FOLFIRINOX Chemotherapy in Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Retrospective and Phase II Studies
Abstract
:1. Introduction
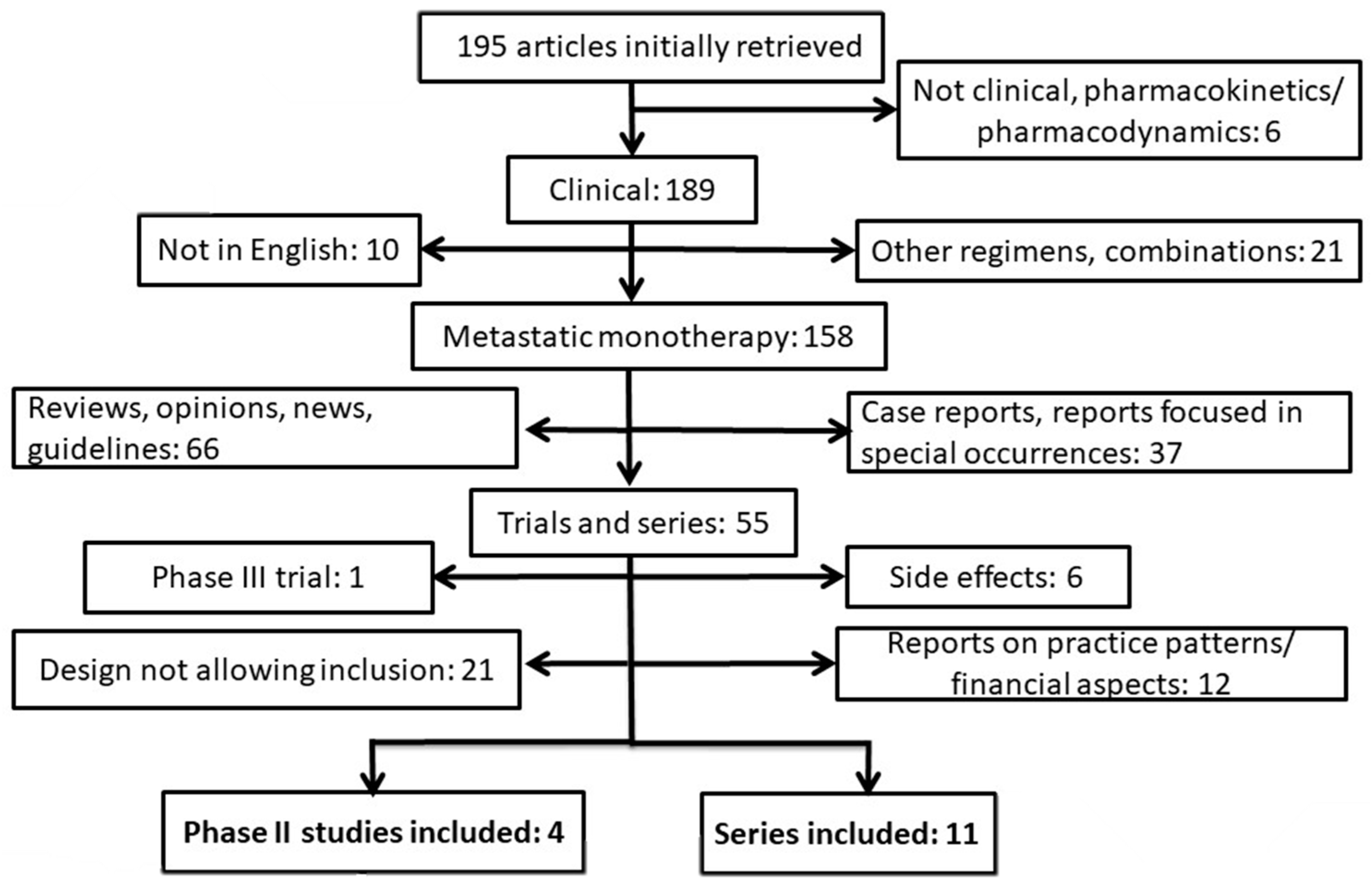
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Mettu, N.B.; Abbruzzese, J.L. Clinical insights into the biology and treatment of pancreatic cancer. J. Oncol. Pract. 2016, 12, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed]
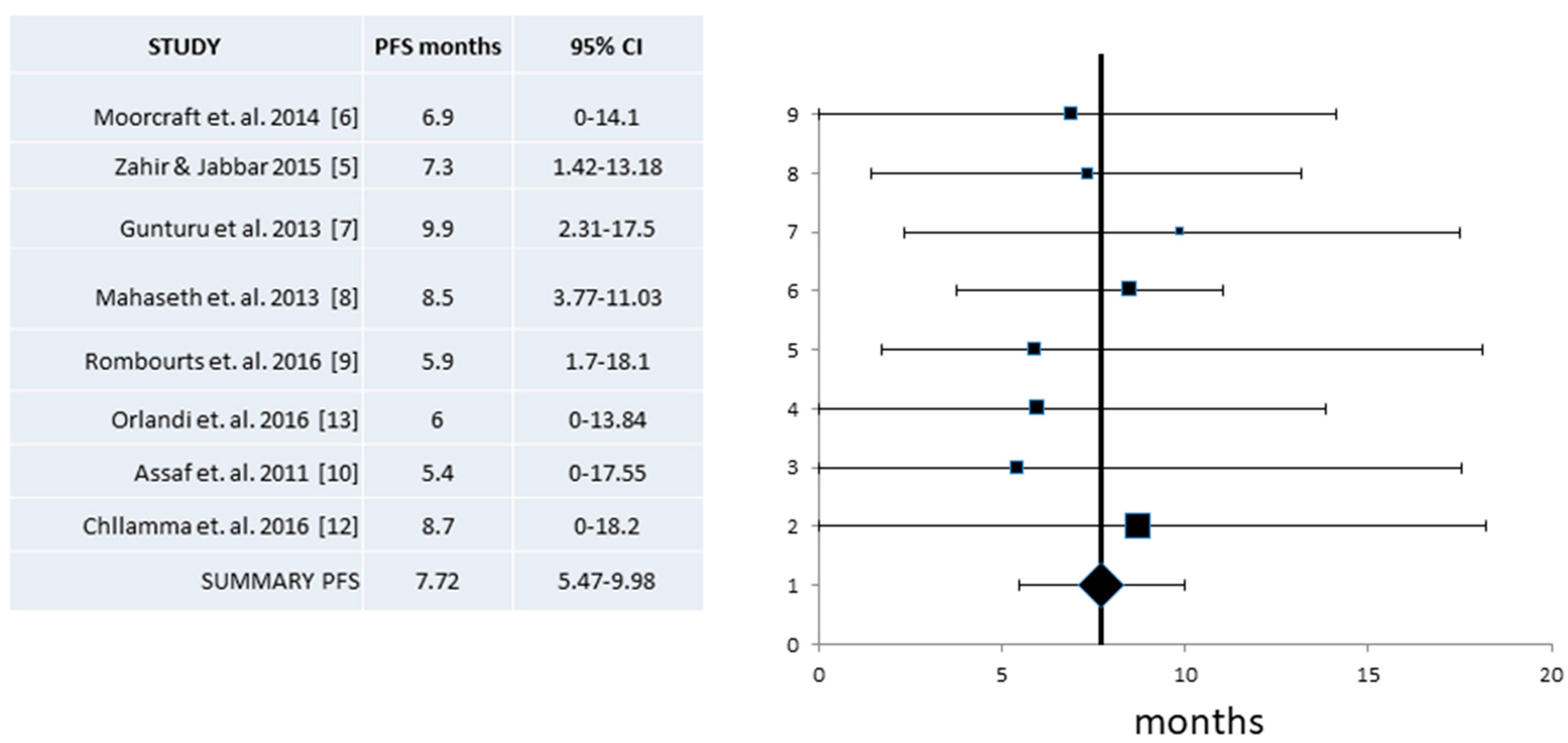
- Zahir, M.N.; Jabbar, A.A. Metastatic pancreatic carcinoma and experience with FOLFIRINOX—A cross sectional analysis from a developing country. Asian Pac. J. Cancer Prev. 2015, 16, 6001–6006. [Google Scholar] [CrossRef] [PubMed]
- Moorcraft, S.Y.; Khan, K.; Peckitt, C.; Watkins, D.; Rao, S.; Cunningham, D.; Chau, I. FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: The Royal Marsden experience. Clin. Colorectal Cancer 2014, 13, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Gunturu, K.S.; Yao, X.; Cong, X.; Thumar, J.R.; Hochster, H.S.; Stein, S.M.; Lacy, J. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: Single institution retrospective review of efficacy and toxicity. Med. Oncol. 2013, 30, 361. [Google Scholar] [CrossRef] [PubMed]
- Mahaseth, H.; Brutcher, E.; Kauh, J.; Hawk, N.; Kim, S.; Chen, Z.; Kooby, D.A.; Maithel, S.K.; Landry, J.; El-Rayes, B.F. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013, 42, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, S.J.; Mungroop, T.H.; Heilmann, M.N.; van Laarhoven, H.W.; Busch, O.R.; Molenaar, I.Q.; Besselink, M.G.; Wilmink, J.W. FOLFIRINOX in locally advanced and metastatic pancreatic cancer: A single centre cohort study. J. Cancer 2016, 7, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Assaf, E.; Verlinde-Carvalho, M.; Delbaldo, C.; Grenier, J.; Sellam, Z.; Pouessel, D.; Bouaita, L.; Baumgaertner, I.; Sobhani, I.; Tayar, C.; et al. 5-Fluorouracil/Leucovorin combined with Irinotecan and Oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology 2011, 80, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Peddi, P.F.; Lubner, S.; McWilliams, R.; Tan, B.R.; Picus, J.; Sorscher, S.M.; Suresh, R.; Lockhart, A.C.; Wang, J.; Menias, C.; et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. J. Pancreas 2012, 13, 497–501. [Google Scholar]
- Chllamma, M.; Cook, N.; Dhani, N.C.; Giby, K.; Dodd, A.; Wang, L.; Hedley, D.W.; Moore, M.J.; Knox, J.J. FOLFIRINOX for advanced pancreatic cancer: The Princess Margaret Cancer Centre experience. Br. J. Cancer 2016, 115, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, A.; Calegari, M.A.; Martini, M.; Cocomazzi, A.; Bagalà, C.; Indellicati, G.; Zurlo, V.; Basso, M.; Cassano, A.; Larocca, L.M.; et al. Gemcitabine versus FOLFIRINOX in patients with advanced pancreatic adenocarcinoma hENT1-positive: Everything was not too bad back when everything seemed worse. Clin. Transl. Oncol. 2016, 18, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Lorgis, V.; Chauffert, B.; Gentil, J.; Ghiringhelli, F. Influence of localization of primary tumor on effectiveness of 5-Fluorouracil/Leucovorin combined with Irinotecan and Oxaliplatin (FOLFIRINOX) in patients with metastatic pancreatic adenocarcinoma: A retrospective study. Anticancer Res. 2012, 32, 4125–4130. [Google Scholar] [PubMed]
- Hann, A.; Bohle, W.; Egger, J.; Zoller, W.G. Improvement in advanced pancreatic cancer survival with novel chemotherapeutic strategies—Experience of a community based hospital. Z. Gastroenterol. 2016, 54, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Paillot, B.; François, E.; Bugat, R.; Jacob, J.-H.; Stein, U.; Nasca, S.; Metges, J.-P.; Rixe, O.; Michel, P.; et al. Irinotecan plus Oxaliplatin and Leucovorin-modulated Fluorouracil in advanced pancreatic cancer—A Groupe Tumeurs Digestives of the Fédération Nationale des Centres de Lutte Contre le Cancer Study. J. Clin. Oncol. 2005, 23, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Shimamura, T.; Tokuhisa, M.; Goto, A.; Endo, I.; Ichikawa, Y. Effect of FOLFIRINOX as a second-line chemotherapy for metastatic pancreatic cancer after gemcitabine-based chemotherapy failure. Medicine 2017, 96, e6769. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.M.; James, E.S.; Deng, Y.; Cong, X.; Kortmansky, J.S.; Li, J.; Staugaard, C.; Indukala, D.; Boustani, A.M.; Patel, V.; et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br. J. Cancer 2016, 114, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Ikeda, M.; Fukutomi, A.; Ioka, T.; Furuse, J.; Ohkawa, S.; Isayama, H.; Boku, N. Phase II study of FOLFIRINOX for chemotherapy-naїve Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014, 105, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
| Study [Reference] | Year of Publication | Country | Number of Patients | RR (%) | DCR (%) |
|---|---|---|---|---|---|
| Zahir & Jabbar [5] | 2015 | Pakistan | 23 | ? | ? |
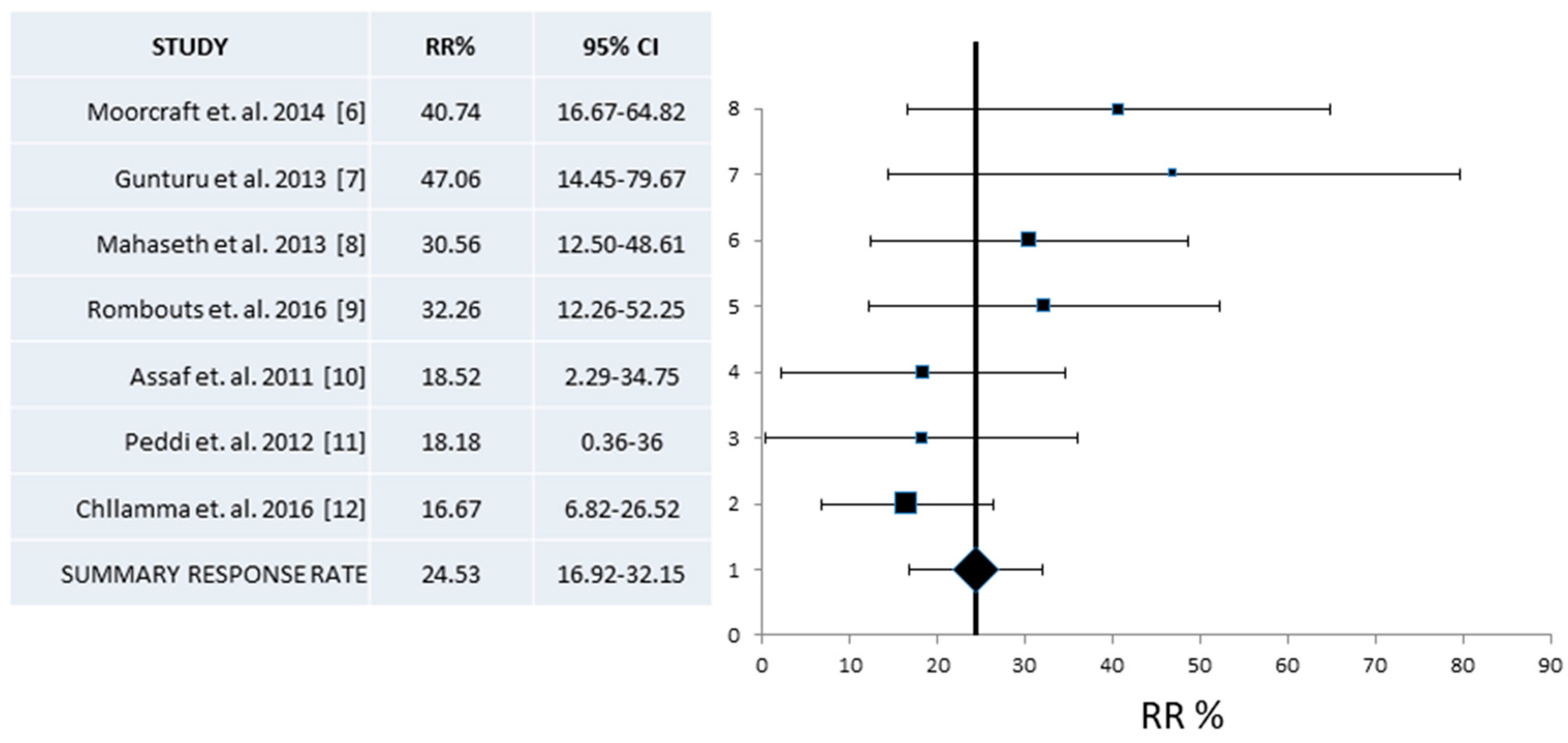
| Moorcraft et al. [6] | 2014 | United Kingdom | 27 | 40.7 | ? |
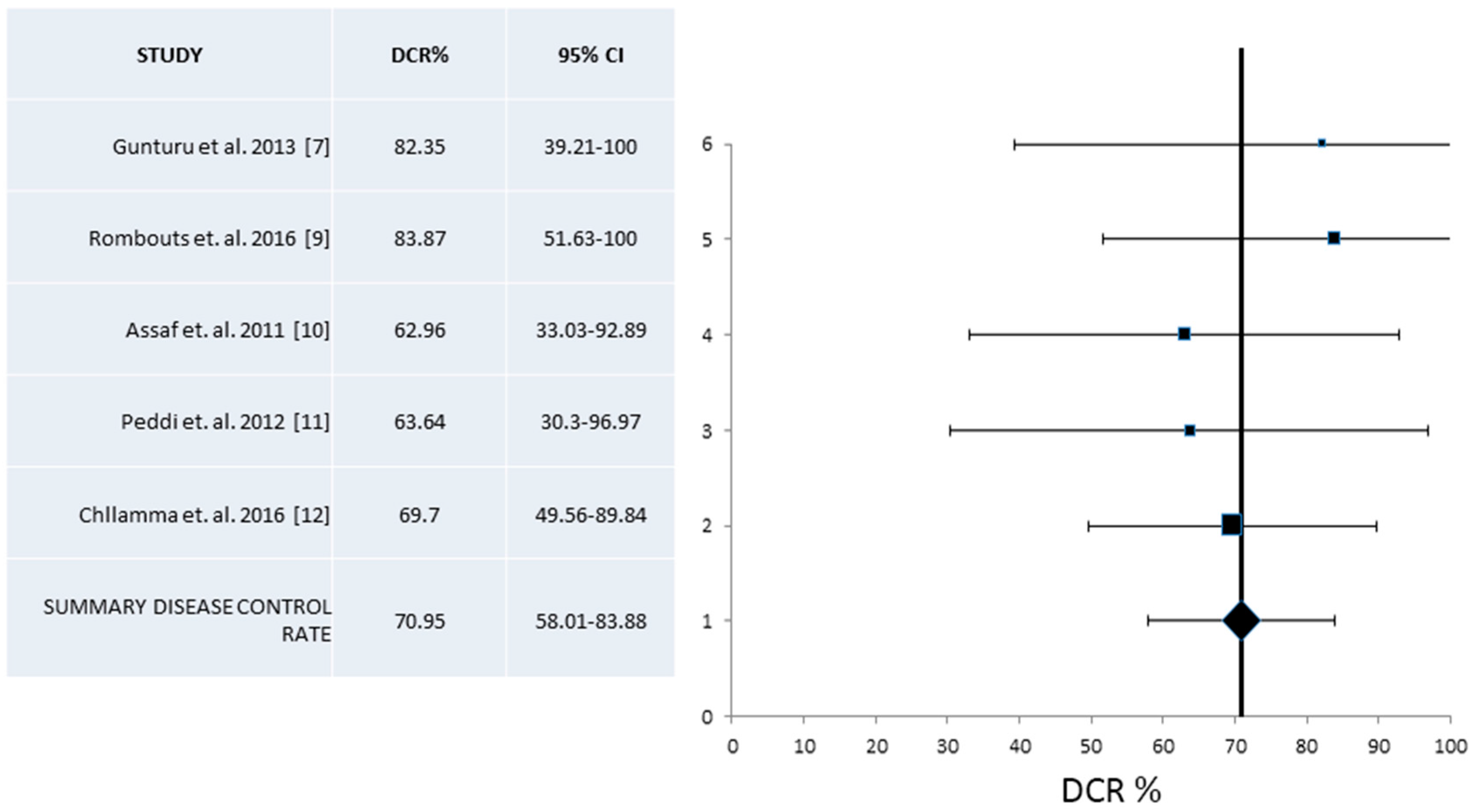
| Guruntu et al. [7] | 2013 | United States | 19 | 47.1 | 82.4 |
| Mahaseth et al. [8] | 2013 | United States | 36 | 30.6 | ? |
| Rambouts et al. [9] | 2016 | The Netherlands | 32 | 32.3 | 83.4 |
| Assaf et al. [10] | 2011 | France | 27 | 18.5 | 63.0 |
| Peddi et al. [11] | 2012 | United States | 22 | 18.2 | 63.6 |
| Chllamma et al. [12] | 2016 | Canada | 66 | 16.7 | 69.7 |
| Orlandi et al. [13] | 2016 | Italy | 36 | ? | ? |
| Lorgis et al. [14] | 2012 | France | 42 | ? | ? |
| Hann et al. [15] | 2016 | Germany | 24 | ? | ? |
| Study [Reference] | Year of Publication | Country | Number of Patients | RR (%) | DCR (%) |
|---|---|---|---|---|---|
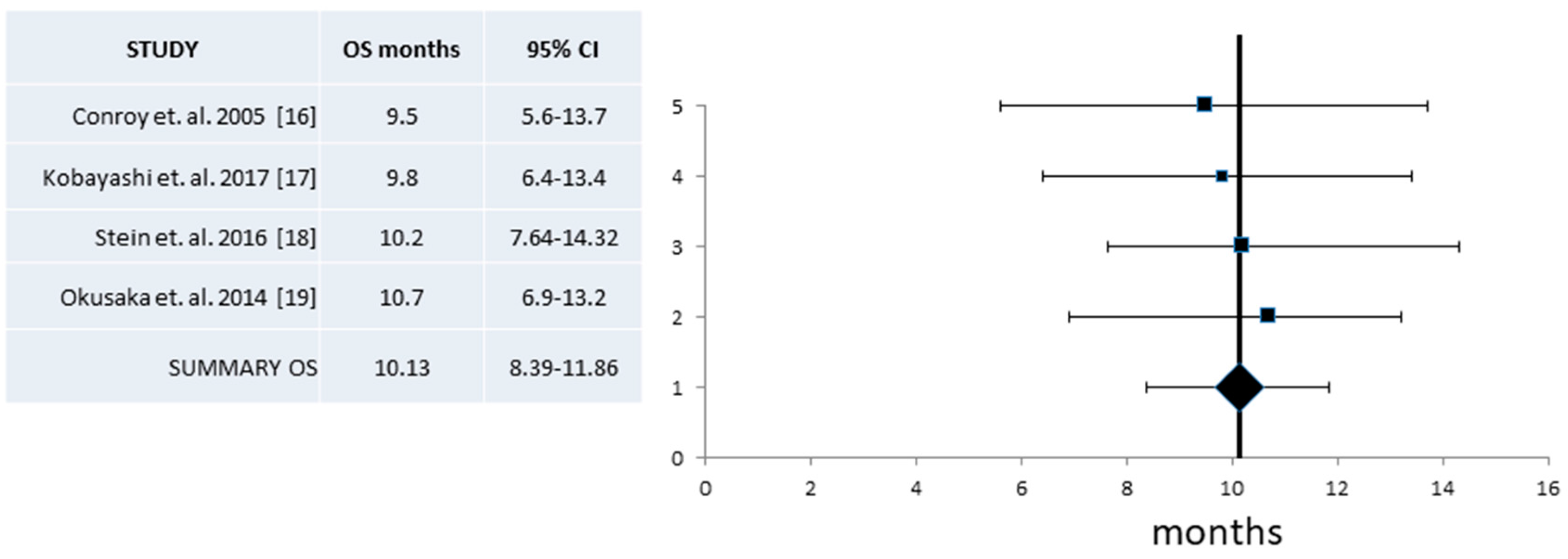
| Conroy et. al. [16] | 2005 | France | 36 | 25 | ? |
| Kobayashi et al. [17] | 2017 | Japan | 18 | 22.2 | 61.1 |
| Stein et. al. [18] | 2016 | United States | 37 | 35.1 | 86.5 |
| Okusaka et. al. [19] | 2014 | Japan | 36 | 38.9 | 69.4 |
| Patients and Disease Variables | Pooled Series Pts (%) | Pooled Series: # of Pts/# of Series | Pooled Phase II Pts (%) | Pooled Phase II: # of Pts/# of Series | Phase III Trial |
|---|---|---|---|---|---|
| Study Size | |||||
| Evaluable Patients | 368 | 368/11 | 126 | 126/4 | 171 |
| Demographics | |||||
| Age—Mean | 52–63 | 101/4 | |||
| Age—Median | 61.5–63 | 91/3 | 61 (25–76) | ||
| Performance Status | |||||
| ECOG 0 | 80 (51.6) | 155/5 | 51 (56.0) | 91/3 | 64 (37.4) |
| ECOG 1 | 56 (36.1) | 155/5 | 40 (44.0) | 91/3 | 106 (62.0) |
| ECOG 2 | 19 (12.3) | 155/5 | 0 | 1 (0.6) | |
| Primary Location | |||||
| Head | 82 (57.3) | 143/5 | 29 (55.8) | 52/3 | 67 (40.6) |
| Body/Tail | 61 (42.7) | 143/5 | 23 (44.2) | 52/3 | 98 (59.4) |
| Metastatic Sites | |||||
| 1 | 49 (46.7) | 105/3 | 2 (Median) | ||
| ≥2 | 56 (53.3) | 105/3 | 1–6 (Range) | ||
| Metastatic Organs | |||||
| Liver | 49 (71.0) | 69/2 | 64 (70.3) | 91/3 | 149 (87.1) |
| Lymph Nodes | 29 (42.0) | 69/2 | 39 (42.9) | 91/3 | 49 (28.7) |
| Lung | 21 (30.4) | 69/2 | 13 (14.3) | 91/3 | 33 (19.3) |
| Peritoneal | 18 (26.1) | 69/2 | 14 (15.4) | 91/3 | 33 (19.3) |
| Prior Lines of Chemotherapy | |||||
| 0 | 78 (83.9) | 93/4 | 171 | ||
| ≥1 | 15 (16.1) | 93/4 | 0 | ||
| FOLFIRINOX Regimen | |||||
| Median Cycles | 4–16 | 118/4 | 6–9 | 91/3 | |
| Dose Reductions | 34 (65.4) | 55/2 | 69 | 70/2 | |
| Efficacy | |||||
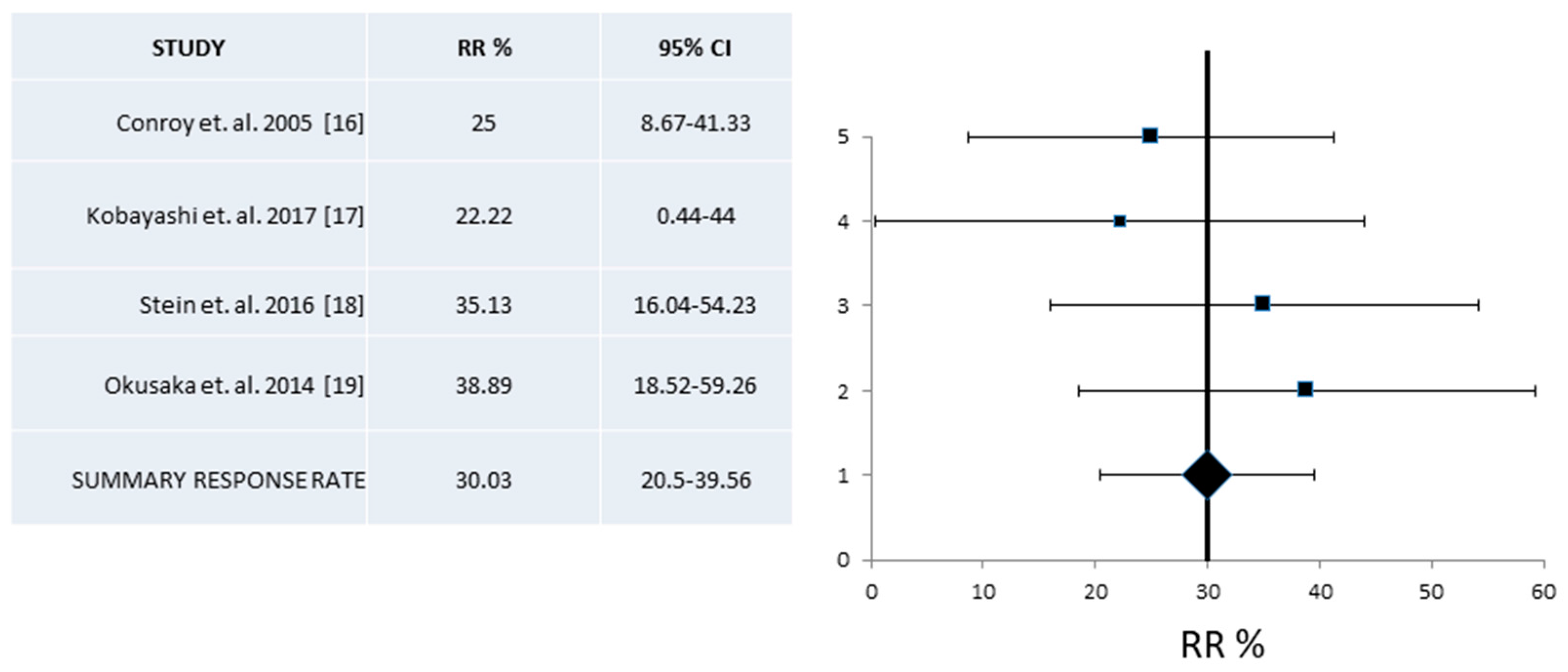
| RR% (95% CI) | 24.5 (16.9–32.1) | 229/7 | 30 (20.5–39.6) | 127/4 | 31.6 (24.7–39.1) |
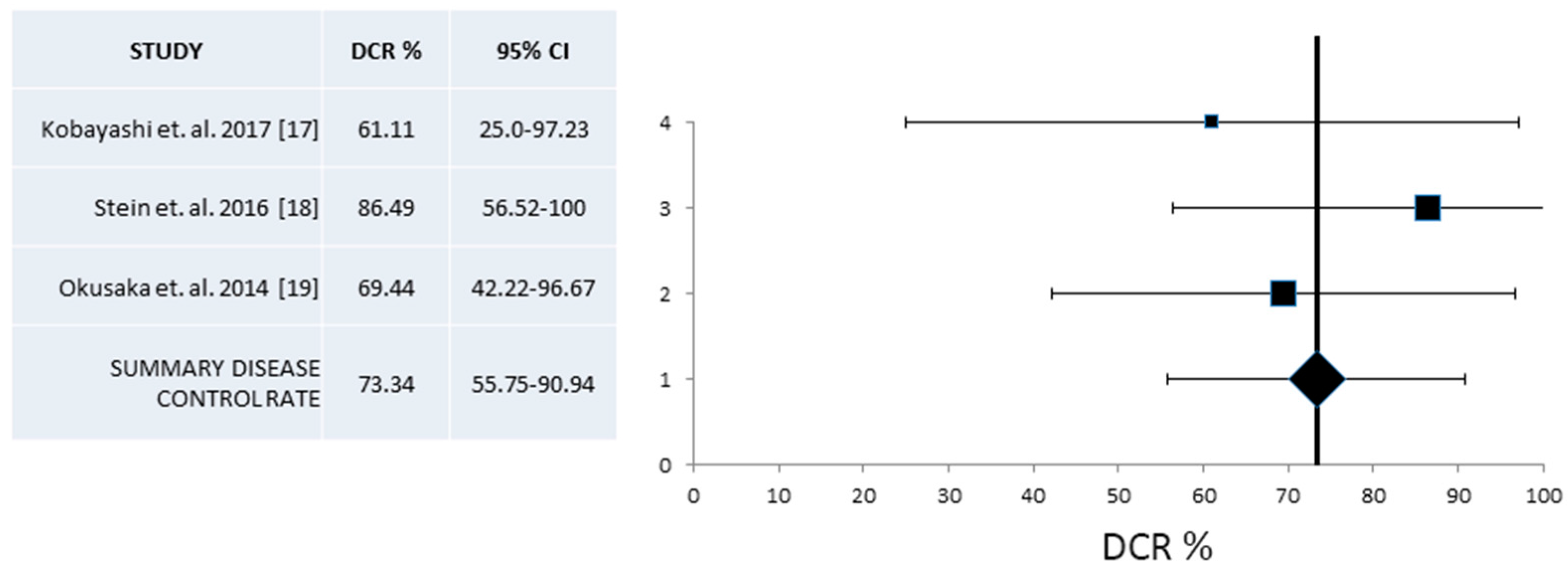
| DCR% (95% CI) | 70.95 (58.0–83.9) | 163/5 | 73.34 (55.7–90.9) | 91/3 | 70.2 (62.7–76.9) |
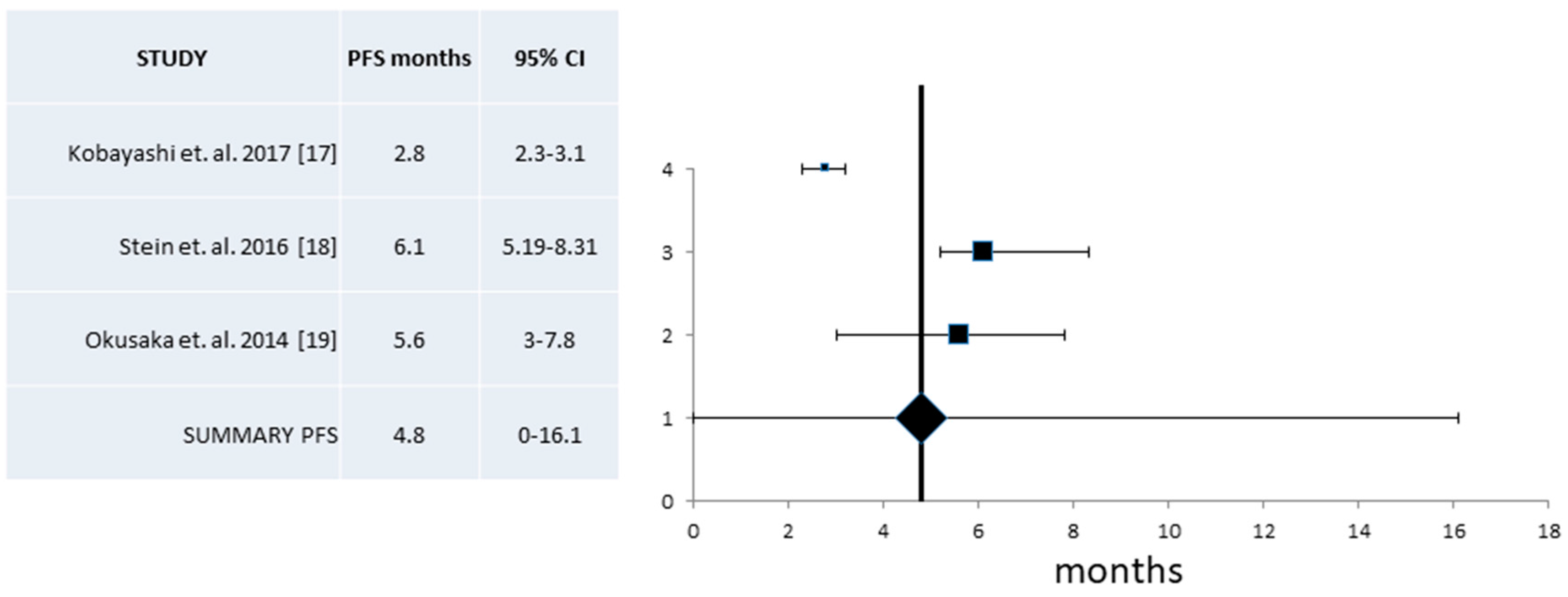
| Median PFS (Mos) (95% CI) | 7.72 (5.47–9.98) | 266/8 | 4.8 (0–16.1) | 91/3 | 6.4 (5.5–7.2) |
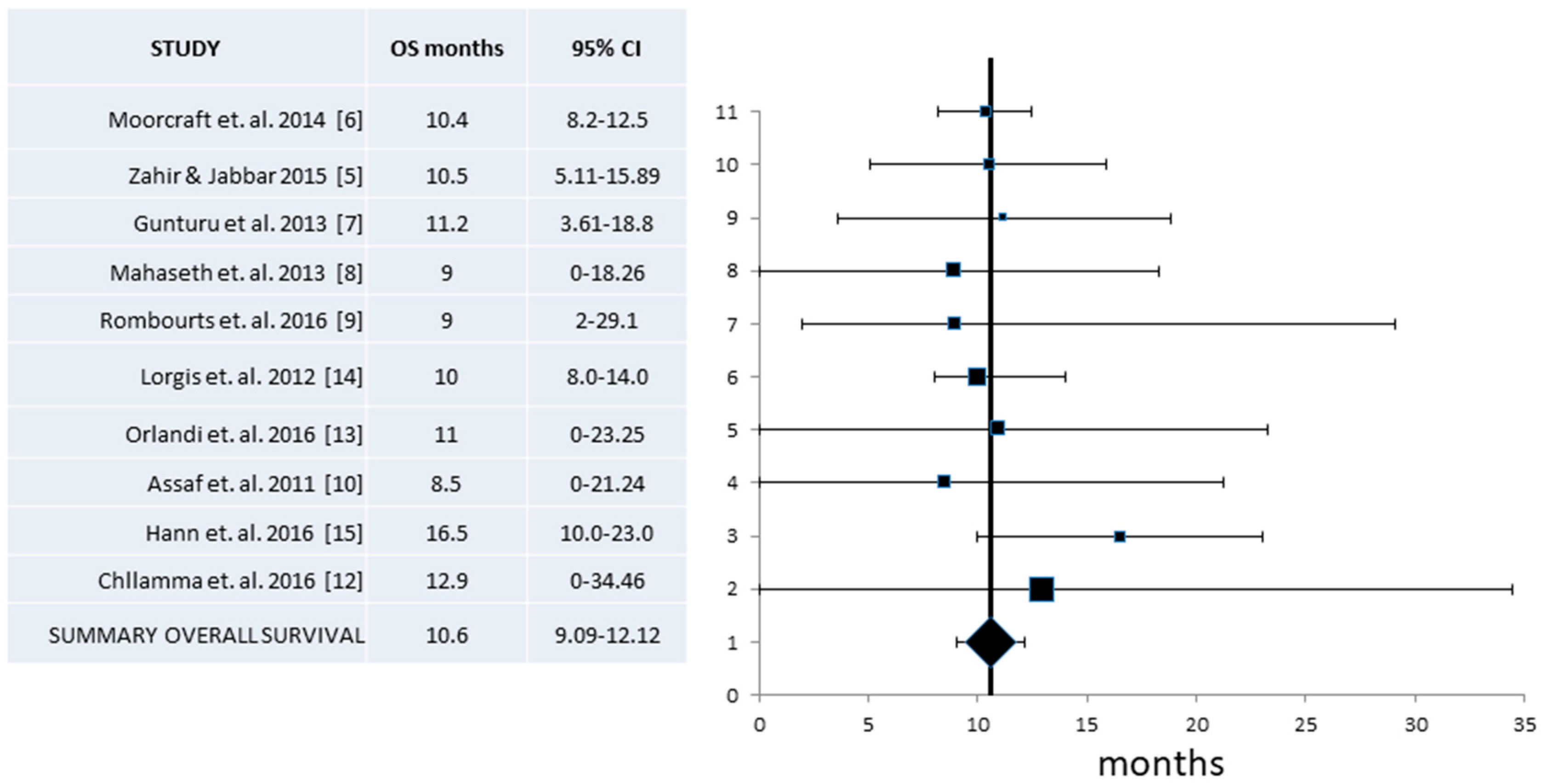
| Median OS (Mos) (95% CI) | 10.6 (9.09–12.12) | 332/10 | 10.13 (8.4–11.9) | 127/4 | 11.1 (9.0–13.1) |
| Toxicity | Pooled Series (%) | Pooled Series # of Pts/# of Series | Pooled Phase II (%) | Pooled Phase II: # of Pts/# of Series | Phase III Trial N = 171 |
|---|---|---|---|---|---|
| Grade 3–4 | |||||
| Neutropenia | 18.1 | 514/10 | 18.3 | 174/4 | 45.7 |
| Febrile Neutropenia | 4.3 | 352/9 | 3.5 | 174/4 | 5.4 |
| Anemia | 2.6 | 371/7 | 4.8 | 174/4 | 7.8 |
| Thrombocytopenia | 4.7 | 537/11 | 4.0 | 174/4 | 9.1 |
| Neuropathy | 4.1 | 272/7 | 2.8 | 174/4 | 9.0 |
| Nausea/Vomiting | 10.2 | 457/10 | 5.5 | 174/4 | 14.5 |
| Diarrhea | 6.3 | 457/10 | 5.8 | 174/4 | 12.7 |
| Asthenia/Fatigue | 4.8 | 296/5 | 23.6 | ||
| Mucositis | 4.0 | 132/3 | |||
| Sepsis | 2.5 | 69/2 | |||
| All Grades | |||||
| Neutropenia | 31.2 | 156/5 | 24.0 | 54/2 | |
| Febrile Neutropenia | 4.0 | 190/3 | 4.5 | 54/2 | |
| Anemia | 35.7 | 171/3 | 24.0 | 54/2 | |
| Thrombocytopenia | 27.4 | 282/5 | 22.0 | 54/2 | |
| Neuropathy | 27.3 | 141/3 | 15.0 | 54/2 | |
| Nausea/Vomiting | 35.0 | 271/4 | 24.0 | 54/2 | |
| Diarrhea | 17.8 | 282/5 | 21.5 | 54/2 | |
| Asthenia/Fatigue | 23.4 | 282/5 | |||
| Mucositis | 9.0 | 99/2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thibodeau, S.; Voutsadakis, I.A. FOLFIRINOX Chemotherapy in Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Retrospective and Phase II Studies. J. Clin. Med. 2018, 7, 7. https://doi.org/10.3390/jcm7010007
Thibodeau S, Voutsadakis IA. FOLFIRINOX Chemotherapy in Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Retrospective and Phase II Studies. Journal of Clinical Medicine. 2018; 7(1):7. https://doi.org/10.3390/jcm7010007
Chicago/Turabian StyleThibodeau, Stephane, and Ioannis A. Voutsadakis. 2018. "FOLFIRINOX Chemotherapy in Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Retrospective and Phase II Studies" Journal of Clinical Medicine 7, no. 1: 7. https://doi.org/10.3390/jcm7010007
APA StyleThibodeau, S., & Voutsadakis, I. A. (2018). FOLFIRINOX Chemotherapy in Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Retrospective and Phase II Studies. Journal of Clinical Medicine, 7(1), 7. https://doi.org/10.3390/jcm7010007





