am. J. Med. Genet. A 2014, 164, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, F.H.; Bishop, N.J.; Plotkin, H.; Chabot, G.; Lanoue, G.; Travers, R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N. Engl. J. Med. 1998, 339, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Semler, O.; Beccard, R.; Palmisano, D.; Demant, A.; Fricke, O.; Schoenau, E.; Koerber, F. Reshaping of Vertebrae during Treatment with Neridronate or Pamidronate in Children with Osteogenesis Imperfecta. Horm. Res. Paediatr. 2011, 76, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Land, C.; Rauch, F.; Travers, R.; Glorieux, F.H. Osteogenesis imperfecta type VI in childhood and adolescence: Effects of cyclical intravenous pamidronate treatment. Bone 2007, 40, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.; Adami, S.; Ahmed, S.F.; Antón, J.; Arundel, P.; Burren, C.P.; Devogelaer, J.P.; Hangartner, T.; Hosszú, E.; Lane, J.M.; et al. Risedronate in children with osteogenesis imperfecta: A randomised, double-blind, placebo-controlled trial. Lancet 2013, 382, 1402–1432. [Google Scholar] [CrossRef]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.L.; Wang, H.; Liu, Y.; San Martin, J. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J. Clin. Endocrinol. Metab. 2008, 93, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Miller, P.D.; McClung, M.R.; Cohen, S.B.; Bolognese, M.A.; Liu, Y.; Wang, A.; Siddhanti, S.; Fitzpatrick, L.A. AMG 162 Bone Loss Study Group. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J. Bone Miner. Res. 2007, 22, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Nakamura, Y.; Takahashi, J.; Kamimura, M.; Ikegami, S.; Suzuki, T.; Uchiyama, S.; Kosho, T.; Kato, H. Efficacy of denosumab for osteoporosis in three female patients with osteogenesis imperfecta. Tohoku J. Med. 2017, 242, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Semler, O.; Netzer, C.; Hoyer-Kuhn, H.; Becker, J.; Eysel, P.; Schoenau, E. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J. Musculoskelet. Neuronal Interact. 2012, 12, 183–188. [Google Scholar] [PubMed]
- Hoyer-Kuhn, H.; Netzer, C.; Koerber, F.; Schoenau, E.; Semler, O. Two years’ experience with denosumab for children with osteogenesis imparcta type VI. Orphanet J. Rare Dis. 2014, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Hoyer-Kuhn, H.; Franklin, J.; Allo, G.; Kron, M.; Netzer, C.; Eysel, P.; Hero, B.; Schoenau, E.; Semler, O. Safety and efficacy of denosumab in children with osteogenesis imperfect—A first prospective trial. J. Musculoskelet. Neuronal Interact. 2016, 16, 24–32. [Google Scholar] [PubMed]
- Orimo, H.; Hayashi, Y.; Fukunaga, M.; Sone, T.; Fujiwara, S.; Shiraki, M.; Hagino, H.; Hosoi, T.; Ohta, H.; Yoneda, T.; et al. Diagnostic criteria of primary osteoporosis: Year 2000 revision. Jpn. J. Bone Metab. 2001, 16, 139–150. [Google Scholar] [CrossRef]
- Major, C.C.; Borggren, C.L.; Devries, R.M. Traumatic hand fracture in a patient with osteogenesis imperfecta. J. Chiropr. Med. 2008, 7, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Nakamura, Y.; Takahashi, J.; Kamimura, M.; Isobe, F.; Yamaguchi, T.; Kosho, T.; Uchiyama, S.; Suzuki, T.; Kato, H. Efficacy of denosumab therapy for neurofibromatosis type 1 with osteoporosis and history of fractures: A case report. Ther. Clin. Risk Manag. 2018, 14, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Nakamura, Y.; Takahashi, J.; Suzuki, T.; Kato, H. Efficacy of denosumab in two cases with multiple-system atrophy and osteoporosis. Ther. Clin. Risk Manag. 2018, 14, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Burrage, L.C.; Jiang, M.M.; Lee, Y.C.; Bertin, T.; Chen, Y.; Tran, A.; Gibbs, R.A.; Jhangiani, S.; Sutton, V.R.; et al. Whole-exome sequencing identifies an intronic cryptic splice site in serpinf1 causing osteogenesis imperfecta type VI. JBMR Plus 2018, 2, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Suzuki, T.; Kamimura, M.; Murakami, K.; Ikegami, S.; Uchiyama, S.; Kato, H. Vitamin D and calcium are required at the time of denosumab administration during osteoporosis treatment. Bone Res. 2017, 5, 17021. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Bolognese, M.A.; Lewiecki, E.M.; McClung, M.R.; Ding, B.; Austin, M.; Liu, Y.; San Martin, J. Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, and restarting of therapy: A randomized blinded phase 2 clinical trial. Bone 2008, 43, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.L.; Miller, P.D.; Yang, Y.C.; Grazette, L.; San Martin, J.; Gallagher, J.C. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J. Clin. Endocrinol. Metab. 2011, 96, 972–980. [Google Scholar] [CrossRef] [PubMed]
| Age, Years at Study Onset | Gender | OI Type | Height, cm at Study Onset | BMI at Study Onset | Previous Treatment | Occurrence of Fracture before Study | Occurrence of Fracture during Study | Causative Mutation | Dose, Frequency, and Duration of Denosumab Administration | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 42 | F | Ib | 138 | 24.1 | None | >10 | None | COL1A1 c.G769A; p.G257R | 60 mg every 6 months for 54 months |
| Patient 2 | 40 | F | I similar | 156 | 21.1 | None | >10 | None | Unidentified | 60 mg every 6 months for 36 months |
| Patient 3 | 14 | F | I similar | 159 | 22.2 | BP | >10 | None | Unidentified | 60 mg every 6 months for 36 months |
| Patient 4 | 22 | F | Ia | 134.5 | 23.6 | BP | >10 | None | COL1A1 c.G1963C; p.G655R | 60 mg every 6 months for 18 months |
| Patient 5 | 3 | F | Ib | 90.2 | 14.8 | None | 1 | 1 | COL1A1 c.G769A; p.G257R | 15 mg every 6 months for 18 months |
| Patient 6 | 51 | F | Ia | 155 | 20.8 | None | >10 | None | COL1A1 c.1243C>T; p.R415X | 60 mg every 6 months for 12 months |
| Patient 7 | 37 | M | Ia | 166 | 25.4 | BP | >10 | None | COL1A1 c.G607T; p.G203C | 60 mg every 6 months for 4 months |
| Patient 8 | 9 | F | Ia | 111.7 | 15.2 | None | None | None | COL1A1 c.607G>T; p.G203C | 30 mg every 6 months for 6 months |
| Serum Albumin-Corrected Ca Level (mg/dL) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before (Reference Value: 8.5–10.2) | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | 9.5 | 9.7 | 9.4 | 9.1 | 9.2 | 9.9 | 10 | 9.6 | 9.6 | 9.6 | 9.6 | 9.5 | 9.4 | ||
| Patient 2 | 9.2 | 9.2 | 9.4 | 8.9 | 9.0 | 9.4 | 9.2 | 9.4 | 9.0 | 9.2 | |||||
| Patient 3 | 9.6 | 9.5 | 9.5 | 9.4 | 9.9 | 9.7 | 9.7 | 9.8 | 9.4 | 9.5 | |||||
| Patient 4 | 9.6 | 9.6 | 9.4 | 9.7 | 9.3 | 9.4 | |||||||||
| Patient 5 | 10.2 | 10.2 | 10.5 | 10.6 | 11.4 | ||||||||||
| Patient 6 | 9.6 | 9.6 | 9.6 | 9.7 | 9.7 | ||||||||||
| Patient 7 | 9.0 | 8.7 | 9.1 | ||||||||||||
| Patient 8 | 9.7 | 7.3 | 9.6 | 9.5 | |||||||||||
| Serum P (mg/dL) | |||||||||||||||
| Before (Reference value: 2.4–4.3) | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | 3.2 | 4.2 | 3.8 | 3.8 | 3.8 | 3.8 | 3.3 | 4.1 | 4.0 | 3.3 | 3.0 | 3.1 | 3.4 | ||
| Patient 2 | 3.9 | 3.2 | 3.0 | 3.5 | 2.7 | 3.0 | 3.2 | 4.1 | 3.0 | 3.7 | |||||
| Patient 3 | 3.8 | 3.1 | 3.8 | 3.5 | 3.8 | 3.9 | 3.5 | 4.2 | 4.6 | 3.7 | |||||
| Patient 4 | 4.1 | 3.4 | 4.2 | 3.7 | 3.0 | 3.7 | |||||||||
| Patient 5 | 5.3 | 5.1 | 5.6 | 6.0 | 5.8 | ||||||||||
| Patient 6 | 4.0 | 3.7 | 4.3 | 3.7 | 3.8 | ||||||||||
| Patient 7 | 2.8 | 2.6 | 2.3 | ||||||||||||
| Patient 8 | 6.3 | 5.9 | 5.0 | 5.1 | |||||||||||
| Whole PTH (pg/dL) | |||||||||||||||
| Before (Reference Value: 8.3–38.7) | 1M | 2-4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | 40.6 | 35.5 | 21 | 40.6 | 35.3 | 35.4 | 73.5 | 29.3 | 37.6 | ||||||
| Patient 2 | 17.1 | 13.9 | 27.4 | 19 | 19.6 | 26.1 | 22.3 | 17.3 | 16.3 | ||||||
| Patient 3 | 11.8 | 21.9 | 7.1 | 20.2 | 13.7 | 14 | 22.2 | 11.7 | 15.3 | 21.2 | |||||
| Patient 4 | 14.8 | 12.2 | 21.5 | 18.5 | 27.5 | 21.7 | |||||||||
| Patient 5 | 17.3 | 10.4 | 12.8 | 6.3 | 4 | ||||||||||
| Patient 6 | 22.8 | 24.5 | 22.2 | 22.5 | 30.3 | ||||||||||
| Patient 7 | 47.7 | 67.3 | 43.1 | ||||||||||||
| Patient 8 | 16.9 | 114 | 19.2 | 22.1 | |||||||||||
| L-BMD (g/cm2) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | 0.571 | 0.707 | 0.723 | 0.705 | 0.705 | 0.736 | 0.791 | 0.859 | 0.762 | 0.754 | 0.818 | ||||
| Patient 2 | 1.018 | 1.002 | 1.099 | 1.062 | 1.082 | 1.100 | |||||||||
| Patient 3 | 1.070 | 1.113 | 1.154 | 1.162 | 1.166 | 1.15 | 1.215 | ||||||||
| Patient 4 | 0.870 | 0.910 | 0.886 | 0.908 | 0.924 | ||||||||||
| Patient 5 | 0.396 | 0.432 | 0.495 | 0.521 | |||||||||||
| Patient 6 | 0.743 | 0.731 | 0.737 | 0.693 | |||||||||||
| Patient 7 | 1.224 | 1.332 | |||||||||||||
| Patient 8 | 0.428 | 0.468 | 0.559 | ||||||||||||
| L-BMD Z-score | |||||||||||||||
| Before | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | −3.2 | −3 | −3.1 | −2.9 | −2.4 | −2.8 | −2.8 | −2.9 | −2.3 | −2.7 | |||||
| Patient 2 | −0.8 | −1 | −0.6 | −0.4 | −0.6 | −0.4 | −0.6 | ||||||||
| Patient 3 | |||||||||||||||
| Patient 4 | −1.6 | −1.7 | −1.9 | −1.8 | −1.6 | ||||||||||
| Patient 5 | |||||||||||||||
| Patient 6 | −2.2 | −2.2 | −2.1 | −2.4 | |||||||||||
| Patient 7 | 0.7 | 1.4 | |||||||||||||
| Patient 8 | −3.1 | −2.6 | −1.9 | ||||||||||||
| L-BMD T-score | |||||||||||||||
| Before | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | −3.4 | −3.2 | −3.4 | −3.1 | −27 | −2.8 | −2.8 | −2.9 | −2.4 | −2.8 | |||||
| Patient 2 | −0.8 | −0.9 | −0.4 | −0.2 | −0.6 | −0.4 | −0.6 | ||||||||
| Patient 3 | |||||||||||||||
| Patient 4 | −2.2 | −1.7 | −1.9 | −1.8 | −1.6 | ||||||||||
| Patient 5 | |||||||||||||||
| Patient 6 | −2.9 | −3 | −3 | −3.3 | |||||||||||
| Patient 7 | 0.7 | 1.4 | |||||||||||||
| Patient 8 | |||||||||||||||
| H-BMD (g/cm2) | |||||||||||||||
| Before | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | 0.520 | 0.549 | 0.562 | 0.595 | 0.608 | 0.562 | 0.591 | 0.579 | 0.558 | 0.57 | 0.576 | ||||
| Patient 2 | 0.729 | 0.867 | 0.884 | 0.879 | 0.898 | 0.908 | |||||||||
| Patient 3 | 0.946 | 0.964 | 0.988 | 0.977 | 0.993 | 0.996 | 1.027 | ||||||||
| Patient 4 | 0.677 | 0.664 | 0.672 | 0.711 | |||||||||||
| Patient 5 | 0.409 | 0.370 | 0.470 | 0.465 | |||||||||||
| Patient 6 | 0.654 | 0.661 | 0.682 | 0.674 | |||||||||||
| Patient 7 | 0.979 | 0.997 | |||||||||||||
| Patient 8 | 0.54 | 0.531 | 0.589 | ||||||||||||
| H-BMD Z-score | |||||||||||||||
| Before | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | −2.95 | −2.85 | −2.85 | −2.85 | −2.6 | −2.45 | −2.75 | −2.7 | −2.65 | −2.55 | |||||
| Patient 2 | −0.55 | −0.45 | −0.4 | −0.25 | −0.2 | −0.15 | −0.05 | ||||||||
| Patient 3 | |||||||||||||||
| Patient 4 | −2 | −2.25 | −2.25 | −1.9 | −2.25 | ||||||||||
| Patient 5 | |||||||||||||||
| Patient 6 | −1.8 | -1.75 | -1.6 | -1.6 | |||||||||||
| Patient 7 | -0.25 | -0.15 | |||||||||||||
| Patient 8 | |||||||||||||||
| H-BMD T-score | |||||||||||||||
| Before | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | -3.2 | -3.1 | -3.1 | -3.1 | -2.85 | -3.15 | -3.05 | -3 | -2.95 | -2.9 | |||||
| Patient 2 | −0.65 | −0.55 | −0.45 | −0.25 | −0.5 | −0.4 | −0.35 | ||||||||
| Patient 3 | |||||||||||||||
| Patient 4 | −2.15 | −2.25 | −2.25 | −1.9 | −2.25 | ||||||||||
| Patient 5 | |||||||||||||||
| Patient 6 | −2.35 | −2.3 | −2.15 | −2.2 | |||||||||||
| Patient 7 | −0.45 | −0.4 | |||||||||||||
| Patient 8 | |||||||||||||||
| BAP (μg/L) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before (Reference Value) | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | 7.6 (2.9–14.5) | 10.8 | 7.9 | 7.8 | 6.1 | 6 | 6.7 | 6.5 | 6.2 | 7.6 | 7 | 5.2 | 7.9 | 7.9 | |
| Patient 2 | 8.4 (2.9–14.5) | 7.6 | 5.8 | 5.9 | 5.1 | 5.1 | 7.2 | 6.9 | 6.8 | 6.6 | |||||
| Patient 3 | 16.7 (2.9–14.5) | 11.9 | 7.8 | 11.5 | 8.7 | 6.9 | 11.5 | 5.8 | 11.4 | 6.6 | |||||
| Patient 4 | 14.7 (2.9–14.5) | 13.4 | 7 | 7.9 | 6.3 | 4.9 | |||||||||
| Patient 5 | 62.2 (2.9–14.5) | 52.6 | 35.6 | 43.4 | 60.9 | ||||||||||
| Patient 6 | 14.2 (2.9–14.5) | 12 | 7.6 | 8.5 | 8.7 | ||||||||||
| Patient 7 | 20.3 (3.7–20.9) | 11.7 | 9.5 | ||||||||||||
| Patient 8 | 84.4 (2.9–14.5) | 55.8 | 58.8 | 74.9 | |||||||||||
| Urinary NTX (nmol BCE/mmol Cr) | |||||||||||||||
| Before (Reference value) | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | 19.3 (9.3–54.3) | 18.1 | 9.7 | 9.9 | 29.7 | 5.4 | 8.5 | 18.9 | 12 | 6.2 | 10.1 | 10.1 | 38.4 | 38.6 | |
| Patient 2 | 24.2 (9.3–54.3) | 4.7 | 16.8 | 22.8 | 12.6 | 13.9 | 27.9 | 10.4 | 31.9 | 23.5 | |||||
| Patient 3 | 32.9 (9.3–54.3) | 12.9 | 18.1 | 79.7 | 48.4 | 44 | 52.3 | 14.4 | 181.2 | 25.8 | |||||
| Patient 4 | 30.4 (9.3–54.3) | 15.4 | 21.3 | 24.3 | |||||||||||
| Patient 5 | 1562.1 (9.3–54.3) | 616.9 | 338 | 587.3 | 612.6 | ||||||||||
| Patient 6 | 35.4 (9.3–54.3) | 13.2 | 21 | 5 | 17.5 | ||||||||||
| Patient 7 | 79.8 (13.0–66.2) | 13.1 | 14 | ||||||||||||
| Patient 8 | 518.2 (9.3–54.3) | 207.9 | 320.3 | 601.8 | |||||||||||
| TRACP-5b (mU/dL) | |||||||||||||||
| Before (reference value) | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | 66 (120–420) | 62 | 106 | 133 | 70 | 61 | 112 | 69 | 71 | 65 | 75 | 153 | 196 | ||
| Patient 2 | 198 (120–420) | 48 | 62 | 83 | 59 | 72 | 144 | 72 | 149 | 83 | |||||
| Patient 3 | 414 (120–420) | 60 | 114 | 432 | 356 | 256 | 484 | 64 | 586 | 142 | |||||
| Patient 4 | 260 (120–420) | 33 | 52 | 123 | 62 | 38 | |||||||||
| Patient 5 | 1500 (120–420) | 1500 | 1420 | 1500 | 1500 | ||||||||||
| Patient 6 | 319 (120–420) | 103 | 123 | 120 | 203 | ||||||||||
| Patient 7 | 465 (170–590) | 83 | 136 | ||||||||||||
| Patient 8 | 1500 (120–420) | 894 | 1500 | 1500 | |||||||||||
| 1,25 (OH)2D (pg/mL) | |||||||||||||||
| Before (Reference Value) | 1M | 2–4M | 6M | 12M | 18M | 24M | 27M | 30M | 33M | 36M | 39M | 42M | 49M | 54M | |
| Patient 1 | 47.9 (20.0–60.0) | 20.3 | 55.7 | 47.9 | 26.1 | 52.4 | 45.5 | 61.4 | 85.8 | 49.1 | 61.4 | 48.9 | 31.8 | ||
| Patient 2 | 49 (20.0–60.0) | 90.1 | 60.3 | 55.5 | 53.7 | 36.8 | 52.8 | 50.9 | 43.3 | 33.1 | |||||
| Patient 3 | 57.2 (20.0–70.0) | 131 | 54.1 | 34.7 | 65.6 | 47.3 | 35.2 | 67 | 47 | 75.9 | |||||
| Patient 4 | 45.9 (20.0–60.0) | 60.6 | 64.1 | 54.3 | 36.8 | 68.3 | |||||||||
| Patient 5 | 58.9 (20.0–70.0) | 41.3 | 29.2 | 5.6 | 10.7 | ||||||||||
| Patient 6 | 30.8 (20.0–60.0) | 47.1 | 35.5 | 27.7 | 25.7 | ||||||||||
| Patient 7 | 69.8 (20.0–60.0) | 81.6 | 83.8 | ||||||||||||
| Patient 8 | 90.7 (20.0–70.0) | 275 | 61.7 | 75.2 | |||||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Nakamura, Y.; Suzuki, T.; Yamaguchi, T.; Takeda, R.; Takagi, M.; Hasegawa, T.; Kosho, T.; Kato, H. Efficacy and Safety of Denosumab Therapy for Osteogenesis Imperfecta Patients with Osteoporosis—Case Series. J. Clin. Med. 2018, 7, 479. https://doi.org/10.3390/jcm7120479
Kobayashi T, Nakamura Y, Suzuki T, Yamaguchi T, Takeda R, Takagi M, Hasegawa T, Kosho T, Kato H. Efficacy and Safety of Denosumab Therapy for Osteogenesis Imperfecta Patients with Osteoporosis—Case Series. Journal of Clinical Medicine. 2018; 7(12):479. https://doi.org/10.3390/jcm7120479
Chicago/Turabian StyleKobayashi, Tsukasa, Yukio Nakamura, Takako Suzuki, Tomomi Yamaguchi, Ryojun Takeda, Masaki Takagi, Tomonobu Hasegawa, Tomoki Kosho, and Hiroyuki Kato. 2018. "Efficacy and Safety of Denosumab Therapy for Osteogenesis Imperfecta Patients with Osteoporosis—Case Series" Journal of Clinical Medicine 7, no. 12: 479. https://doi.org/10.3390/jcm7120479
APA StyleKobayashi, T., Nakamura, Y., Suzuki, T., Yamaguchi, T., Takeda, R., Takagi, M., Hasegawa, T., Kosho, T., & Kato, H. (2018). Efficacy and Safety of Denosumab Therapy for Osteogenesis Imperfecta Patients with Osteoporosis—Case Series. Journal of Clinical Medicine, 7(12), 479. https://doi.org/10.3390/jcm7120479






