Pioglitazone Reduces Dementia Risk in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Analysis
Abstract
1. Introduction
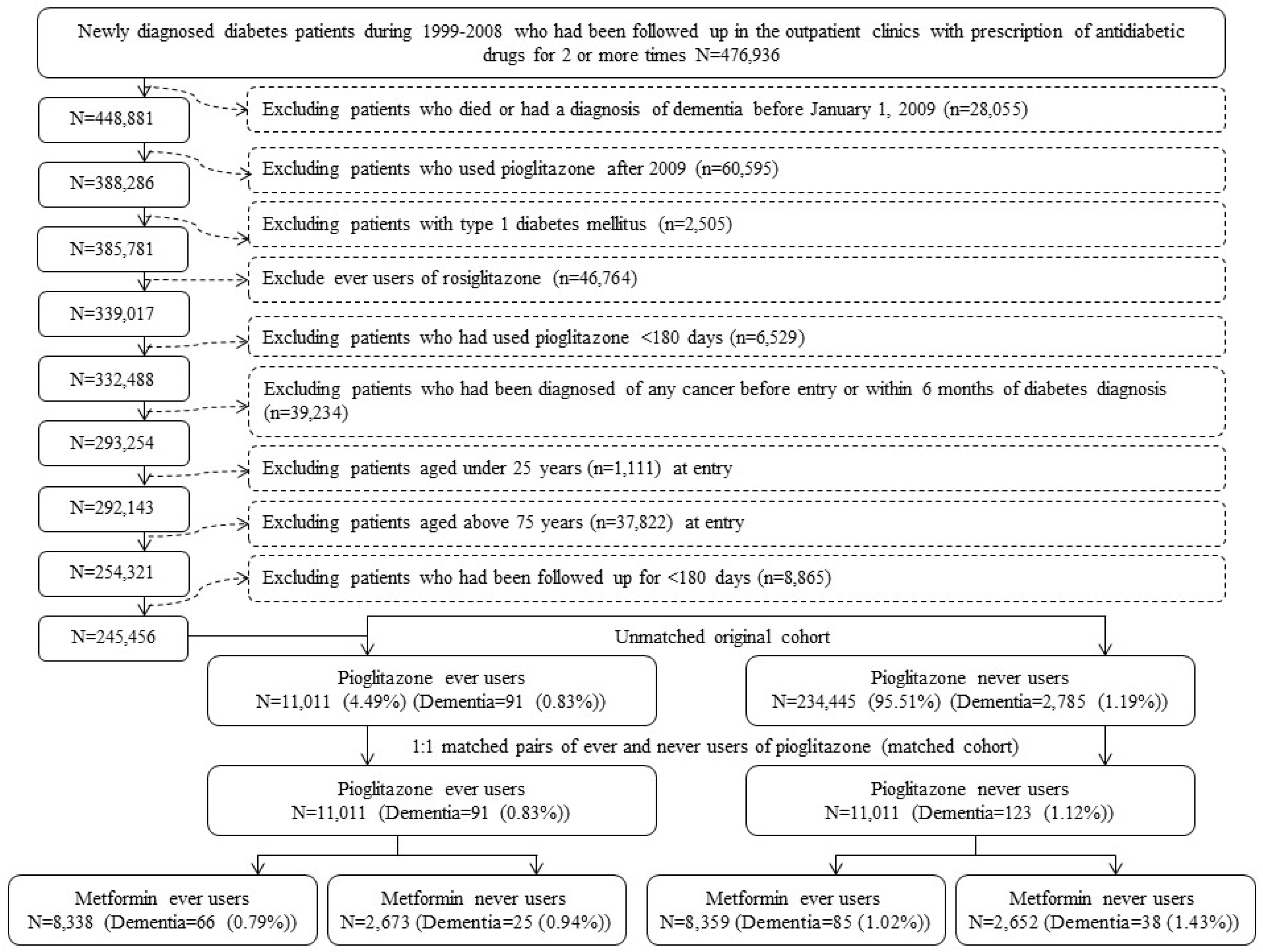
2. Materials and Methods
3. Results
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- De la Monte, S.M.; Tong, M.; Wands, J.R. The 20-year voyage aboard the Journal of Alzheimer’s Disease: Docking at ‘type 3 diabetes’, environmental/exposure factors, pathogenic mechanisms, and potential treatments. J. Alzheimers Dis. 2018, 62, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, D.; Leng, S.X. Link between type 2 diabetes and Alzheimer’s disease: From epidemiology to mechanism and treatment. Clin. Interv. Aging 2015, 10, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Willem, M.; Tahirovic, S.; Busche, M.A.; Ovsepian, S.V.; Chafai, M.; Kootar, S.; Hornburg, D.; Evans, L.D.; Moore, S.; Daria, A.; et al. η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature 2015, 526, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Hanke, A.; Dewachter, I.; Kuiperi, C.; O’Banion, K.; Klockgether, T.; Van Leuven, F.; Landreth, G.E. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1–42 levels in APPV717I transgenic mice. Brain 2005, 128, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Shi, Y.; Ohli, J.; Schüller, U.; Dorostkar, M.M.; Herms, J. Neuroinflammation impairs adaptive structural plasticity of dendritic spines in a preclinical model of Alzheimer’s disease. Acta Neuropathol. 2016, 131, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Lin, Z.Y.; Zhu, Y.G.; Lin, N.; Zhang, J.; Pan, X.D.; Chen, X.C. Ginsenoside Rg1 attenuates β-amyloid generation via suppressing PPARγ-regulated BACE1 activity in N2a-APP695 cells. Eur. J. Pharmacol. 2012, 675, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pohland, M.; Hagl, S.; Pellowska, M.; Wurglics, M.; Schubert-Zsilavecz, M.; Eckert, G.P. MH84: A novel γ-secretase modulator/PPARγ agonist—Improves mitochondrial dysfunction in a cellular model of Alzheimer’s disease. Neurochem. Res. 2016, 41, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.L.; Pee, H.N.; Yang, S.; Ho, P.C. Influence of drug transporters and stereoselectivity on the brain penetration of pioglitazone as a potential medicine against Alzheimer’s disease. Sci. Rep. 2015, 5, 9000. [Google Scholar] [CrossRef] [PubMed]
- Takeda News Releases. Takeda and Zinfandel Pharmaceuticals Discontinue TOMMORROW Trial Following Planned Futility Analysis. Available online: https://www.takeda.com/newsroom/newsreleases/2018/takeda-tommorrow-trial/ (accessed on 19 July 2018).
- Lu, C.H.; Yang, C.Y.; Li, C.Y.; Hsieh, C.Y.; Ou, H.T. Lower risk of dementia with pioglitazone, compared with other second-line treatments, in metformin-based dual therapy: A population-based longitudinal study. Diabetologia 2018, 61, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and the risk of dementia in type 2 diabetes patients. Aging Dis. 2017, in press. [Google Scholar]
- Orkaby, A.R.; Cho, K.; Cormack, J.; Gagnon, D.R.; Driver, J.A. Metformin vs. sulfonylurea use and risk of dementia in US veterans aged ≥65 years with diabetes. Neurology 2017, 89, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.M. Metabolic alterations associated to brain dysfunction in diabetes. Aging Dis. 2015, 6, 304–321. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and lung cancer risk in patients with type 2 diabetes mellitus. Oncotarget 2017, 8, 41132. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: A retrospective cohort analysis. Diabetes Metab. 2017, 43, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L.S. Performing a 1:N Case-Control Match on Propensity Score. Available online: http://www2.sas.com/proceedings/sugi29/165-29.pdf (accessed on 19 July 2018).
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Karlo, J.C.; Caprariello, A.; Blankenship, D.; Dechant, A.; Landreth, G.E. The PPARγ agonist pioglitazone crosses the blood-brain barrier and reduces tumor growth in a human xenograft model. Cancer Chemother. Pharmacol. 2013, 71, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Barrera, J.; Subramanian, S.; Chiba-Falek, O. Probing the role of PPARγ in the regulation of late-onset Alzheimer’s disease-associated genes. PLoS ONE 2018, 13, e0196943. [Google Scholar] [CrossRef] [PubMed]
- Łabuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Béjot, Y.; Reis, J.; Giroud, M.; Feigin, V. A review of epidemiological research on stroke and dementia and exposure to air pollution. Int. J. Stroke 2018. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.; Bousser, M.G.; Betteridge, D.J.; Schernthaner, G.; Pirags, V.; Kupfer, S.; Dormandy, J. PROactive Investigators. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: Results from PROactive (PROspective pioglitAzone Clinical Trial in macroVascular Events 04). Stroke 2007, 38, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Viscoli, C.M.; Furie, K.L.; Young, L.H.; Inzucchi, S.E.; Gorman, M.; Guarino, P.D.; Lovejoy, A.M.; Peduzzi, P.N.; Conwit, R.; et al. IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 2016, 374, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
| Variables | Never Users | Ever Users | Standardized Difference | ||
|---|---|---|---|---|---|
| (n = 11,011) | (n = 11,011) | ||||
| n | % | n | % | ||
| Demographic data | |||||
| Age (years) | 58.75 | 10.10 | 58.71 | 9.71 | −0.38 |
| Sex (men) | 6218 | 56.47 | 6294 | 57.16 | 1.37 |
| Diabetes duration (years) | 6.46 | 2.78 | 6.41 | 2.58 | −1.94 |
| Occupation | |||||
| I | 4334 | 39.36 | 4403 | 39.99 | |
| II | 2607 | 23.68 | 2593 | 23.55 | −0.21 |
| III | 2074 | 18.84 | 2043 | 18.55 | −0.78 |
| IV | 1996 | 18.13 | 1972 | 17.91 | −0.60 |
| Living region | |||||
| Taipei | 4324 | 39.27 | 4341 | 39.42 | |
| Northern | 1134 | 10.30 | 1115 | 10.13 | −0.62 |
| Central | 1790 | 16.26 | 1759 | 15.97 | −0.79 |
| Southern | 1390 | 12.62 | 1344 | 12.21 | −1.24 |
| Kao-Ping and Eastern | 2373 | 21.55 | 2452 | 22.27 | 1.66 |
| Major comorbidities associated with diabetes mellitus | |||||
| Hypertension | 8927 | 81.07 | 8810 | 80.01 | −2.64 |
| Dyslipidemia | 9507 | 86.34 | 9477 | 86.07 | −0.72 |
| Obesity | 716 | 6.50 | 725 | 6.58 | 0.31 |
| Diabetes-related complications | |||||
| Nephropathy | 2704 | 24.56 | 2600 | 23.61 | −2.22 |
| Eye disease | 3766 | 34.20 | 3749 | 34.05 | −0.33 |
| Stroke | 2321 | 21.08 | 2306 | 20.94 | −0.37 |
| Ischemic heart disease | 4437 | 40.30 | 4383 | 39.81 | −0.91 |
| Peripheral arterial disease | 2427 | 22.04 | 2492 | 22.63 | 1.43 |
| Other major risk factors of dementia | |||||
| Head injury | 366 | 3.32 | 337 | 3.06 | −1.59 |
| Parkinson’s disease | 131 | 1.19 | 130 | 1.18 | −0.10 |
| Hypoglycemia | 301 | 2.73 | 328 | 2.98 | 1.38 |
| Potential risk factors of cancer | |||||
| Chronic obstructive pulmonary disease | 4644 | 42.18 | 4690 | 42.59 | 0.77 |
| Tobacco abuse | 464 | 4.21 | 458 | 4.16 | −0.25 |
| Alcohol-related diagnoses | 615 | 5.59 | 628 | 5.70 | 0.47 |
| Antidiabetic drugs | |||||
| Insulin | 360 | 3.27 | 343 | 3.12 | −0.92 |
| Sulfonylureas | 7892 | 71.67 | 7855 | 71.34 | −0.65 |
| Metformin | 8359 | 75.91 | 8338 | 75.72 | −0.27 |
| Meglitinide | 728 | 6.61 | 739 | 6.71 | 0.50 |
| Acarbose | 1432 | 13.01 | 1500 | 13.62 | 1.81 |
| Medications commonly used in diabetes patients | |||||
| Angiotensin converting enzyme inhibitors/angiotensin receptor blockers | 8028 | 72.91 | 8013 | 72.77 | −0.24 |
| Calcium channel blockers | 6043 | 54.88 | 6011 | 54.59 | −0.54 |
| Statins | 8226 | 74.71 | 8274 | 75.14 | 1.03 |
| Fibrates | 5054 | 45.90 | 5042 | 45.79 | −0.18 |
| Aspirin | 6223 | 56.52 | 6266 | 56.91 | 0.86 |
| Pioglitazone Use | n | N | Person-Years | Incidence Rate (Per 100,000 Person-Years) | HR | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|
| Never users | 123 | 11,011 | 28,378.04 | 433.43 | 1.000 | ||
| Ever users | 91 | 11,011 | 29,612.81 | 307.30 | 0.716 | (0.545–0.940) | 0.0163 |
| Tertiles of cumulative duration of pioglitazone therapy (months) | |||||||
| Never users | 123 | 11,011 | 28,378.04 | 433.43 | 1.000 | ||
| <11.0 | 32 | 3636 | 9537.25 | 335.53 | 0.806 | (0.544–1.193) | 0.2809 |
| 11.0–19.6 | 27 | 3613 | 9746.22 | 277.03 | 0.654 | (0.430–0.994) | 0.0467 |
| >19.6 | 32 | 3762 | 10,329.34 | 309.80 | 0.694 | (0.469–1.026) | 0.0670 |
| Cumulative duration of pioglitazone therapy treated as a continuous variable | |||||||
| For every 1-month increment of pioglitazone use | 0.987 | (0.976–0.998) | 0.0246 | ||||
| Metformin Use/Pioglitazone Use | n | N | Person-Years | Incidence Rate (Per 100,000 Person-Years) | HR | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|
| Metformin ever users | |||||||
| Pioglitazone never users | 85 | 8359 | 21,706.67 | 391.58 | 1.000 | ||
| Pioglitazone ever users | 66 | 8338 | 22,445.80 | 294.04 | 0.802 | (0.580–1.109) | 0.1822 |
| Tertiles of cumulative duration of pioglitazone therapy (months) | |||||||
| Never users | 85 | 8359 | 21,706.67 | 391.58 | 1.000 | ||
| <11.0 | 23 | 2781 | 7319.59 | 314.23 | 0.874 | (0.549–1.392) | 0.5719 |
| 11.0–19.6 | 6 | 2732 | 7379.70 | 81.30 | 0.603 | (0.352–1.032) | 0.0649 |
| >19.6 | 27 | 2825 | 7746.51 | 348.54 | 0.915 | (0.591–1.417) | 0.6920 |
| Cumulative duration of pioglitazone therapy treated as a continuous variable | |||||||
| For every 1-month increment of pioglitazone use | 0.991 | (0.978–1.004) | 0.1772 | ||||
| Metformin never users | |||||||
| Pioglitazone never users | 38 | 2652 | 6671.38 | 569.60 | 1.000 | ||
| Pioglitazone ever users | 25 | 2673 | 7167.01 | 348.82 | 0.494 | (0.284–0.857) | 0.0121 |
| Tertiles of cumulative duration of pioglitazone therapy (months) | |||||||
| Never users | 38 | 2652 | 6671.38 | 569.60 | 1.000 | ||
| <11.0 | 9 | 855 | 2217.66 | 405.83 | 0.588 | (0.272–1.273) | 0.1778 |
| 11.0–19.6 | 11 | 881 | 2366.52 | 464.82 | 0.690 | (0.338–1.409) | 0.3084 |
| >19.6 | 5 | 937 | 2582.84 | 193.59 | 0.265 | (0.102–0.688) | 0.0064 |
| Cumulative duration of pioglitazone therapy treated as a continuous variable | |||||||
| For every 1-month increment of pioglitazone use | 0.974 | (0.952–0.998) | 0.0306 | ||||
| Major Risk Factor/Pioglitazone Use | n | N | Person-Years | HR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Stroke (+)/Pioglitazone (−) | 72 | 2321 | 5986.79 | 1.000 | ||
| Stroke (+)/Pioglitazone (+) | 46 | 2306 | 6153.77 | 0.617 | (0.425–0.895) | 0.0110 |
| Stroke (−)/Pioglitazone (−) | 51 | 8690 | 22,391.25 | 0.317 | (0.217–0.463) | <0.0001 |
| Stroke (−)/Pioglitazone (+) | 45 | 8705 | 23,459.04 | 0.271 | (0.183–0.402) | <0.0001 |
| Hypoglycemia (+)/Pioglitazone (−) | 13 | 301 | 789.68 | 1.000 | ||
| Hypoglycemia (+)/Pioglitazone (+) | 11 | 328 | 869.14 | 0.775 | (0.346–1.737) | 0.5356 |
| Hypoglycemia (−)/Pioglitazone (−) | 110 | 10,710 | 27,588.36 | 0.430 | (0.239–0.773) | 0.0048 |
| Hypoglycemia (−)/Pioglitazone (+) | 80 | 10,683 | 28,743.67 | 0.304 | (0.167–0.554) | 0.0001 |
| Head injury (+)/Pioglitazone (−) | 6 | 366 | 926.96 | 1.000 | ||
| Head injury (+)/Pioglitazone (+) | 8 | 337 | 891.60 | 1.365 | (0.472–3.951) | 0.5655 |
| Head injury (−)/Pioglitazone (−) | 117 | 10,645 | 27,451.08 | 0.954 | (0.417–2.180) | 0.9102 |
| Head injury (−)/Pioglitazone (+) | 83 | 10,674 | 28,721.21 | 0.652 | (0.283–1.502) | 0.3149 |
| Parkinson’s disease (+)/Pioglitazone (−) | 8 | 131 | 330.96 | 1.000 | ||
| Parkinson’s disease (+)/Pioglitazone (+) | 7 | 130 | 351.72 | 0.768 | (0.276–2.132) | 0.6119 |
| Parkinson’s disease (−)/Pioglitazone (−) | 115 | 10,880 | 28,047.08 | 0.396 | (0.191–0.822) | 0.0129 |
| Parkinson’s disease (−)/Pioglitazone (+) | 84 | 10,881 | 29,261.09 | 0.282 | (0.135–0.591) | 0.0008 |
| Any of the four (+)/Pioglitazone (−) | 80 | 2779 | 7168.34 | 1.000 | ||
| Any of the four (+)/Pioglitazone (+) | 52 | 2784 | 7430.66 | 0.635 | (0.447–0.903) | 0.0113 |
| Any of the four (−)/Pioglitazone (−) | 43 | 8232 | 21,209.70 | 0.282 | (0.192–0.415) | <0.0001 |
| Any of the four (−)/Pioglitazone (+) | 39 | 8227 | 22,182.15 | 0.249 | (0.167–0.372) | <0.0001 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-H. Pioglitazone Reduces Dementia Risk in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Analysis. J. Clin. Med. 2018, 7, 306. https://doi.org/10.3390/jcm7100306
Tseng C-H. Pioglitazone Reduces Dementia Risk in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Analysis. Journal of Clinical Medicine. 2018; 7(10):306. https://doi.org/10.3390/jcm7100306
Chicago/Turabian StyleTseng, Chin-Hsiao. 2018. "Pioglitazone Reduces Dementia Risk in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Analysis" Journal of Clinical Medicine 7, no. 10: 306. https://doi.org/10.3390/jcm7100306
APA StyleTseng, C.-H. (2018). Pioglitazone Reduces Dementia Risk in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Analysis. Journal of Clinical Medicine, 7(10), 306. https://doi.org/10.3390/jcm7100306





