Inhibitory Effects of Intranasal Administration of Insulin on Fat Oxidation during Exercise Are Diminished in Young Overweight Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
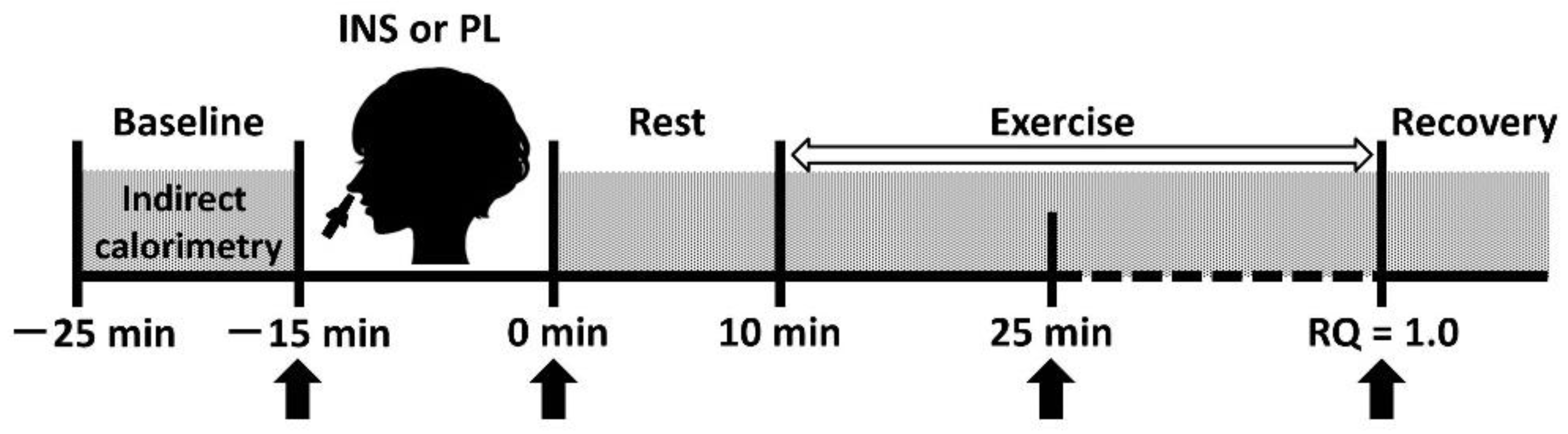
2.2. Study Design
2.3. Determination of Peak VO2
2.4. Intranasal Administration
2.5. Respiratory Gas Analysis during the Graded Exercise Test to Assess Fat Oxidation Rate
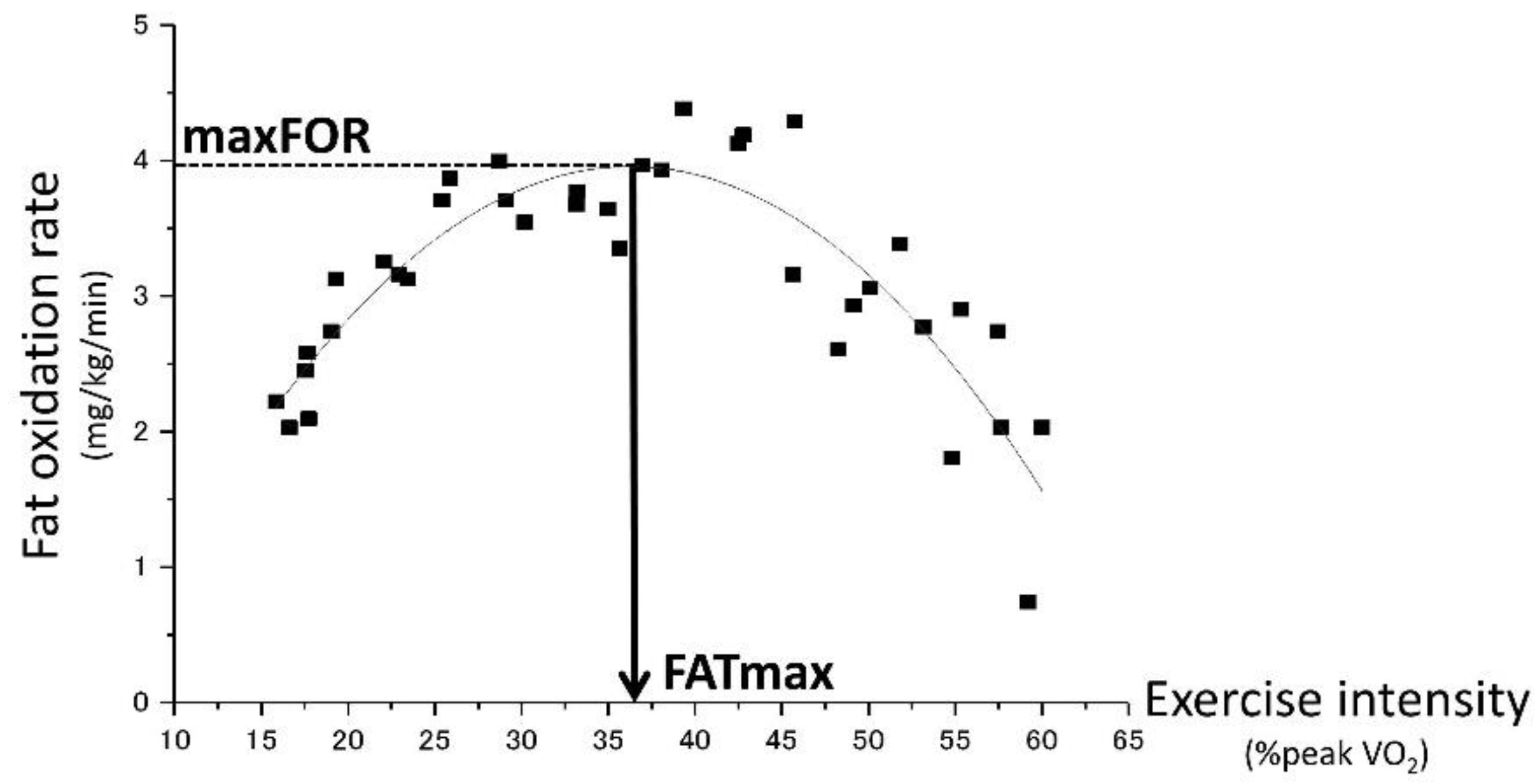
2.6. Estimation of maxFOR and FATmax
2.7. Anthropometrical Measurements
2.8. Laboratory Measurements
2.9. Statistical Analyses
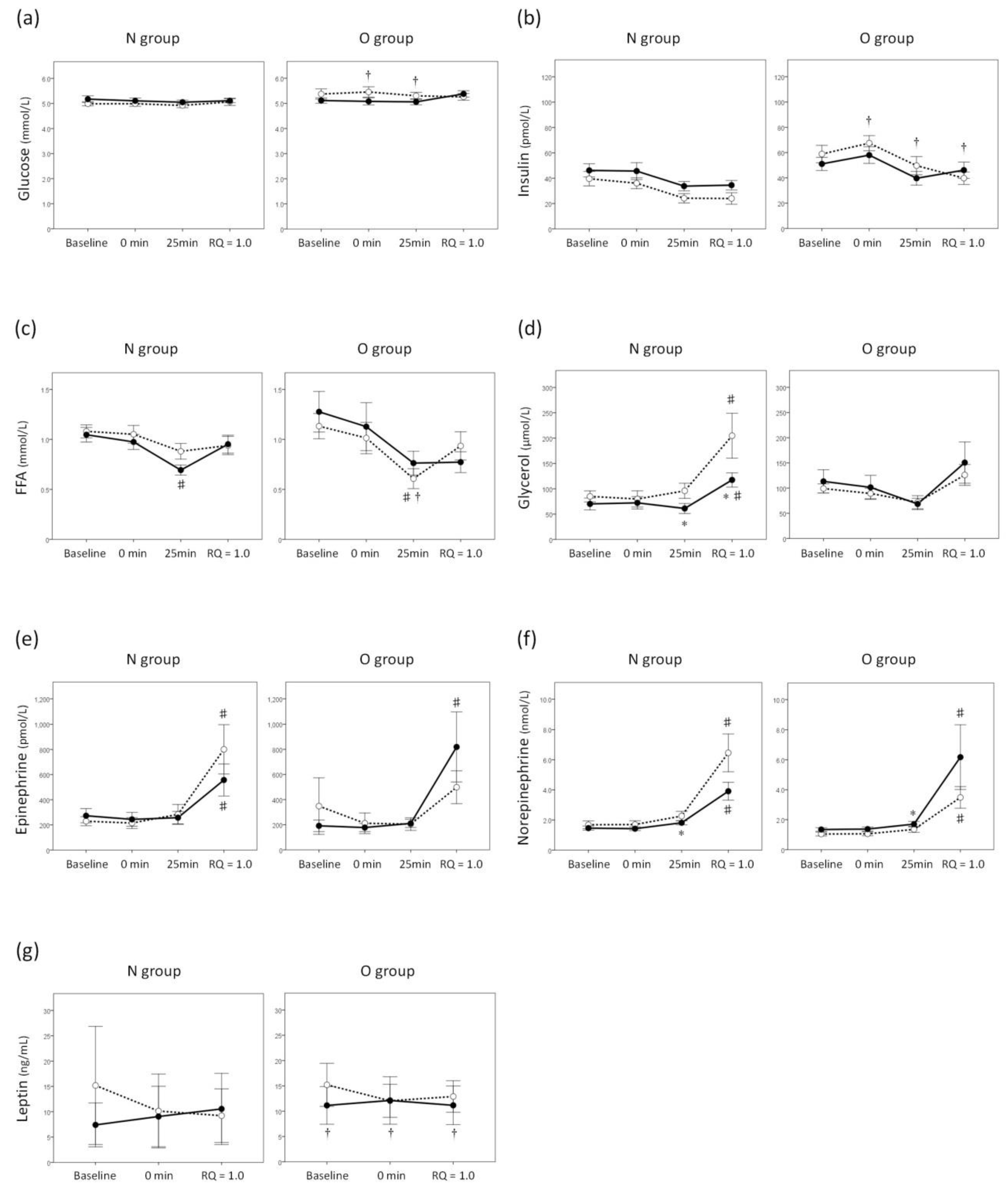
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.H., 3rd; Klein, S. Use of endogenous carbohydrate and fat as fuels during exercise. Proc. Nutr. Soc. 1998, 57, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, J.; Cao, L.; Guo, Z.; Wang, Y. Positive effect of exercise training at maximal fat oxidation intensity on body composition and lipid metabolism in overweight middle-aged women. Clin. Physiol. Funct. Imaging 2016, 36, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Isacco, L.; Duche, P.; Thivel, D.; Meddahi-Pelle, A.; Lemoine-Morel, S.; Duclos, M.; Boisseau, N. Fat mass localization alters fuel oxidation during exercise in normal weight women. Med. Sci. Sports Exerc. 2013, 45, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Lanzi, S.; Codecasa, F.; Cornacchia, M.; Maestrini, S.; Salvadori, A.; Brunani, A.; Malatesta, D. Fat oxidation, hormonal and plasma metabolite kinetics during a submaximal incremental test in lean and obese adults. PLoS ONE 2014, 9, e88707. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.J.; Baskin, D.G.; Schwartz, M.W. Insulin and the blood-brain barrier. Curr. Pharm. Des. 2003, 9, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Rask-Madsen, C.; Banks, W.A. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schulingkamp, R.J.; Pagano, T.C.; Hung, D.; Raffa, R.B. Insulin receptors and insulin action in the brain: Review and clinical implications. Neurosci. Biobehav. Rev. 2000, 24, 855–872. [Google Scholar] [CrossRef]
- Unger, J.W.; Livingston, J.N.; Moss, A.M. Insulin receptors in the central nervous system: Localization, signalling mechanisms and functional aspects. Prog. Neurobiol. 1991, 36, 343–362. [Google Scholar] [CrossRef]
- Porte, D., Jr.; Woods, S.C. Regulation of food intake and body weight by insulin. Diabetologia 1981, 20, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Obici, S.; Zhang, B.B.; Karkanias, G.; Rossetti, L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 2002, 8, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Frey, W.H., 2nd; Craft, S.; Danielyan, L.; Hallschmid, M.; Schioth, H.B.; Benedict, C. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Hallschmid, M.; Schultes, B.; Marshall, L.; Molle, M.; Kern, W.; Bredthauer, J.; Fehm, H.L.; Born, J. Transcortical direct current potential shift reflects immediate signaling of systemic insulin to the human brain. Diabetes 2004, 53, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Xiao, C.; Morgantini, C.; Koulajian, K.; Lewis, G.F. Is insulin action in the brain relevant in regulating blood glucose in humans? J. Clin. Endocrinol. Metab. 2015, 100, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Jauch-Chara, K.; Friedrich, A.; Rezmer, M.; Melchert, U.H.; Scholand-Engler, H.G.; Hallschmid, M.; Oltmanns, K.M. Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes 2012, 61, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Hallschmid, M.; Benedict, C.; Schultes, B.; Fehm, H.L.; Born, J.; Kern, W. Intranasal insulin reduces body fat in men but not in women. Diabetes 2004, 53, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Weltman, A.; Katch, V.; Sady, S.; Freedson, P. Onset of metabolic-acidosis (anaerobic threshold) as a criterion measure of submaximum fitness. Res. Q. 1978, 49, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Emoto, M.; Fujiwara, S.; Motoyama, K.; Morioka, T.; Komatsu, M.; Tahara, H.; Koyama, H.; Shoji, T.; Inaba, M.; et al. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment are useful indexes of insulin resistance in type 2 diabetic patients with wide range of fasting plasma glucose. J. Clin. Endocrinol. Metab. 2004, 89, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hallschmid, M.; Higgs, S.; Thienel, M.; Ott, V.; Lehnert, H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 2012, 61, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Kern, W.; Schultes, B.; Born, J.; Hallschmid, M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J. Clin. Endocrinol. Metab. 2008, 93, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Guthoff, M.; Grichisch, Y.; Canova, C.; Tschritter, O.; Veit, R.; Hallschmid, M.; Haring, H.U.; Preissl, H.; Hennige, A.M.; Fritsche, A. Insulin modulates food-related activity in the central nervous system. J. Clin. Endocrinol. Metab. 2010, 95, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Brede, S.; Schioth, H.B.; Lehnert, H.; Schultes, B.; Born, J.; Hallschmid, M. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes 2011, 60, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Jeukendrup, A.E.; Wagenmakers, A.J.; Saris, W.H. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am. J. Physiol. 1997, 273, E268–E275. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Bergman, R.N.; Kahn, S.E.; Taborsky, G.J., Jr.; Fisher, L.D.; Sipols, A.J.; Woods, S.C.; Steil, G.M.; Porte, D., Jr. Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J. Clin. Investig. 1991, 88, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Coates, P.A.; Ismail, I.S.; Luzio, S.D.; Griffiths, I.; Ollerton, R.L.; Volund, A.; Owens, D.R. Intranasal insulin: The effects of three dose regimens on postprandial glycaemic profiles in type ii diabetic subjects. Diabet. Med. 1995, 12, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Frauman, A.G.; Jerums, G.; Louis, W.J. Effects of intranasal insulin in non-obese type ii diabetics. Diabetes Res. Clin. Pract. 1987, 3, 197–202. [Google Scholar] [CrossRef]
- Bartness, T.J.; Song, C.K. Thematic review series: Adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J. Lipid Res. 2007, 48, 1655–1672. [Google Scholar] [CrossRef] [PubMed]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef] [PubMed]
- De Glisezinski, I.; Larrouy, D.; Bajzova, M.; Koppo, K.; Polak, J.; Berlan, M.; Bulow, J.; Langin, D.; Marques, M.A.; Crampes, F.; et al. Adrenaline but not noradrenaline is a determinant of exercise-induced lipid mobilization in human subcutaneous adipose tissue. J. Physiol. 2009, 587, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Banni, S.; Carta, G.; Murru, E.; Cordeddu, L.; Giordano, E.; Marrosu, F.; Puligheddu, M.; Floris, G.; Asuni, G.P.; Cappai, A.L.; et al. Vagus nerve stimulation reduces body weight and fat mass in rats. PLoS ONE 2012, 7, e44813. [Google Scholar] [CrossRef] [PubMed]
- Malbert, C.H.; Picq, C.; Divoux, J.L.; Henry, C.; Horowitz, M. Obesity-associated alterations in glucose metabolism are reversed by chronic bilateral stimulation of the abdominal vagus nerve. Diabetes 2017, 66, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martin, A.; Dumortier, M.; Raynaud, E.; Brun, J.F.; Fedou, C.; Bringer, J.; Mercier, J. Balance of substrate oxidation during submaximal exercise in lean and obese people. Diabetes Metab. 2001, 27, 466–474. [Google Scholar] [PubMed]
- Isacco, L.; Thivel, D.; Duclos, M.; Aucouturier, J.; Boisseau, N. Effects of adipose tissue distribution on maximum lipid oxidation rate during exercise in normal-weight women. Diabetes Metab. 2014, 40, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Harris, M.B. Abdominal fat reducing outcome of exercise training: Fat burning or hydrocarbon source redistribution? Can. J. Physiol. Pharmacol. 2016, 94, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, B.; Fields, D.A.; Klein, S. Excess body fat in men decreases plasma fatty acid availability and oxidation during endurance exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E354–E362. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Wolfe, R.R.; Kelley, D.E. Effects of obesity on substrate utilization during exercise. Obes. Res. 2002, 10, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Heni, M.; Wagner, R.; Kullmann, S.; Veit, R.; Mat Husin, H.; Linder, K.; Benkendorff, C.; Peter, A.; Stefan, N.; Haring, H.U.; et al. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 2014, 63, 4083–4088. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Heni, M.; Veit, R.; Scheffler, K.; Machann, J.; Haring, H.U.; Fritsche, A.; Preissl, H. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care 2015, 38, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Gancheva, S.; Koliaki, C.; Bierwagen, A.; Nowotny, P.; Heni, M.; Fritsche, A.; Haring, H.U.; Szendroedi, J.; Roden, M. Effects of intranasal insulin on hepatic fat accumulation and energy metabolism in humans. Diabetes 2015, 64, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Tschritter, O.; Preissl, H.; Hennige, A.M.; Stumvoll, M.; Porubska, K.; Frost, R.; Marx, H.; Klosel, B.; Lutzenberger, W.; Birbaumer, N.; et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: A magnetoencephalographic study. Proc. Natl. Acad. Sci. USA 2006, 103, 12103–12108. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Salameh, T.S.; Logsdon, A.F.; Hanson, A.J.; Erickson, M.A.; Banks, W.A. Blood-brain barriers in obesity. AAPS J. 2017, 19, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Sajja, R.K.; Naik, P.; Cucullo, L. Diabetes mellitus and blood-brain barrier dysfunction: An overview. J. Pharmacovigil 2014, 2, 125. [Google Scholar] [PubMed]
- Ott, V.; Benedict, C.; Schultes, B.; Born, J.; Hallschmid, M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes. Metab. 2012, 14, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Obici, S.; Feng, Z.; Karkanias, G.; Baskin, D.G.; Rossetti, L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002, 5, 566–572. [Google Scholar] [CrossRef] [PubMed]
| N Group | O Group | p | ||||||
|---|---|---|---|---|---|---|---|---|
| n (male/female) | --- | 15 (7/8) | 8 (4/4) | 0.879 § | ||||
| Age | (years) | 20.6 | ± | 3.7 | 23.6 | ± | 7.1 | 0.191 |
| BW | (kg) | 53.8 | ± | 8.8 | 74.5 | ± | 7.6 | <0.001 |
| BMI | (kg/m2) | 19.2 | ± | 1.5 | 27.2 | ± | 2.5 | <0.001 |
| Body fat | (%) | 16.7 | ± | 5.3 | 33.6 | ± | 9.2 | <0.001 |
| Peak VO2 | (ml/kg/min) | 37.7 | ± | 7.7 | 31.9 | ± | 9.1 | 0.119 |
| Glucose | (mmol/L) | 5.1 | ± | 0.4 | 5.4 | ± | 0.6 | 0.162 |
| Insulin | (pmol/L) | 41.7 | ± | 22.9 | 59.0 | ± | 19.4 | 0.079 |
| Glycated albumin | (%) | 13.4 | ± | 1.3 | 12.9 | ± | 0.9 | 0.348 |
| HOMA-IR | --- | 1.38 | ± | 0.81 | 1.99 | ± | 0.61 | 0.072 |
| N Group | O Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | INS | PL | INS | ||||||||||
| Baseline | Rest | 25 min | Baseline | Rest | 25 min | Baseline | Rest | 25 min | Baseline | Rest | 25 min | ||
| SBP | (mmHg) | 111 ± 15 | 111 ± 15 | 118 ± 24 | 113 ± 13 | 109 ± 19 | 123 ± 19 | 116 ± 20 | 122 ± 8 | 129 ± 15 | 118 ± 17 | 117 ± 16 | 130 ± 18 |
| DBP | (mmHg) | 73 ± 10 | 75 ± 11 | 71 ± 17 | 74 ± 12 | 77 ± 11 | 70 ± 12 | 88 ± 16 † | 80 ± 16 | 83 ± 8 | 83 ± 15 | 83 ± 19 | 79 ± 16 |
| HR | (beats/min) | 74 ± 10 | 75 ± 12 | 102 ± 16 | 77 ± 13 | 79 ± 12 | 104 ± 17 | 74 ± 9 | 76 ± 9 | 96 ± 12 | 77 ± 13 | 77 ± 12 | 99 ± 11 |
| N Group | O Group | p | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | INS | PL | INS | Group | Trial | Group × Trial Interaction | ||||||||||
| MaxFOR | (mg/kg/min) | 3.72 | ± | 0.30 | 3.00 | ± | 0.20 * | 2.44 | ± | 0.19 † | 2.69 | ± | 0.27 | 0.035 | 0.166 | 0.007 |
| FATmax | (%peak VO2) | 38.5 | ± | 2.5 | 32.1 | ± | 1.9 * | 33.0 | ± | 3.4 | 36.0 | ± | 4.4 | 0.837 | 0.354 | 0.017 |
| Total exercise time | (min) | 35.5 | ± | 3.9 | 28.1 | ± | 3.2 * | 34.0 | ± | 4.4 | 38.3 | ± | 5.8 | 0.463 | 0.447 | 0.009 |
| Total amount of FO | (g) | 6.9 | ± | 1.5 | 4.4 | ± | 0.9 * | 5.8 | ± | 1.3 | 7.4 | ± | 2.0 ** | 0.626 | 0.451 | 0.004 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoyama, H.; Takeda, R.; Kawai, E.; Ota, A.; Morita, E.; Imai, D.; Suzuki, Y.; Morioka, T.; Emoto, M.; Inaba, M.; et al. Inhibitory Effects of Intranasal Administration of Insulin on Fat Oxidation during Exercise Are Diminished in Young Overweight Individuals. J. Clin. Med. 2018, 7, 308. https://doi.org/10.3390/jcm7100308
Yokoyama H, Takeda R, Kawai E, Ota A, Morita E, Imai D, Suzuki Y, Morioka T, Emoto M, Inaba M, et al. Inhibitory Effects of Intranasal Administration of Insulin on Fat Oxidation during Exercise Are Diminished in Young Overweight Individuals. Journal of Clinical Medicine. 2018; 7(10):308. https://doi.org/10.3390/jcm7100308
Chicago/Turabian StyleYokoyama, Hisayo, Ryosuke Takeda, Eriko Kawai, Akemi Ota, Emiko Morita, Daiki Imai, Yuta Suzuki, Tomoaki Morioka, Masanori Emoto, Masaaki Inaba, and et al. 2018. "Inhibitory Effects of Intranasal Administration of Insulin on Fat Oxidation during Exercise Are Diminished in Young Overweight Individuals" Journal of Clinical Medicine 7, no. 10: 308. https://doi.org/10.3390/jcm7100308
APA StyleYokoyama, H., Takeda, R., Kawai, E., Ota, A., Morita, E., Imai, D., Suzuki, Y., Morioka, T., Emoto, M., Inaba, M., & Okazaki, K. (2018). Inhibitory Effects of Intranasal Administration of Insulin on Fat Oxidation during Exercise Are Diminished in Young Overweight Individuals. Journal of Clinical Medicine, 7(10), 308. https://doi.org/10.3390/jcm7100308






