Hand Laser Perfusion Imaging to Assess Radial Artery Patency: A Pilot Study
Abstract
1. Introduction
2. Experimental Section
2.1. Patient Selection
2.2. Clinical Evaluation
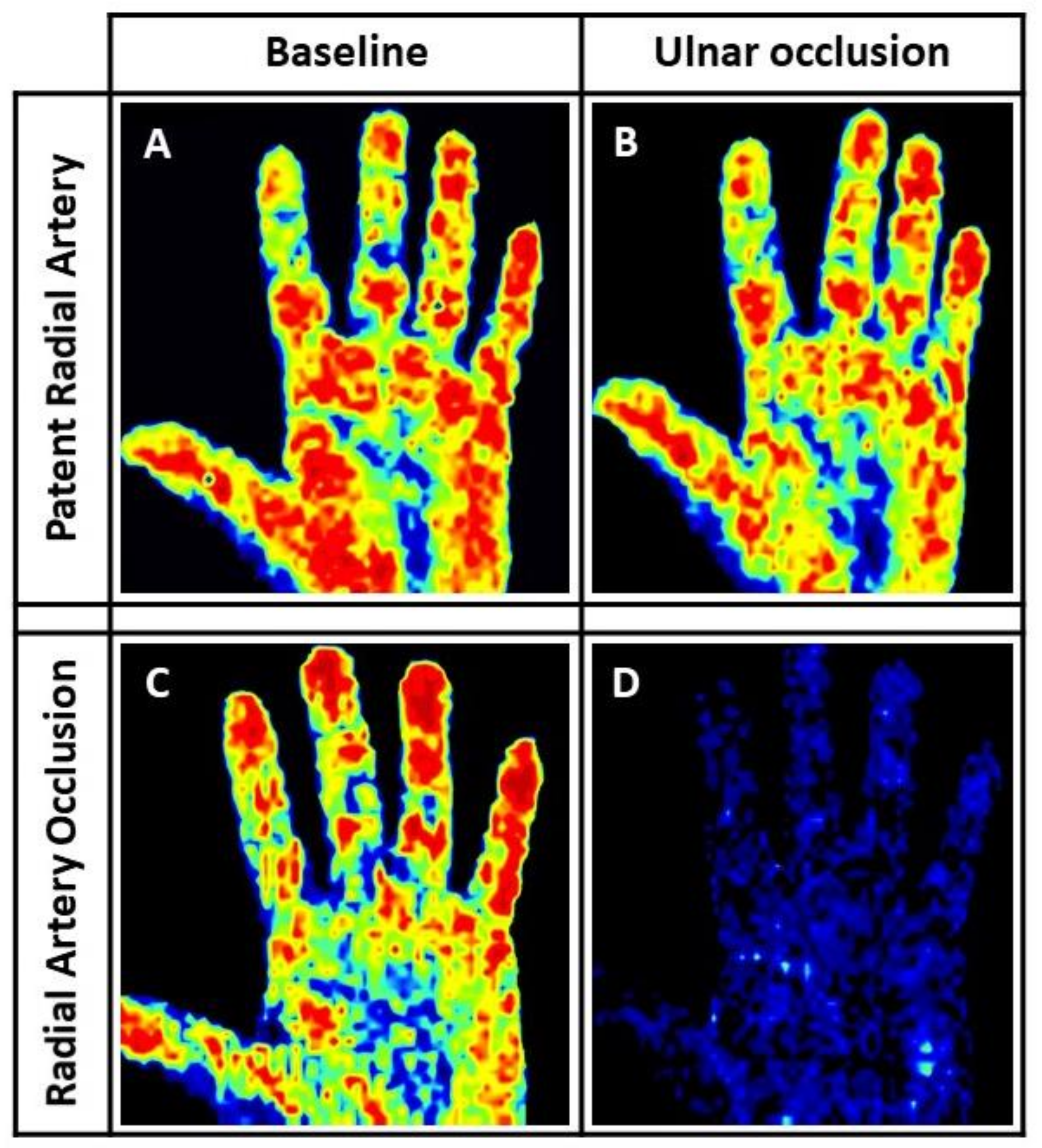
2.3. Laser Perfusion Imaging Evaluation
2.4. Vascular Duplex
2.5. Coronary Angiography
2.6. Periprocedural Antithrombotic Treatment
2.7. Hemostasis
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. Procedural Data
3.3. Post-Procedure Evaluation
3.4. Characterization of Patients with Post-Procedural RAO
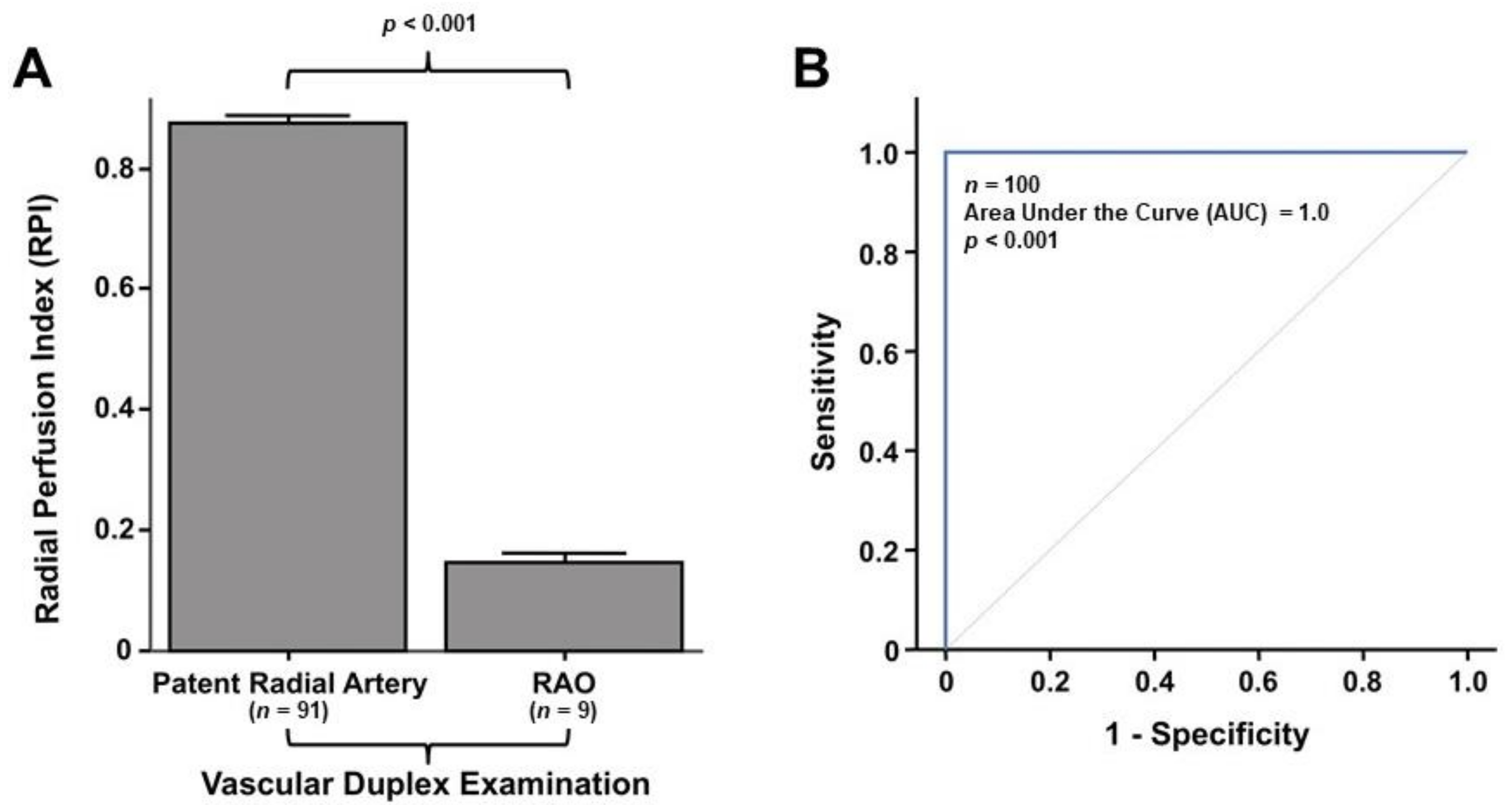
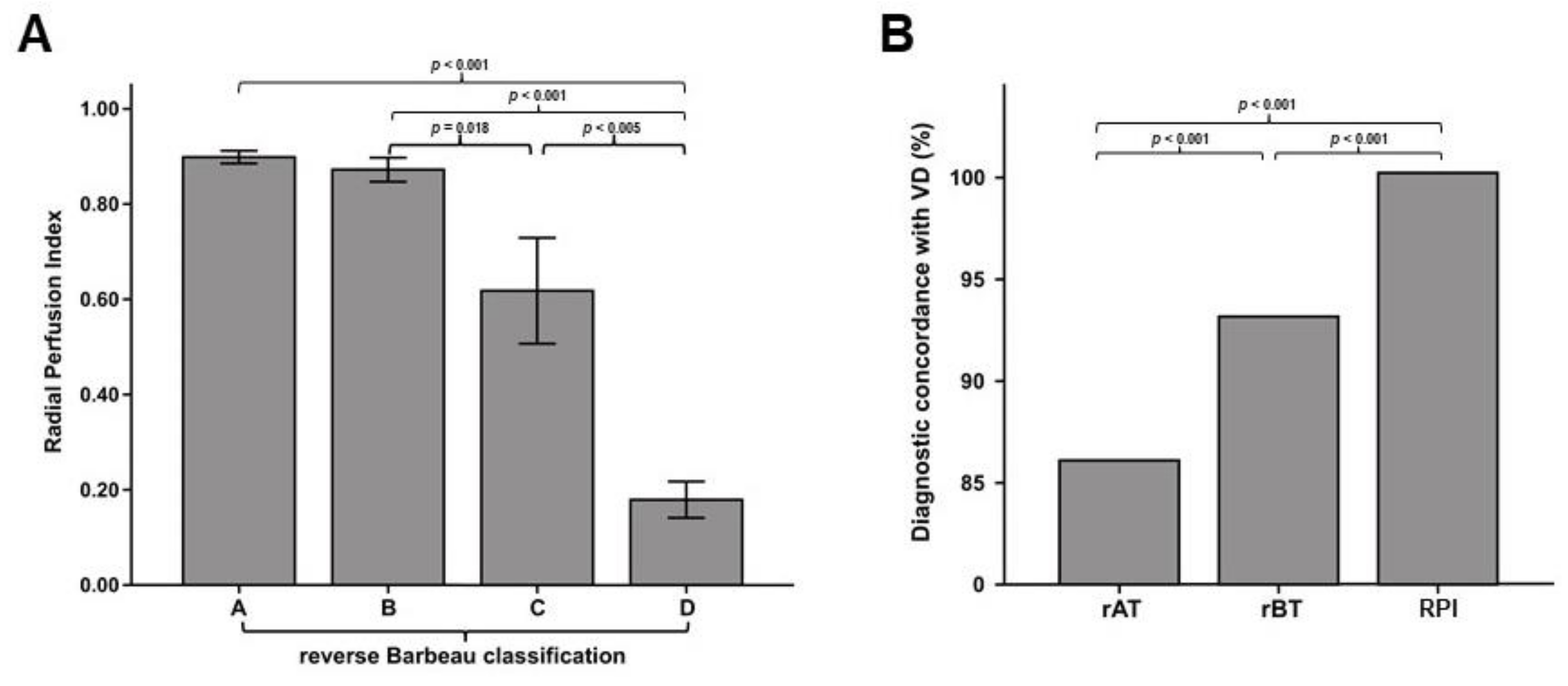
3.5. Laser Perfusion Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jolly, S.S.; Yusuf, S.; Cairns, J.; Niemelä, K.; Xavier, D.; Widimsky, P.; Budaj, A.; Niemelä, M.; Valentin, V.; Lewis, B.S.; et al. RIVAL trial group. Radial vs. femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet 2011, 377, 1409–1420. [Google Scholar] [CrossRef]
- Jolly, S.S.; Amlani, S.; Hamon, M.; Yusuf, S.; Mehta, S.R. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: A systematic review and meta-analysis of randomized trials. Am. Heart J. 2009, 157, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodriguez, E.; Asfour, A.; Lolay, G.; Ziada, K.M.; Abdel-Latif, A.K. Systematic Review and Meta-Analysis of Major Cardiovascular Outcomes for Radial Versus Femoral Access in Patients With Acute Coronary Syndrome. South. Med. J. 2016, 109, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Gagnor, A.; Calabró, P.; Frigoli, E.; Leonardi, S.; Zaro, T.; Rubartelli, P.; Briguori, C.; Andò, G.; Repetto, A.; et al. MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet 2015, 385, 2465–2476. [Google Scholar] [CrossRef]
- Lehmann, R.; Ehrlich, J.R.; Weber, V.; de Rosa, S.; Gotarda, M.N.; SchÄchinger, V.; Zeiher, A.M.; Fichtlscherer, S. Implementation of the transradial approach for coronary procedures is not associated with an elevated complication rate and elevated radiation patient exposure. J. Interv. Cardiol. 2011, 24, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Rigattieri, S.; Sciahbasi, A.; Brilakis, E.S.; Burzotta, F.; Rathore, S.; Pugliese, F.R.; Fedele, S.; Ziakas, A.G.; Zhou, Y.J.; Guzman, L.A. Meta-Analysis of Radial Versus Femoral Artery Approach for Coronary Procedures in Patients With Previous Coronary Artery Bypass Grafting. Am. J. Cardiol. 2016, 117, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Lundqvist, C.B.; Borger, M.A.; Mario, C.D.; Dickstein, K.; Ducrocq, G.; Fernandez-Aviles, F.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.; Pristipino, C.; Di Mario, C.; Nolan, J.; Ludwig, J.; Tubaro, M.; Sabate, M.; Mauri-Ferré, J.; Huber, K.; Niemelä, K.; et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: Position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Coronary Care and Thrombosis of the European Society of Cardiology. EuroIntervention 2013, 8, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Kanei, Y.; Kwan, T.; Nakra, N.C.; Liou, M.; Huang, Y.; Vales, L.L.; Fox, J.T.; Chen, J.P.; Saito, S. Transradial cardiac catheterization: A review of access site complications. Catheter. Cardiovasc. Interv. 2011, 78, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, V.K.; Vidovich, M.I.; Shroff, A.R. Complications of transradial catheterization. Cardiovasc. Revasc. Med. 2012, 13, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Passafaro, F.; Mongiardo, A.; Spaccarotella, C.; Torella, D.; Sorrentino, S.; Polimeni, A.; Emanuele, V.; Curcio, A.; De Rosa, S. Delayed sudden radial artery rupture after left transradial coronary catheterization: A case report. Medicine 2015, 94, e634. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Kwok, C.S.; Pancholy, S.; Chugh, S.; Kedev, S.A.; Bernat, I.; Ratib, K.; Large, A.; Fraser, D.; Nolan, N. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002686. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, A.; Passafaro, F.; De Rosa, S.; Sorrentino, S.; Torella, D.; Spaccarotella, C.; Mongiardo, A.; Indolfi, C. Clinical and Procedural Outcomes of 5-French versus 6-French Sheaths in Transradial Coronary Interventions. Medicine 2015, 94, e2170. [Google Scholar] [CrossRef] [PubMed]
- Rhyne, D.; Mann, T. Hand ischemia resulting from a transradial intervention: Successful management with radial artery angioplasty. Catheter. Cardiovasc. Interv. 2010, 76, 383–386. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Passafaro, F.; Polimeni, A.; Sorrentino, S.; Indolfi, C. A novel quick and easy test for radial artery occlusion with the laser Doppler scan. JACC Cardiovasc. Interv. 2014, 7, e89–e90. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, O.F.; Rao, S.V.; Pancholy, S.; Jolly, S.S.; Rodés-Cabau, J.; Larose, É.; Costerousse, O.; Hamon, M.; Mann, T. Transradial approach for coronary angiography and interventions: Results of the first international transradial practice survey. JACC Cardiovasc. Interv. 2010, 3, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.V. Thromboangiitis obliterans: Methods of diagnosis of chronic occlusive arterial lesions distal to the wrist with illustrative cases. Am. J. Med. Sci. 1929, 178, 237–244. [Google Scholar] [CrossRef]
- Wright, I.S. Vascular Diseases in Clinical Practice, 2nd ed.; Year Book Publishers: Chicago, IL, USA, 1952. [Google Scholar]
- Kotowycz, M.A.; Dzavík, V. Radial artery patency after transradial catheterization. Circ. Cardiovasc. Interv. 2012, 5, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, G.R.; Arsenault, F.; Dugas, L.; Simard, S.; Lariviere, M.M. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: Comparison with the Allen’s test in 1010 patients. Am. Heart J. 2004, 147, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Campo, G.; Penzo, C.; Tebaldi, M.; Biscaglia, S.; Ferrari, R. Transradial coronary catheterization and intervention across the whole spectrum of Allen test results. J. Am. Coll. Cardiol. 2014, 63, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Mongiardo, A.; Spaccarotella, C.; Torella, D.; Caiazzo, G.; Polimeni, A.; Sorrentino, S.; Micieli, M.; Sabatino, J.; Curcio, A.; et al. The instantaneous wave-free ratio (iFR) for evaluation of non-culprit lesions in patients with acute coronary syndrome and multivessel disease. Int. J. Cardiol. 2015, 178, 46–54. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Torella, D.; Caiazzo, G.; Giampà, S.; Indolfi, C. Left radial access for percutaneous coronary procedures: From neglected to performer? A meta-analysis of 14 studies including 7603 procedures. Int. J. Cardiol. 2014, 171, 66–72. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Seeger, F.H.; Honold, J.; Fischer-Rasokat, U.; Lehmann, R.; Fichtlscherer, S.; Schächinger, V.; Dimmeler, S.; Zeiher, A.M.; Assmus, B. Procedural safety and predictors of acute outcome of intracoronary administration of progenitor cells in 775 consecutive procedures performed for acute myocardial infarction or chronic heart failure. Circ. Cardiovasc. Interv. 2013, 6, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Abe, S.; Sato, T.; Hozawa, K.; Yuki, K.; Hanashima, K.; Tomoike, H.; Tomoike, H. Ultrasonic assessment of vascular complications in coronary angiography and angioplasty after transradial approach. Am. J. Cardiol. 1999, 83, 180–186. [Google Scholar] [CrossRef]
- Zankl, A.R.; Andrassy, M.; Volz, C.; Ivandic, B.; Krumsdorf, U.; Katus, H.A.; Blessing, E. Radial artery thrombosis following trasradial coronary angiography: Incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins. Clin. Res. Cardiol. 2010, 99, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Bernat, I.; Bertrand, O.F.; Rokyta, R.; Kacer, M.; Pesek, J.; Koza, J.; Smid, S.; Bruhova, H.; Sterbakova, G.; Stepankova, L.; et al. Efficacy and safety of transient ulnar artery compression to recanalize acute radial artery occlusion after transradial catheterization. Am. J. Cardiol. 2011, 107, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chu, Y.S.; Sun, J.; Jiang, T.M. Ulnar artery compression: A feasible and effective approach to prevent the radial artery occlusion after coronary intervention. Chin. Med. J. (Engl) 2015, 128, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Pancholy, S.B.; Bernat, I.; Bertrand, O.F.; Patel, T.M. Prevention of Radial Artery Occlusion After Transradial Catheterization: The PROPHET-II Randomized Trial. JACC Cardiovasc. Interv. 2016, 9, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Saito, S.; Takahashi, A.; Bernat, I.; Jobe, R.L.; Kajiya, T.; Gilchrist, I.C.; Louvard, Y.; Kiemeneij, F.; van Royen, N.; et al. Impact of sheath size and hemostasis time on radial artery patency after transradial coronary angiography and intervention in Japanese and non-Japanese patients: A substudy from RAP and BEAT (Radial Artery Patency and Bleeding, Efficacy, Adverse evenT) randomized multicenter trial. Catheter. Cardiovasc. Interv. 2018. [Google Scholar] [CrossRef]
- Pancholy, S.B.; Patel, T.M. Effect of duration of hemostatic compression on radial artery occlusion after transradial access. Catheter. Cardiovasc. Interv. 2012, 79, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.; Gordin, J.; Sallam, T.; Wachsner, R.; Meymandi, S.; Traina, M. Facilitated patent haemostasis after transradial catheterisation to reduce radial artery occlusion. EuroIntervention 2015, 11, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Dangoisse, V.; Guedès, A.; Chenu, P.; Hanet, C.; Albert, C.; Robin, V.; Tavier, L.; Dury, C.; Piraux, O.; Domange, D.; et al. Usefulness of a gentle and short hemostasis using the transradial band device after transradial access for percutaneous coronary angiography and interventions to reduce the radial artery occlusion rate (from the prospective and randomized CRASOC I., II, and III studies). Am. J. Cardiol. 2017, 120, 374–379. [Google Scholar] [PubMed]
- Lavi, S.; Cheema, A.; Yadegari, A.; Israeli, Z.; Levi, Y.; Wall, S.; Alemayehu, M.; Parviz, Y.; Murariu, B.D.; McPherson, T.; et al. Randomized Trial of Compression Duration After Transradial Cardiac Catheterization and Intervention. J. Am. Heart Assoc. 2017, 6, e005029. [Google Scholar] [CrossRef] [PubMed]
- Buturak, A.; Gorgulu, S.; Norgaz, T.; Voyvoda, N.; Sahingoz, Y.; Degirmencioglu, A.; Dagdelen, S. The long-term incidence and predictors of radial artery occlusion following a transradial coronary procedure. Cardiol. J. 2014, 21, 350–356. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 100) | Occluded (n = 9) | Not Occluded (n = 91) | p * | |
|---|---|---|---|---|
| Female | 32% | 4 (44.0%) | 28 (31.0%) | 0.40 |
| Age (years, mean ± SD) | 66.8 ± 11.4 | 73.9 ± 11.7 | 66.1 ± 11.2 | 0.051 |
| Family history of CAD | 30% | 2 (22.2%) | 28 (30.8%) | 0.59 |
| BMI (kg/m2, mean ± SD) | 27.9 ± 4.4 | 25.7 ± 3.3 | 28.2 ± 4.4 | 0.11 |
| Hypertension | 84% | 7 (77.8%) | 77 (84.6%) | 0.59 |
| Dyslipidaemia | 51% | 4 (44.4%) | 47 (51.6%) | 0.68 |
| Diabetes | 29% | 1 (11.1%) | 28 (30.8%) | 0.22 |
| Smokers | 25% | 2 (22.2%) | 23 (25.3%) | 0.84 |
| Previous PCI | 26% | 3 (33.3%) | 23 (25.3%) | 0.60 |
| Previous trans-radial access (>3 months) | 18% | 2 (22.2%) | 16 (17.6%) | 0.73 |
| Atrial Fibrillation | 12% | 1 (11.1%) | 11 (12.1%) | 0.93 |
| LVEF (%, mean ± SD) | 51.5 ± 8.2 | 52.7 ± 6.9 | 51.4 ± 8.3 | 0.65 |
| NYHA Class II-IV | 61% | 6 (66.7%) | 55 (60.4%) | 0.72 |
| anti-P2Y12 loading dose | 15% | 1 (11.1%) | 14 (15.4%) | 0.73 |
| DAPT (Cath-lab) | 71% | 9 (100.0%) | 62 (68.1%) | 0.044 |
| ACE-Is/ARBs | 75% | 6 (66.7%) | 69 (77.5%) | 0.46 |
| Beta Blockers | 58% | 5 (55.6%) | 53 (59.6%) | 0.82 |
| Nitrate | 23% | 3 (33.3%) | 20 (22.5%) | 0.46 |
| Oral Hypoglycemics | 22% | 1 (11.1%) | 21 (23.1%) | 0.41 |
| Insulin | 6% | 0 (0.0%) | 6 (6.6%) | 0.43 |
| Statin | 73% | 6 (66.7%) | 67 (73.6%) | 0.65 |
| GFR (mL/min, mean ± SD) | 84.2 ± 32.7 | 69.8 ± 30.6 | 85.7 ± 32.7 | 0.17 |
| Hemoglobin (g/dL, mean ± SD) | 13.7 ± 1.59 | 13.2 ± 1.28 | 13.8 ± 1.61 | 0.29 |
| Hematocrit (%, mean ± SD) | 42.0 ± 4.3 | 41.1 ± 4.6 | 42.1 ± 4.3 | 0.50 |
| Platelets (×103/μL, mean ± SD) | 211 ± 54 | 242 ± 52 | 208 ± 54 | 0.068 |
| MPV (fL, mean ± SD) | 8.1 ± 1.0 | 7.4 ± 0.6 | 8.2 ± 1.0 | 0.035 |
| Glycemia (mg/dL, mean ± SD) | 122 ± 45 | 109 ± 18 | 122 ± 47 | 0.41 |
| Total Cholesterol (mg/dL, mean ± SD) | 172.0 ± 36.9 | 158.1 ± 31.9 | 173.3 ± 37.2 | 0.24 |
| LDL Cholesterol (mg/dL, mean ± SD) | 108.4 ± 32.4 | 95.8 ± 29.9 | 109.7 ± 32.5 | 0.22 |
| HDL Cholesterol (mg/dL, mean ± SD) | 50.4 ± 18.2 | 49.9 ± 12.6 | 50.5 ± 18.7 | 0.93 |
| Triglycerides (mg/dL, mean ± SD) | 143.3 ± 79.8 | 114.8 ± 54.2 | 146.1 ± 81.5 | 0.26 |
| Creatinine (mg/dL, mean ± SD) | 0.93 ± 0.22 | 0.93 ± 0.28 | 0.93 ± 0.22 | 0.98 |
| Total (n = 100) | Occluded (n = 9) | Not Occluded (n = 91) | p * | |
|---|---|---|---|---|
| Procedural time (min, mean ± SD) | 51.0 ± 32.8 | 70.6 ± 38.6 | 49.0 ± 31.7 | 0.060 |
| Access complications | ||||
| Challenging access (puncture) | 14% | 2 (22.2%) | 12 (13.2%) | 0.46 |
| Spasm | 7% | 1 (11.1%) | 6 (6.6%) | 0.61 |
| Dissection | 0% | 0 (0.0%) | 0 (0.0%) | - |
| PCI performed | 21% | 3 (33.3%) | 18 (19.8%) | 0.34 |
| Nitroglicerin i.a. | 21% | 3 (33.3%) | 18 (19.8%) | 0.34 |
| UFH (IUs, mean ± SD) | 3530 ± 893 | 3666 ± 1000 | 3516 ± 886 | 0.63 |
| TR-band time (h, mean ± SD) | 5.32 ± 1.52 | 6.67 ± 2.21 | 5.18 ± 1.38 | 0.004 |
| Symptoms | ||||
| Transient paresthesia | 23% | 2 (22.2%) | 21 (23.1%) | 0.95 |
| Local pain (VAS, mean ± SD) | 0.92 ± 1.80 | 2.33 ± 2.96 | 0.78 ± 1.60 | 0.013 |
| Local pain (VAS ≥ 5) | 10% | 3 (33.3%) | 7 (7.7%) | 0.014 |
| Banding (post TR-band removal) | ||||
| None | 35% | 1 (11.1%) | 34 (37.4%) | 0.12 |
| Non compressive | 49% | 5 (55.6%) | 44 (48.4%) | 0.68 |
| Compressive Banding | 16% | 3 (33.3%) | 13 (14.3%) | 0.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indolfi, C.; Passafaro, F.; Sorrentino, S.; Spaccarotella, C.; Mongiardo, A.; Torella, D.; Polimeni, A.; Sabatino, J.; Curcio, A.; De Rosa, S. Hand Laser Perfusion Imaging to Assess Radial Artery Patency: A Pilot Study. J. Clin. Med. 2018, 7, 319. https://doi.org/10.3390/jcm7100319
Indolfi C, Passafaro F, Sorrentino S, Spaccarotella C, Mongiardo A, Torella D, Polimeni A, Sabatino J, Curcio A, De Rosa S. Hand Laser Perfusion Imaging to Assess Radial Artery Patency: A Pilot Study. Journal of Clinical Medicine. 2018; 7(10):319. https://doi.org/10.3390/jcm7100319
Chicago/Turabian StyleIndolfi, Ciro, Francesco Passafaro, Sabato Sorrentino, Carmen Spaccarotella, Annalisa Mongiardo, Daniele Torella, Alberto Polimeni, Jolanda Sabatino, Antonio Curcio, and Salvatore De Rosa. 2018. "Hand Laser Perfusion Imaging to Assess Radial Artery Patency: A Pilot Study" Journal of Clinical Medicine 7, no. 10: 319. https://doi.org/10.3390/jcm7100319
APA StyleIndolfi, C., Passafaro, F., Sorrentino, S., Spaccarotella, C., Mongiardo, A., Torella, D., Polimeni, A., Sabatino, J., Curcio, A., & De Rosa, S. (2018). Hand Laser Perfusion Imaging to Assess Radial Artery Patency: A Pilot Study. Journal of Clinical Medicine, 7(10), 319. https://doi.org/10.3390/jcm7100319






