The Comparative Associations of Ultrasound and Computed Tomography Estimates of Muscle Quality with Physical Performance and Metabolic Parameters in Older Men
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Participants
2.3. Outcome Measures
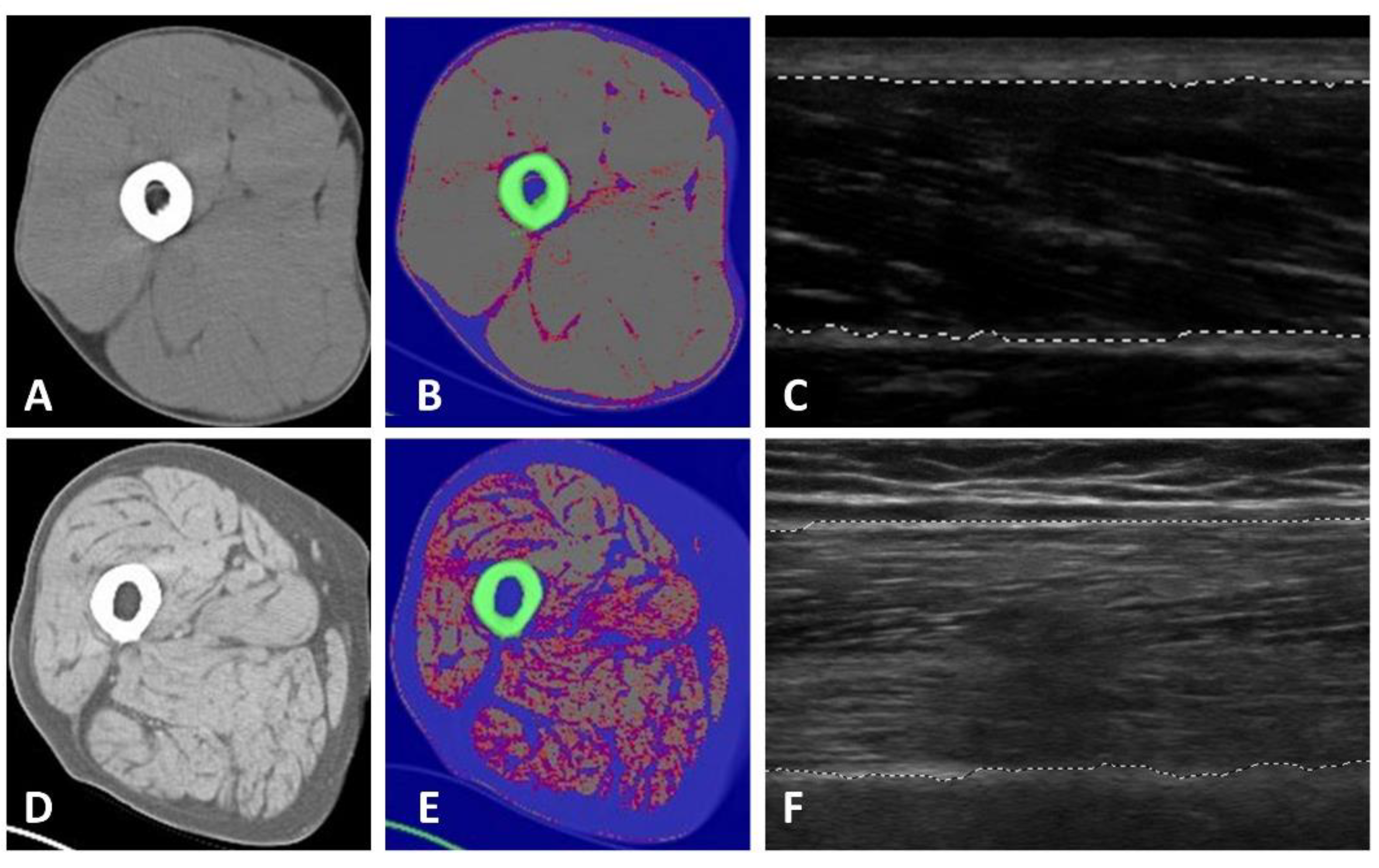
2.3.1. Tissue and Body Composition Analyses
2.3.2. Blood Sampling: Metabolic Parameters
Oral Glucose Tolerance Test (OGTT)
Glucose and Insulin
Lipids
2.3.3. Physical Performance: Strength and Mobility
3. Statistical Analysis
4. Results
4.1. Surrogate Measures of Muscle Quality and Body Composition Estimates
4.2. Surrogate Measures of Muscle Quality, Body Composition, and Metabolic Parameters
4.3. Surrogate Measures of Muscle Quality, Body Composition, and Physical Performance
4.4. Surrogate Measures of Muscle Quality as Discriminators of Physical Performance
5. Discussion
5.1. Surrogate Measures of Muscle Quality Are Associated with Strength and Physical Performance
5.2. The Comparative Use of Ultrasound, Computed Tomography (CT), and Magnetic Resonance Imaging (MRI) for Muscle Tissue Composition Estimates
5.3. Quantitative Ultrasound Muscle Measures Are Linked to Tissue Properties and Health Outcomes
5.4. Limitations and Future Work
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heard, R.S.M.; Ramsay, G.; Hildebrand, D.R. Sarcopaenia in surgical populations: A review. Surgeon 2017, 15, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Alessa, A. Sarcopenia: Prevalence and prognostic significance in hospitalized patients. Clin. Nutr. 2013, 32, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging and Body Composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Gonzalez, M.C.; Lu, J.; Jia, G.; Zheng, J. Skeletal muscle mass and quality: Evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc. 2015, 74, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Teigen, L.M.; Kuchnia, A.J.; Mourtzakis, M.; Earthman, C.P. The use of technology for estimating body composition: Strengths and weaknesses of common modalities in a clinical setting. Nutr. Clin. Pract. 2017, 32, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Sanada, K.; Kearns, C.F.; Midorikawa, T.; Abe, T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur. J. Appl. Physiol. 2006, 96, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kondo, M.; Kawakami, Y.; Fukunaga, T. Prediction equations for body composition of Japanese adults by B-mode ultrasound. Am. J. Hum. Biol. 1994, 6, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Monfaredi, R.; Ismail, C.; Blackman, M.R.; Cleary, K. Quantitative ultrasound: Measurement considerations for the assessment of muscular dystrophy and sarcopenia. Front. Aging Neurosci. 2014, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Pillen, S.; van Alfen, N. Skeletal muscle ultrasound. Neurol. Res. 2011, 33, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Correa-de-Araujo, R.; Harris-Love, M.O.; Miljkovic, I.; Fragala, M.S.; Anthony, B.W.; Manini, T.M. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: A symposium report. Front. Physiol. 2017, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Iftekharuddin, K.M.; Awwal, A.A.S. Field Guide to Image Processing; SPIE Field Guides; SPIE Press: Bellingham, WA, USA, 2012; ISBN 978-0-8194-9021-6. [Google Scholar]
- Sipilä, S.; Suominen, H. Muscle ultrasonography and computed tomography in elderly trained and untrained women. Muscle Nerve 1993, 16, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Pillen, S.; Verrips, A.; van Alfen, N.; Arts, I.M.P.; Sie, L.T.L.; Zwarts, M.J. Quantitative skeletal muscle ultrasound: Diagnostic value in childhood neuromuscular disease. Neuromuscul. Disord. 2007, 17, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-F.; Chen, C.P.-C.; Tsai, W.-C.; Hu, L.-L.; Hsu, C.-C.; Tseng, S.-T.; Shau, Y.-W. Quantification of skeletal muscle fibrosis at different healing stages using sonography: A morphologic and histologic study in an animal model. J. Ultrasound Med. 2012, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Reimers, K.; Reimers, C.D.; Wagner, S.; Paetzke, I.; Pongratz, D.E. Skeletal muscle sonography: A correlative study of echogenicity and morphology. J. Ultrasound Med. 1993, 12, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Akima, H.; Hioki, M.; Yoshiko, A.; Koike, T.; Sakakibara, H.; Takahashi, H.; Oshida, Y. Intramuscular adipose tissue determined by T1-weighted MRI at 3T primarily reflects extramyocellular lipids. Magn. Reson. Imaging 2016, 34, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Tateuchi, H.; Ikezoe, T.; Tsukagoshi, R.; Akiyama, H.; So, K.; Kuroda, Y.; Ichihashi, N. Effects of high-velocity resistance training on muscle function, muscle properties, and physical performance in individuals with hip osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2014, 28, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ismail, C.; Zabal, J.; Hernandez, H.J.; Woletz, P.; Manning, H.; Teixeira, C.; DiPietro, L.; Blackman, M.R.; Harris-Love, M. Diagnostic ultrasound estimates of muscle mass and muscle quality discriminate between women with and without sarcopenia. Front. Physiol. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Ikezoe, T.; Yamada, Y.; Tsukagoshi, R.; Nakamura, M.; Mori, N.; Kimura, M.; Ichihashi, N. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur. J. Appl. Physiol. 2012, 112, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Yamada, Y.; Fukumoto, Y.; Ishihara, T.; Yokoyama, K.; Yoshida, T.; Miyake, M.; Yamagata, E.; Kimura, M. Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin. Interv. Aging 2013, 8, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Hairi, N.N.; Cumming, R.G.; Naganathan, V.; Handelsman, D.J.; Le Couteur, D.G.; Creasey, H.; Waite, L.M.; Seibel, M.J.; Sambrook, P.N. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: The Concord Health and Ageing in Men Project. J. Am. Geriatr. Soc. 2010, 58, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Fox, K.M.; Gandra, S.R.; Delmonico, M.J.; Chiou, C.-F.; Anthony, M.S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S.R.; et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J. Am. Geriatr. Soc. 2009, 57, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Kuipers, A.L.; Cauley, J.A.; Prasad, T.; Lee, C.G.; Ensrud, K.E.; Cawthon, P.M.; Hoffman, A.R.; Dam, T.-T.; Gordon, C.L.; et al. Greater skeletal muscle fat infiltration is associated with higher all-cause and cardiovascular mortality in older men. J. Gerontol. A. Biol. Sci. Med. Sci. 2015, 70, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Fielding, R.; Visser, M.; Loon, L.J.; Rolland, Y.; Orwoll, E.; Reid, K.; Boonen, S.; Dere, W.; Epstein, S.; et al. Tools in the assessment of sarcopenia. Calcif. Tissue Int. 2013, 93, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Seamon, B.A.; Teixeira, C.; Ismail, C. Ultrasound estimates of muscle quality in older adults: Reliability and comparison of Photoshop and ImageJ for the grayscale analysis of muscle echogenicity. PeerJ 2016, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.R.; Eastlack, M.; Hicks, G.E.; Alley, D.E.; Shardell, M.D.; Orwig, D.L.; Goodpaster, B.H.; Chomentowski, P.J.; Hawkes, W.G.; Hochberg, M.C.; et al. Asymmetry in CT scan measures of thigh muscle 2 months after hip fracture: The Baltimore Hip Studies. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Cauley, J.A.; Tylavsky, F.; Bauer, D.; Cummings, S.; Harris, T.B. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: The Health, Aging, and Body Composition Study. J. Bone Miner. Res. 2010, 25, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Alley, D.E.; Shardell, M.D.; Peters, K.W.; McLean, R.R.; Dam, T.-T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Grip strength cutpoints for the identification of clinically relevant weakness. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Verdijk, L.B.; de Groot, L.C.P.G.M.; van Loon, L.J.C. Handgrip strength does not represent an appropriate measure to evaluate changes in muscle strength during an exercise intervention program in frail older people. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, A.Y.; Meskers, C.G.M.; van Heemst, D.; Westendorp, R.G.J.; de Craen, A.J.M.; Maier, A.B. Diagnostic criteria for sarcopenia relate differently to insulin resistance. Age 2013, 35, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Thaete, F.L.; Kelley, D.E. Composition of skeletal muscle evaluated with computed tomography. Ann. N. Y. Acad. Sci. 2000, 904, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Jaric, S. Role of body size in the relation between muscle strength and movement performance. Exerc. Sport Sci. Rev. 2003, 31, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Jaric, S. Muscle strength testing—Use of normalisation for body size. Sports Med. 2002, 32, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, S.; Sallinen, J.; Koster, A.; Rantanen, T.; Sainio, P.; Heliövaara, M.; Koskinen, S. Association between obesity history and hand grip strength in older adults—Exploring the roles of inflammation and insulin resistance as mediating factors. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.M.; Bürger, A.; Rickert, M.; Crispin, A.; Schulz, C.U. Grip strength in healthy Caucasian adults: Reference values. J. Hand Surg. 2008, 33, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, J.; Neyens, J.C.L.; Spreeuwenberg, M.D.; van Rossum, E.; Hewson, D.J.; de Witte, L.P. Measuring grip strength in older adults: Comparing the grip-ball with the Jamar dynamometer. J. Geriatr. Phys. Ther. 2015, 38, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O. Safety and efficacy of submaximal eccentric strength training for a subject with polymyositis. Arthritis Rheum. 2005, 53, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.; Lephart, S.; Karunakara, R. Reliability and precision of isokinetic strength and muscular endurance for the quadriceps and hamstrings. Int. J. Sports Med. 1997, 18, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Knols, R.; Murer, K.; de Bruin, E.D. Reproducibility of an isokinetic strength-testing protocol of the knee and ankle in older adults. Gerontology 2009, 55, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Andrews, A.W.; Thomas, M.W. Walking speed: Reference values and correlates for older adults. J. Orthop. Sports Phys. Ther. 1996, 24, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Catlin, P.A.; Gage, K.; Gurucharri, K.; Robertson, R.; Stephen, K. Establishing the reliability and validity of measurements of walking time using the Emory Functional Ambulation Profile. Phys. Ther. 1999, 79, 1122–1133. [Google Scholar] [PubMed]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2009; ISBN 978-0-13-171640-7. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS; Sage: Los Angeles, CA, 2009; ISBN 978-1-84787-906-6. [Google Scholar]
- Miljkovic-Gacic, I.; Gordon, C.L.; Goodpaster, B.H.; Bunker, C.H.; Patrick, A.L.; Kuller, L.H.; Wheeler, V.W.; Evans, R.W.; Zmuda, J.M. Adipose tissue infiltration in skeletal muscle: Age patterns and association with diabetes among men of African ancestry. Am. J. Clin. Nutr. 2008, 87, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.L.; Cone, J.D. Validity issues in clinical assessment. Psychol. Assess. 1995, 7, 248–260. [Google Scholar] [CrossRef]
- Harris-Love, M.O.; Adams, B.; Ismail, C.; Hernandez, H.J.; McIntosh, V.; Yang, J.; Chacko, L.; Blackman, M.R.; Garra, B.S. Ultrasound proxy measures of muscle quality are associated with strength and functional performance in older men. J. Frailty Aging 2015, 4, 55–56. [Google Scholar] [CrossRef]
- Roseveare, D. World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (WCO-IOF-ESCEO 2018): Poster Abstracts. Osteoporos. Int. 2018, 29, 149–565. [Google Scholar] [CrossRef] [PubMed]
- Yorke, A.M.; Curtis, A.B.; Shoemaker, M.; Vangsnes, E. Grip strength values stratified by age, gender, and chronic disease status in adults aged 50 years and older. J. Geriatr. Phys. Ther. 2015, 38, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Abellan van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Health, Aging, and Body Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Avila, N.A.; Adams, B.; Ismail, C.; Zaidi, S.; Kassner, C.; Liu, F.; Blackman, M.R. Ultrasound echogenicity at the rectus femoris predicts attenuation on computed tomography in the evaluation of thigh tissue composition. J. Frailty Aging 2015, 4, 55. [Google Scholar] [CrossRef]
- Akazawa, N.; Okawa, N.; Tamura, K.; Moriyama, H. Relationships between intramuscular fat, muscle strength and gait independence in older women: A cross-sectional study. Geriatr. Gerontol. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Wilhelm, E.N.; Rech, A.; Minozzo, F.; Radaelli, R.; Pinto, R.S. Echo intensity independently predicts functionality in sedentary older men. Muscle Nerve 2017, 55, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.M.; El-Ansary, D.; Cartwright, M.S.; Sarwal, A.; Berney, S.; Koopman, R.; Annoni, R.; Puthucheary, Z.; Gordon, I.R.; Morris, P.E.; et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J. Crit. Care 2015, 30, 1151.e9–1151.e14. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, N.C.; Fabbri, E.; Ferrucci, L.; Shardell, M.; Simonsick, E.M.; Studenski, S. Muscle quality, strength, and lower extremity physical performance in the Baltimore Longitudinal Study of Aging. J. Frailty Aging 2017, 6, 183–187. [Google Scholar] [CrossRef]
- Rech, A.; Radaelli, R.; Goltz, F.R.; da Rosa, L.H.T.; Schneider, C.D.; Pinto, R.S. Echo intensity is negatively associated with functional capacity in older women. Age 2014, 36, 9708. [Google Scholar] [CrossRef] [PubMed]
- Cady, E.B.; Gardener, J.E. Tissue Characterization of Normal and Dystrophic Muscle Using Broad-Band Backscattered, R.F. Data. In Ultrasound Interactions in Biology and Medicine; Millner, R., Rosenfeld, E., Cobet, U., Eds.; Springer: Boston, MA, USA, 1983; pp. 77–84. ISBN 978-1-4684-8386-4. [Google Scholar]
- Cady, E.B.; Gardener, J.E.; Edwards, R.H.T. Ultrasonic tissue characterisation of skeletal muscle. Eur. J. Clin. Investig. 1983, 13, 469–473. [Google Scholar] [CrossRef]
- Sipilä, S.; Suominen, H. Ultrasound imaging of the quadriceps muscle in elderly athletes and untrained men. Muscle Nerve 1991, 14, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Adams, B.; Hernandez, H.J.; DiPietro, L.; Blackman, M.R. Disparities in the consequences of sarcopenia: Implications for African American Veterans. Front. Physiol. 2014, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Cauley, J.A.; Petit, M.A.; Ensrud, K.E.; Strotmeyer, E.; Sheu, Y.; Gordon, C.L.; Goodpaster, B.H.; Bunker, C.H.; Patrick, A.L.; et al. Osteoporotic Fractures in Men Research Group; Tobago Health Studies Research Group Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. J. Clin. Endocrinol. Metab. 2009, 94, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Borkan, G.A.; Hults, D.E.; Gerzof, S.G.; Robbins, A.H.; Silbert, C.K. Age changes in body composition revealed by computed tomography. J. Gerontol. 1983, 38, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Young, H.-J.; Jenkins, N.T.; Zhao, Q.; Mccully, K.K. Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve 2015, 52, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Files, D.C.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Gregory, H.; Stone, J.; Lyles, M.F.; Dhar, S.; Marsh, A.P.; et al. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C.; Kreis, R. Observation of intramyocellular lipids by 1H-magnetic resonance spectroscopy. Ann. N. Y. Acad. Sci. 2000, 904, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C. Musculoskeletal spectroscopy. J. Magn. Reson. Imaging 2007, 25, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Therkelsen, K.E.; Pedley, A.; Speliotes, E.K.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Dufour, S.; Petersen, K.F.; LeBon, V.; Enoksson, S.; Ma, Y.-Z.; Savoye, M.; Rothman, D.L.; Shulman, G.I.; Caprio, S. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: Relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 2002, 51, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Thaete, F.L.; Simoneau, J.A.; Kelley, D.E. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997, 46, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Zmuda, J.M. Epidemiology of myosteatosis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Strasser, E.M.; Draskovits, T.; Praschak, M.; Quittan, M.; Graf, A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013, 35, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
| Participant Characteristics (n = 30) | |
|---|---|
| Age (years) | 62.5 ± 9.2 |
| Racial/ethnic group | |
| African American | 24 (80.0%) |
| Caucasian | 6 (20.0%) |
| Body mass index | 26.3 ± 3.8 |
| Echogenicity (GSL) a | 31.67 ± 9.41 |
| Body composition b | |
| aLM/ht2 (kg/m2) | 8.57 ± 1.12 |
| Body fat (%) | 27.8 ± 7.4 |
| Muscle tissue composition (mid-thigh CSA) c | |
| Lean mass (%) | 53.1 ± 12.1 |
| Subcutaneous fat (%) | 28.6 ± 8.3 |
| IMAT (%) | 17.1 (14.18, 22.56) |
| Muscle strength d | |
| Grip strength 0°/s (kg) | 39.5 ± 9.2 |
| Grip strength 0°/s (Adj.) | 0.49 ± 0.98 |
| Knee extensors 60°/s (Nm) | 194.8 ± 65.7 |
| Knee extensors 60°/s (Adj.) | 0.58 ± 0.16 |
| Knee extensors 180°/s (Nm) | 126.2 ± 50.7 |
| Knee extensors 180°/s (Adj.) | 0.37 ± 0.12 |
| Mobility | |
| Customary gait speed (m/s) | 1.23 ± 0.34 |
| Fast gait speed (m/s) | 1.62 ± 0.41 |
| Metabolic parameters | |
| OGTT (mg/dL) e | 105.00 (77.75, 123.50) |
| Insulin (mg/dL) | 3.74 (2.72, 6.78) |
| Glucose (uM/mL) | 92.00 (88.00, 99.25) |
| QUICKI | 0.39 (.36, 0.41) |
| TC (mg/dL) | 185.15 ± 37.79 |
| LDL (mg/dL) | 114.50 ± 36.80 |
| HDL (mg/dL) | 54.45 ± 13.48 |
| TG (mg/dL) | 81.92 ± 32.99 |
| Echogenicity a | IMAT b | Subcutaneous Fat b | Total Body Fat c | Lean Body Mass d | ||
|---|---|---|---|---|---|---|
| Echogenicity a | r | 1 | 0.73 | 0.47 | 0.51 | −0.29 |
| p-value | <0.001 * | 0.008 * | 0.004 * | 0.126 | ||
| IMAT b | r | 1 | 0.38 | 0.54 | 0.03 | |
| p-value | 0.038 * | 0.002 * | 0.882 | |||
| Subcutaneous fat b | r | 1 | 0.62 * | 0.11 | ||
| p-value | <0.001 | 0.553 | ||||
| Total body fat c | r | 1 | 0.18 | |||
| p-value | 0.328 | |||||
| Lean body mass d | r | 1 | ||||
| p-value |
| Insulin c | Glucose c | QUICKI c | OGTT c,f | TC | LDL | HDL | TG | ||
|---|---|---|---|---|---|---|---|---|---|
| Echogenicity a | r | −0.20 | −0.33 | 0.31 | 0.43 | −0.29 | −0.13 | −0.52 | −0.03 |
| p-value | 0.438 | 0.116 | 0.161 | 0.018 * | 0.114 | 0.496 | 0.003 * | 0.875 | |
| IMAT b,c | r | −0.44 | −0.14 | 0.13 | 0.38 | −0.14 | 0.06 | −0.61 | 0.22 |
| p-value | 0.825 | 0.514 | 0.57 | 0.038 * | 0.550 | 0.745 | <0.001 * | 0.237 | |
| Subcutaneous fat b | r | 0.15 | −0.24 | −0.17 | 0.12 | 0.05 | 0.14 | −0.27 | 0.02 |
| p-value | 0.438 | 0.255 | 0.44 | 0.519 | 0.799 | 0.465 | 0.155 | 0.937 | |
| Total body fat d | r | 0.06 | −0.21 | −0.17 | 0.40 | 0.06 | 0.21 | -0.059 | 0.35 |
| p-value | 0.751 | 0.327 | 0.44 | 0.029 * | 0.738 | 0.265 | 0.001 * | 0.061 | |
| Lean body mass e | r | 0.17 | 0.46 | −0.28 | −0.13 | 0.30 | 0.33 | −0.11 | 0.17 |
| p-value | 0.401 | 0.023 * | 0.20 | 0.503 | 0.111 | 0.076 | 0.574 | 0.382 |
| Grip | Adj. Grip | 60°/s Knee Ext. | Adj. 60°/s Knee Ext. | 180°/s Knee Ext. | Adj. 180°/s Knee Ext. | ||
|---|---|---|---|---|---|---|---|
| Echogenicity a | r | −0.41 | −0.50 | −0.38 | −0.47 | −0.41 | −0.49 |
| p-value | 0.026 * | 0.005 * | 0.038 * | 0.008 * | 0.025 * | 0.006 * | |
| IMAT b | r | −0.13 | −0.45 | −0.14 | −0.34 | −0.21 | −0.40 |
| p-value | 0.496 | 0.013 * | 0.476 | 0.069 | 0.275 | 0.029 * | |
| Subcutaneous fat b | r | 0.12 | −0.16 | 0.14 | −0.02 | 0.06 | −0.10 |
| p-value | 0.519 | 0.388 | 0.451 | 0.909 | 0.760 | 0.612 | |
| Total body fat c | r | 0.05 | −0.31 | 0.20 | −0.02 | 0.12 | −0.07 |
| p-value | 0.809 | 0.091 | 0.285 | 0.921 | 0.527 | 0.709 | |
| Lean body mass d | r | 0.50 | 0.00 | 0.53 | 0.24 | 0.49 | 0.21 |
| p-value | 0.005 * | 0.992 | 0.002 * | 0.198 | 0.006 * | 0.274 |
| Peak Grip Force | Peak Knee Extensor Torque | ||||||
|---|---|---|---|---|---|---|---|
| 0°/s (kg) | Adj. 0°/s | 60°/s (Nm) | Adj. 60°/s | 180°/s (Nm) | Adj. 180°/s | ||
| IMAT: 1st and 3rd tertiles (df = 19) | |||||||
| <15.7% | Mean ± SD | 40.3 ± 4.8 | 0.54 ± 0.07 | 194.2 ± 53.9 | 0.61 ± 0.14 | 129.7 ± 48.4 | 0.40 ± 0.12 |
| ≥20.0% | 36.6 ± 9.9 | 0.44 ± 0.10 | 166.3 ± 61.4 | 0.49 ± 0.16 | 99.2 ± 40.3 | 0.29 ± 0.11 | |
| MD 95% CI | −3.4–10.6 | 0.02–0.18 | −24.7–80.6 | 0.01–0.26 | −10.5–71.36 | 0.01–0.22 | |
| SE | 3.4 | 0.04 | 25.2 | 0.07 | 19.6 | 0.05 | |
| t | 1.09 | 2.63 | 1.11 | 1.90 | 1.56 | 2.21 | |
| p-value | 0.290 | 0.016 * | 0.281 | 0.076 | 0.136 | 0.040 * | |
| Echo: 1st and 3rd tertiles (df = 18) | |||||||
| <28.77 | Mean ± SD | 45.4 ± 7.0 | 0.55 ± 0.08 | 224.7 ± 56.1 | 0.64 ± 0.07 | 153.3 ± 53.0 | 0.43 ± 0.11 |
| ≥34.54 | 36.4 ± 8.2 | 0.46 ± 0.09 | 153.3 ± 59.0 | 0.47 ± 0.18 | 98.7 ± 34.0 | 0.31 ± 0.11 | |
| MD 95% CI | 1.9–16.2 | 0.01–0.17 | 17.3–125.4 | 0.04–0.32 | 7.2–92.0 | 0.02–0.22 | |
| SE | 3.4 | 0.04 | 25.7 | 0.06 | 20.2 | 0.05 | |
| t | 2.64 | 2.43 | 2.77 | 2.94 | 2.46 | 2.50 | |
| p-value | 0.016 * | 0.026 * | 0.013 * | 0.012 * | 0.024 * | 0.022 * | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris-Love, M.O.; Avila, N.A.; Adams, B.; Zhou, J.; Seamon, B.; Ismail, C.; Zaidi, S.H.; Kassner, C.A.; Liu, F.; Blackman, M.R. The Comparative Associations of Ultrasound and Computed Tomography Estimates of Muscle Quality with Physical Performance and Metabolic Parameters in Older Men. J. Clin. Med. 2018, 7, 340. https://doi.org/10.3390/jcm7100340
Harris-Love MO, Avila NA, Adams B, Zhou J, Seamon B, Ismail C, Zaidi SH, Kassner CA, Liu F, Blackman MR. The Comparative Associations of Ultrasound and Computed Tomography Estimates of Muscle Quality with Physical Performance and Metabolic Parameters in Older Men. Journal of Clinical Medicine. 2018; 7(10):340. https://doi.org/10.3390/jcm7100340
Chicago/Turabian StyleHarris-Love, Michael O., Nilo A. Avila, Bernadette Adams, June Zhou, Bryant Seamon, Catheeja Ismail, Syed H. Zaidi, Courtney A. Kassner, Frank Liu, and Marc R. Blackman. 2018. "The Comparative Associations of Ultrasound and Computed Tomography Estimates of Muscle Quality with Physical Performance and Metabolic Parameters in Older Men" Journal of Clinical Medicine 7, no. 10: 340. https://doi.org/10.3390/jcm7100340
APA StyleHarris-Love, M. O., Avila, N. A., Adams, B., Zhou, J., Seamon, B., Ismail, C., Zaidi, S. H., Kassner, C. A., Liu, F., & Blackman, M. R. (2018). The Comparative Associations of Ultrasound and Computed Tomography Estimates of Muscle Quality with Physical Performance and Metabolic Parameters in Older Men. Journal of Clinical Medicine, 7(10), 340. https://doi.org/10.3390/jcm7100340





