Upper Normal Serum Creatinine Concentrations as a Predictor for Chronic Kidney Disease: Analysis of 14 Years’ Korean Genome and Epidemiology Study (KoGES)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Anthropometric and Laboratory Data
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. The Decline Rate of eGFR According to sCr Levels
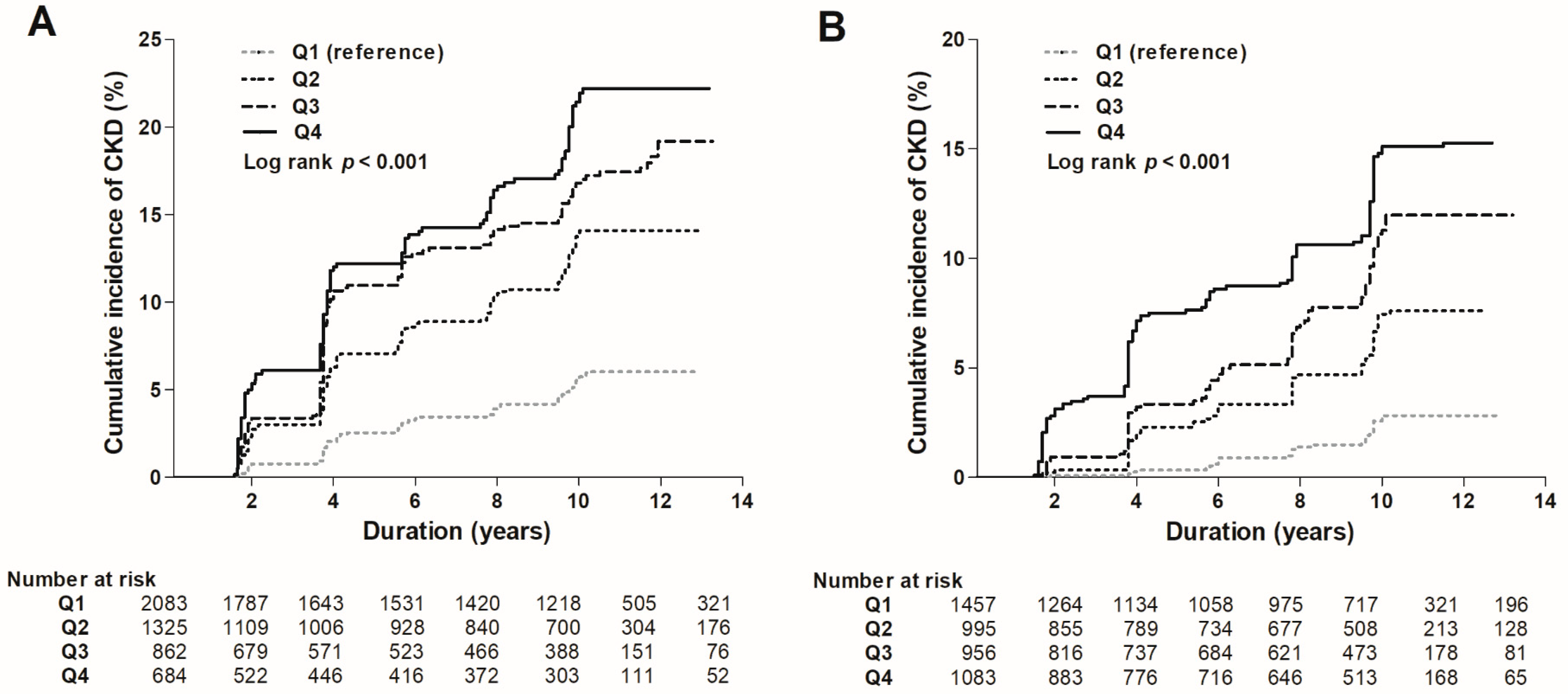
3.3. Risk of the Development of eGFR <60 mL/min/1.73 m2
3.4. Risk of Proteinuria Development
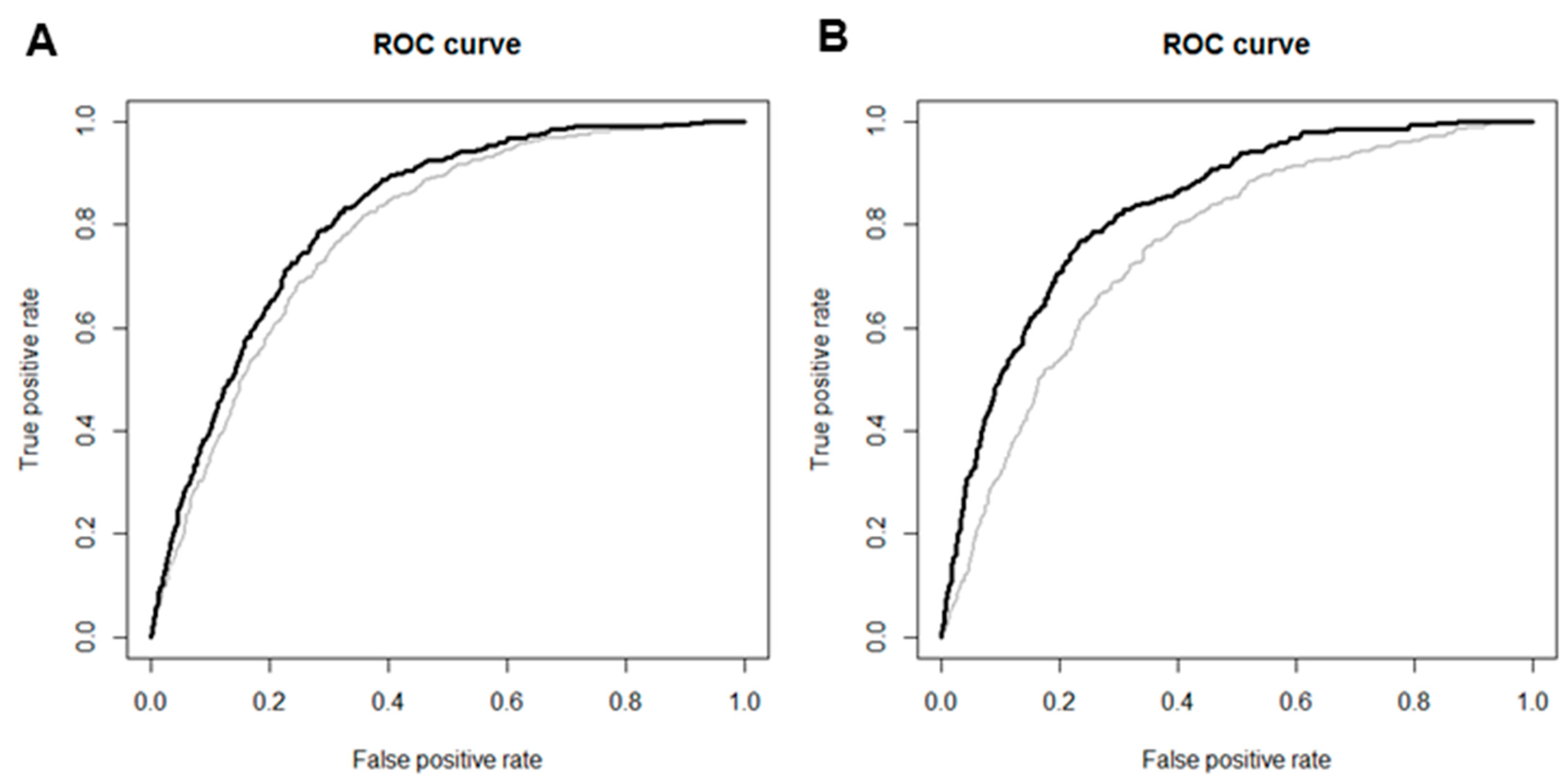
3.5. Predictive Value of sCr Levels
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet (Lond. Engl.) 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Jha, V.; Wang, A.Y.; Wang, H. The impact of ckd identification in large countries: The burden of illness. Nephrol. Dial. Transplant. 2012, 27 (Suppl. 3), 32–38. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, Y.C.; Oh, S.W.; Koo, H.S.; Na, K.Y.; Chae, D.W.; Kim, S.; Chin, H.J. Trends in the prevalence of chronic kidney disease, other chronic diseases and health-related behaviors in an adult korean population: Data from the korean national health and nutrition examination survey (knhanes). Nephrol. Dial. Transplant. 2011, 26, 3975–3980. [Google Scholar] [CrossRef] [PubMed]
- Seul Hyun, O.; Ha Yeon, K.; Chan Young, O.; Min Jee, K.; Chang Seong, K. Prevalence and factors of chronic kidney disease. Kidney Res. Clin. Pract. 2010, 29, 441–449. [Google Scholar]
- National Clinical Guideline Centre. National Clinical Guideline Centre. National institute for health and care excellence: Clinical guidelines. In Chronic Kidney Disease (Partial Update): Early Identification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care; National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Kim, H.S.; Shin, D.W.; Lee, W.C.; Kim, Y.T.; Cho, B. National screening program for transitional ages in korea: A new screening for strengthening primary prevention and follow-up care. J. Korean Med. Sci. 2012, 27, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Practice Guidelines for Assessments and Recommendations of Laboratory and Physical Measurements in Korea National Screening Program. Available online: http://www.cdc.go.kr/CDC/cms/content/mobile/36/12436_view.html (accessed on 1 September 2009).
- An Evaluation of National Screening Program for Chronic Kidney Disease; Korea Centers for Disease Control & Prevention: Seoul, Korea, 2016.
- K/doqi clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266.
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Kdigo 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 134–135.
- Kim, Y.; Han, B.G. Cohort profile: The Korean genome and epidemiology study (koges) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- Junge, W.; Wilke, B.; Halabi, A.; Klein, G. Determination of reference intervals for serum creatinine, creatinine excretion and creatinine clearance with an enzymatic and a modified jaffe method. Clin. Chim. Acta Int. J. Clin. Chem. 2004, 344, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Ceriotti, F.; Boyd, J.C.; Klein, G.; Henny, J.; Queralto, J.; Kairisto, V.; Panteghini, M. Reference intervals for serum creatinine concentrations: Assessment of available data for global application. Clin. Chem. 2008, 54, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Mahmoodi, B.K.; Woodward, M.; Emberson, J.R.; Jafar, T.H.; Jee, S.H.; Polkinghorne, K.R.; Shankar, A.; Smith, D.H.; Tonelli, M.; et al. Comparison of risk prediction using the ckd-epi equation and the mdrd study equation for estimated glomerular filtration rate. JAMA 2012, 307, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Yang, J.H.; Kim, J.; Cho, L.Y.; Park, B.; Ma, S.H.; Song, S.H.; Min, W.K.; Kim, S.S.; Park, M.S.; et al. Reliability of quadruplicated serological parameters in the korean genome and epidemiology study. Epidemiol. Health 2011, 33, e2011004. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function--measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin c. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Shaikh, S.; Chaudhry, M. Associations of endogenous markers of kidney function with outcomes: More and less than glomerular filtration rate. Curr. Opin. Nephrol. Hypertens. 2013, 22, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, N.A.; Appel, L.J.; Kusek, J.W.; Contreras, G.; Bakris, G.; Coresh, J.; Astor, B.C. Comparison of measured gfr, serum creatinine, cystatin c, and beta-trace protein to predict esrd in african americans with hypertensive ckd. Am. J. Kidney Dis. 2011, 58, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Inker, L.A.; Tighiouart, H.; Sorensen, E.; Menon, V.; Beck, G.; Shlipak, M.; Coresh, J.; Levey, A.S.; Sarnak, M.J. Filtration markers may have prognostic value independent of glomerular filtration rate. J. Am. Soc. Nephrol. JASN 2012, 23, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Spanaus, K.S.; Kollerits, B.; Ritz, E.; Hersberger, M.; Kronenberg, F.; von Eckardstein, A. Serum creatinine, cystatin c, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin. Chem. 2010, 56, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.; Rodriguez-Iturbe, B. Stimulation of tubular secretion of creatinine in health and in conditions associated with reduced nephron mass. Evidence for a tubular functional reserve. Nephrol. Dial. Transplant. 1998, 13, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.; Avila, E.; Marin, C.; Rodriguez-Iturbe, B. Impaired creatinine secretion after an intravenous creatinine load is an early characteristic of the nephropathy of sickle cell anaemia. Nephrol. Dial. Transplant. 2002, 17, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, O.; Golbetz, H.; Kriss, J.P.; Myers, B.D. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985, 28, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Rahn, K.H.; Heidenreich, S.; Bruckner, D. How to assess glomerular function and damage in humans. J. Hypertens. 1999, 17, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Bostom, A.G.; Kronenberg, F.; Ritz, E. Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J. Am. Soc. Nephrol. JASN 2002, 13, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Dalton, R.N. Serum creatinine and glomerular filtration rate: Perception and reality. Clin. Chem. 2010, 56, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Reich, H.N. Risk stratification of patients with iga nephropathy. Am. J. Kidney Dis. 2012, 59, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Coylewright, M.; Reckelhoff, J.F.; Ouyang, P. Menopause and hypertension: An age-old debate. Hypertension (Dallas TX 1979) 2008, 51, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Lindheimer, M.D.; Taler, S.J.; Cunningham, F.G. Hypertension in pregnancy. J. Am. Soc. Hypertens. JASH 2008, 2, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Chasan-Taber, L.; Willett, W.C.; Manson, J.E.; Spiegelman, D.; Hunter, D.J.; Curhan, G.; Colditz, G.A.; Stampfer, M.J. Prospective study of oral contraceptives and hypertension among women in the united states. Circulation 1996, 94, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wassertheil-Smoller, S.; Anderson, G.; Psaty, B.M.; Black, H.R.; Manson, J.; Wong, N.; Francis, J.; Grimm, R.; Kotchen, T.; Langer, R.; et al. Hypertension and its treatment in postmenopausal women: Baseline data from the women’s health initiative. Hypertension (Dallas TX 1979) 2000, 36, 780–789. [Google Scholar] [CrossRef]
- Drawz, P.; Rahman, M. Chronic kidney disease. Ann. Intern. Med. 2015, 162, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Stevens, L.A.; Griffith, J.; Tighiouart, H.; Djurdjev, O.; Naimark, D.; Levin, A.; Levey, A.S. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011, 305, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Grams, M.E.; Levey, A.S.; Coresh, J.; Appel, L.J.; Astor, B.C.; Chodick, G.; Collins, A.J.; Djurdjev, O.; Elley, C.R.; et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: A meta-analysis. JAMA 2016, 315, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult us population: Third national health and nutrition examination survey. Am. J. Kidney Dis. 2003, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Rizk, D.V.; Meier, D.; Sandoval, R.M.; Chacana, T.; Reilly, E.S.; Seegmiller, J.C.; DeNoia, E.; Strickland, J.S.; Muldoon, J.; Molitoris, B.A. A novel method for rapid bedside measurement of gfr. J. Am. Soc. Nephrol. JASN 2018, 29, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Greene, T.; Schluchter, M.D.; Cleary, P.A.; Teschan, P.E.; Lorenz, R.A.; Molitch, M.E.; Mitch, W.E.; Siebert, C.; Hall, P.M.; et al. Glomerular filtration rate measurements in clinical trials. Modification of diet in renal disease study group and the diabetes control and complications trial research group. J. Am. Soc. Nephrol. JASN 1993, 4, 1159–1171. [Google Scholar] [PubMed]
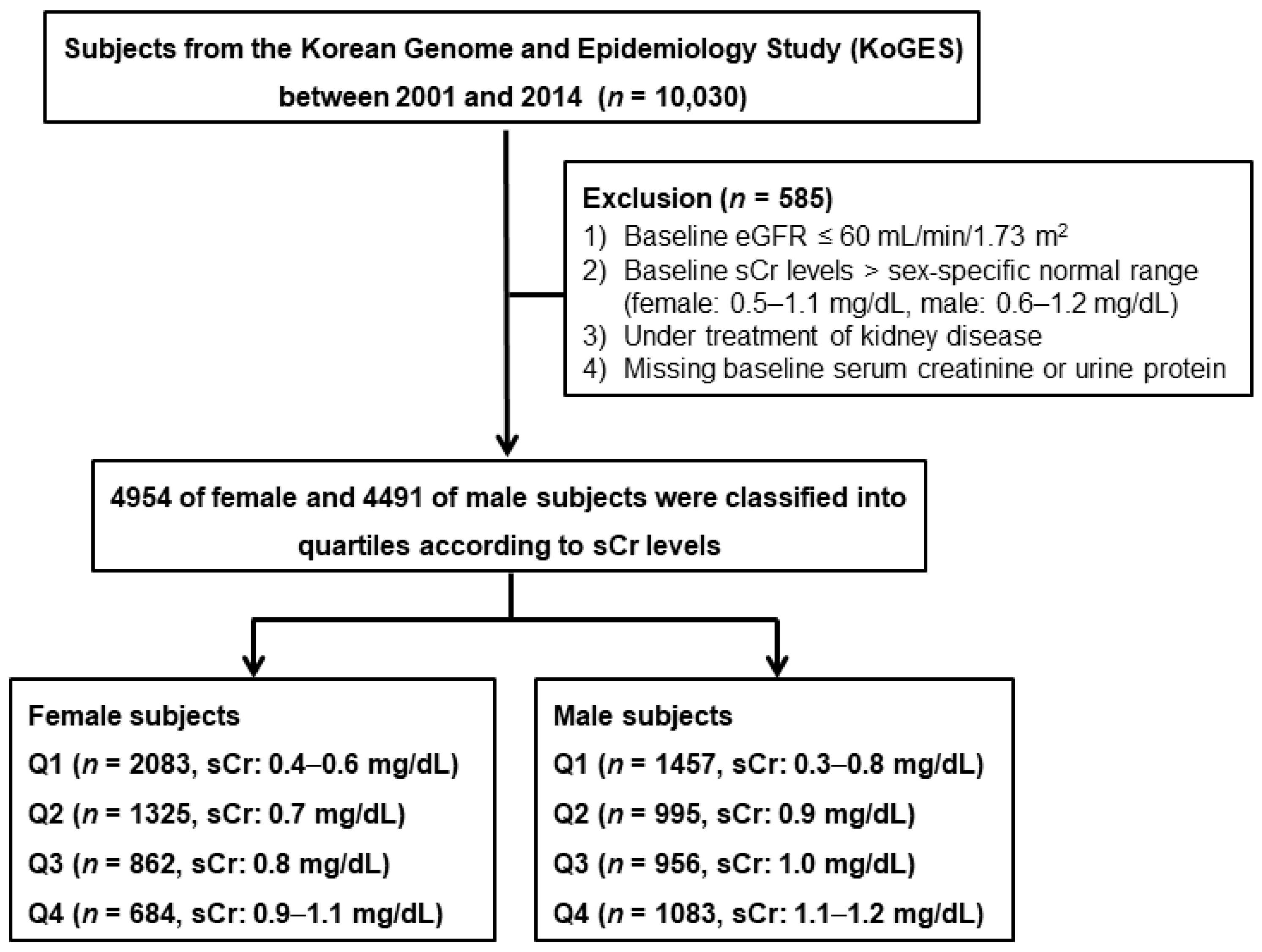

| Total (n = 9445) | Female (n = 4954) | Male (n = 4491) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 2083) | Q2 (n = 1325) | Q3 (n = 862) | Q4 (n = 684) | p | Q1 (n = 1457) | Q2 (n = 995) | Q3 (n = 956) | Q4 (n = 1083) | p | ||
| Demographic data | |||||||||||
| Age, years | 52.1 ± 8.9 | 51.7 ± 8.8 | 52.3 ± 9.0 | 53.4 ± 9.1 | 53.2 ± 8.7 | <0.001 | 53.8 ± 8.8 | 51.7 ± 8.5 | 51.1 ± 8.8 | 49.8 ± 8.5 | <0.001 |
| BMI, kg/m2 | 24.5 ± 3.1 | 24.7 ± 3.3 | 24.9 ± 3.2 | 25.0 ± 3.5 | 25.2 ± 3.3 | 0.025 | 23.5 ± 2.9 | 24.3 ± 2.8 | 24.3 ± 2.9 | 24.7 ± 2.7 | <0.001 |
| Body muscle mass, kg | 43.5 ± 8.0 | 37.3 ± 4.2 | 37.7 ± 4.2 | 37.9 ± 4.4 | 38.4 ± 4.2 | <0.001 | 48.0 ± 6.1 | 50.0 ± 5.7 | 50.5 ± 5.8 | 51.4 ± 5.9 | <0.001 |
| Smoking status, n (%) | 3866 (41.5) | 101 (5.0) | 83 (6.4) | 36 (4.3) | 34 (5.0) | 0.730 | 1186 (82.2) | 780 (78.8) | 778 (81.7) | 868 (80.1) | 0.396 |
| Alcohol status, n (%) | 5061 (54.1) | 570 (27.7) | 394 (30.1) | 258 (30.3) | 189 (27.8) | 0.525 | 1198 (82.9) | 790 (79.7) | 789 (83.0) | 873 (80.8) | 0.396 |
| SBP, mmHg | 121.9 ± 18.6 | 121.8 ± 19.9 | 119.9 ± 19.4 | 121.5 ± 20.5 | 121.1 ± 19.1 | 0.062 | 123.9 ± 17.3 | 122.4 ± 16.9 | 123.3 ± 18.1 | 121.1 ± 16.2 | <0.001 |
| DBP, mmHg | 80.5 ± 11.7 | 79.2 ± 12.3 | 78.4 ± 11.8 | 78.9 ± 12.2 | 79.4 ± 11.1 | 0.178 | 81.8 ± 10.8 | 81.7 ± 10.7 | 82.9 ± 11.5 | 82.8 ± 11.2 | 0.007 |
| Comorbidities, n (%) | |||||||||||
| Hypertension | 1356 (14.4) | 297 (14.3) | 221 (16.7) | 160 (18.6) | 117 (17.1) | 0.008 | 166 (11.4) | 118 (11.9) | 122 (12.8) | 155 (14.3) | 0.026 |
| DM | 611 (6.5) | 124 (6.0) | 60 (4.5) | 49 (5.7) | 44 (6.4) | 0.775 | 132 (9.1) | 68 (6.8) | 66 (6.9) | 68 (6.3) | 0.009 |
| Dyslipidemia | 223 (2.4) | 39 (1.9) | 24 (1.8) | 12 (1.4) | 16 (2.3) | 0.816 | 34 (2.3) | 33 (3.3) | 27 (2.8) | 38 (3.5) | 0.131 |
| CVDs | 148 (1.6) | 25 (1.2) | 21 (1.6) | 12 (1.4) | 10 (1.5) | 0.570 | 22 (1.5) | 14 (1.4) | 17 (1.8) | 27 (2.5) | 0.062 |
| Laboratory data | |||||||||||
| Creatinine, mg/dL | 0.81 ± 0.18 | 0.57 ± 0.04 | 0.70 ± 0.00 | 0.80 ± 0.00 | 0.92 ± 0.04 | <0.001 | 0.75 ± 0.06 | 0.90 ± 0.00 | 1.00 ± 0.00 | 1.13 ± 0.05 | <0.001 |
| BUN, mg/dL | 13.8 ± 3.6 | 11.9 ± 3.2 | 13.3 ± 3.0 | 14.2 ± 3.4 | 14.9 ± 3.9 | <0.001 | 13.4 ± 3.4 | 14.6 ± 3.5 | 14.9 ± 3.8 | 15.5 ± 3.6 | <0.001 |
| eGFR, mL/min/1.73 m2 | 94.3 ± 13.9 | 107.0 ± 7.2 | 99.6 ± 6.2 | 84.1 ± 5.3 | 71.1 ± 5.4 | <0.001 | 104.6 ± 7.5 | 98.2 ± 5.8 | 86.9 ± 5.3 | 75.2 ± 5.8 | <0.001 |
| Proteinuria (%) | 702 (7.4) | 118 (5.7) | 93 (7.0) | 61 (7.1) | 57 (8.3) | 0.012 | 123 (8.4) | 82 (8.2) | 81 (8.5) | 87 (8.0) | 0.771 |
| Hemoglobin, g/dL | 13.6 ± 1.6 | 12.5 ± 1.2 | 12.6 ± 1.1 | 12.6 ± 1.1 | 12.5 ± 1.1 | 0.007 | 14.5 ± 1.1 | 14.7 ± 1.1 | 14.8 ± 1.1 | 14.9 ± 1.1 | <0.001 |
| Albumin, g/dL | 4.5 ± 0.2 | 4.4 ± 0.2 | 4.4 ± 0.2 | 4.4 ± 0.3 | 4.5 ± 0.2 | <0.001 | 4.5 ± 0.3 | 4.6 ± 0.3 | 4.6 ± 0.2 | 4.7 ± 0.3 | <0.001 |
| Total cholesterol, mg/dL | 198.0 ± 36.5 | 192.9 ± 35.2 | 199.6 ± 36.1 | 203.0 ± 37.1 | 208.9 ± 36.7 | <0.001 | 186.3 ± 36.5 | 195.9 ± 33.6 | 203.2 ± 35.5 | 207.9 ± 36.2 | <0.001 |
| LDL-C, mg/dL | 117.9 ± 34.4 | 114.5 ± 31.5 | 119.9 ± 32.1 | 125.2 ± 32.3 | 131.4 ± 34.1 | <0.001 | 103.4 ± 35.2 | 113.4 ± 33.9 | 121.5 ± 35.6 | 128.2 ± 3.43 | <0.001 |
| HDL-C, mg/dL | 49.6 ± 11.9 | 50.9 ± 11.9 | 52.1 ± 12.1 | 51.1 ± 11.5 | 50.1 ± 11.8 | 0.002 | 49.8 ± 13.0 | 48.6 ± 11.6 | 47.3 ± 10.7 | 45.5 ± 9.7 | <0.001 |
| Triglyceride, mg/dL | 152.2 ± 109.9 | 137.5 ± 93.5 | 137.6 ± 90.8 | 133.6 ± 82.6 | 137.3 ± 94.9 | 0.717 | 165.7 ± 128.9 | 169.7 ± 136.9 | 172.0 ± 128.7 | 171.2 ± 104.7 | 0.587 |
| Fasting glucose, mg/dL | 92.3 ± 22.6 | 90.4 ± 21.4 | 89.9 ± 19.8 | 89.1 ± 15.2 | 91.3 ± 23.3 | 0.193 | 95.2 ± 27.1 | 93.3 ± 22.8 | 94.0 ± 19.2 | 95.8 ± 27.1 | 0.099 |
| HbA1c, % | 5.8 ± 0.9 | 5.8 ± 0.9 | 5.7 ± 0.8 | 5.8 ± 0.9 | 5.8 ± 0.9 | 0.279 | 5.9 ± 1.0 | 5.7 ± 0.8 | 5.7 ± 0.9 | 5.8 ± 0.9 | 0.005 |
| CRP (IQR), mg/dL | 0.14 (0.07–0.25) | 0.14 (0.06–0.24) | 0.14 (0.17–0.24) | 0.14 (0.06–0.25) | 0.12 (0.06–0.23) | 0.042 | 0.15 (0.07–0.25) | 0.14 (0.07–0.25) | 0.15 (0.07–0.26) | 0.14 (0.07–0.25) | 0.804 |
| Model 1 | Model 2 | Model 3 | Model 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence of CKD (%) a | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Quartiles of sCr | |||||||||
| Female | |||||||||
| Q1 (n = 2083) | 96 (4.6) | Reference | Reference | Reference | Reference | ||||
| Q2 (n = 1325) | 146 (11.0) | 2.50 (1.93–3.24) | <0.001 | 2.59 (1.90–3.53) | <0.001 | 2.62 (1.91–3.59) | <0.001 | 2.57 (1.83–3.60) | <0.001 |
| Q3 (n = 862) | 133 (15.4) | 3.89 (2.99–5.06) | <0.001 | 3.51 (2.56–4.81) | <0.001 | 3.56 (2.58–4.92) | <0.001 | 3.79 (1.83–5.35) | <0.001 |
| Q4 (n = 684) | 112 (16.4) | 4.20 (3.20–5.51) | <0.001 | 4.21 (3.04–5.84) | <0.001 | 4.19 (3.00–5.85) | <0.001 | 4.71 (3.29–6.74) | <0.001 |
| Male | |||||||||
| Q1 (n = 1457) | 28 (1.9) | Reference | Reference | Reference | Reference | ||||
| Q2 (n = 995) | 56 (5.6) | 2.88 (1.83–4.53) | <0.001 | 3.18 (1.90–5.34) | <0.001 | 3.08 (1.82–5.20) | <0.001 | 3.30 (1.90–5.74) | <0.001 |
| Q3 (n = 956) | 84 (8.8) | 4.64 (3.03–7.12) | <0.001 | 5.79 (3.57–9.39) | <0.001 | 6.04 (3.72–9.81) | <0.001 | 6.75 (4.03–11.30) | <0.001 |
| Q4 (n = 1083) | 124 (11.4) | 6.43 (4.27–9.69) | <0.001 | 10.31 (6.49–16.38) | <0.001 | 11.21 (7.02–17.90) | <0.001 | 12.77 (7.69–21.23) | <0.001 |
| Model 1 | Model 2 | Model 3 | Model 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence of proteinuria (%) a | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Quartiles of sCr | |||||||||
| Female | |||||||||
| Q1 (n = 2083) | 174 (9.1) | Reference | Reference | Reference | Reference | ||||
| Q2 (n = 1325) | 125 (10.5) | 2.50 (1.93–3.24) | <0.001 | 2.59 (1.90–3.53) | <0.001 | 2.59 (1.89–3.55) | <0.001 | 2.60 (1.85–3.64) | <0.001 |
| Q3 (n = 862) | 104 (13.3) | 3.89 (2.99–5.06) | <0.001 | 3.51 (2.56–4.81) | <0.001 | 3.56 (2.58–4.91) | <0.001 | 3.88 (2.76–5.47) | <0.001 |
| Q4 (n = 684) | 119 (19.4) | 4.20 (3.20–5.51) | <0.001 | 4.21 (3.04–5.84) | <0.001 | 4.23 (3.03–5.91) | <0.001 | 4.87 (3.41–6.96) | <0.001 |
| Male | |||||||||
| Q1 (n = 1457) | 150 (11.7) | Reference | Reference | Reference | Reference | ||||
| Q2 (n = 995) | 168 (19.0) | 2.88 (1.83–4.53) | <0.001 | 3.19 (1.90–5.34) | <0.001 | 3.08 (1.82–5.20) | <0.001 | 3.31 (1.90–5.75) | <0.001 |
| Q3 (n = 956) | 225 (26.3) | 4.64 (3.03–7.12) | <0.001 | 5.79 (3.57–9.39) | <0.001 | 6.04 (3.72–9.81) | <0.001 | 6.79 (4.05–11.37) | <0.001 |
| Q4 (n = 1083) | 382 (38.7) | 6.43 (4.27–9.69) | <0.001 | 10.31 (6.49–16.38) | <0.001 | 11.21 (7.02–17.90) | <0.001 | 13.06 (7.86–21.69) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhee, J.H.; Hwang, S.D.; Song, J.H.; Lee, S.W. Upper Normal Serum Creatinine Concentrations as a Predictor for Chronic Kidney Disease: Analysis of 14 Years’ Korean Genome and Epidemiology Study (KoGES). J. Clin. Med. 2018, 7, 463. https://doi.org/10.3390/jcm7110463
Jhee JH, Hwang SD, Song JH, Lee SW. Upper Normal Serum Creatinine Concentrations as a Predictor for Chronic Kidney Disease: Analysis of 14 Years’ Korean Genome and Epidemiology Study (KoGES). Journal of Clinical Medicine. 2018; 7(11):463. https://doi.org/10.3390/jcm7110463
Chicago/Turabian StyleJhee, Jong Hyun, Seun Deuk Hwang, Joon Ho Song, and Seoung Woo Lee. 2018. "Upper Normal Serum Creatinine Concentrations as a Predictor for Chronic Kidney Disease: Analysis of 14 Years’ Korean Genome and Epidemiology Study (KoGES)" Journal of Clinical Medicine 7, no. 11: 463. https://doi.org/10.3390/jcm7110463
APA StyleJhee, J. H., Hwang, S. D., Song, J. H., & Lee, S. W. (2018). Upper Normal Serum Creatinine Concentrations as a Predictor for Chronic Kidney Disease: Analysis of 14 Years’ Korean Genome and Epidemiology Study (KoGES). Journal of Clinical Medicine, 7(11), 463. https://doi.org/10.3390/jcm7110463





