Role of Autoantibodies in the Diagnosis of Connective-Tissue Disease ILD (CTD-ILD) and Interstitial Pneumonia with Autoimmune Features (IPAF)
Abstract
1. Background
2. Diagnosis of CTD-ILD
2.1. Clinical Features of CTD-ILD
2.2. Radiological Features of CTD-ILD
2.3. Histopathological Features of CTD-ILD
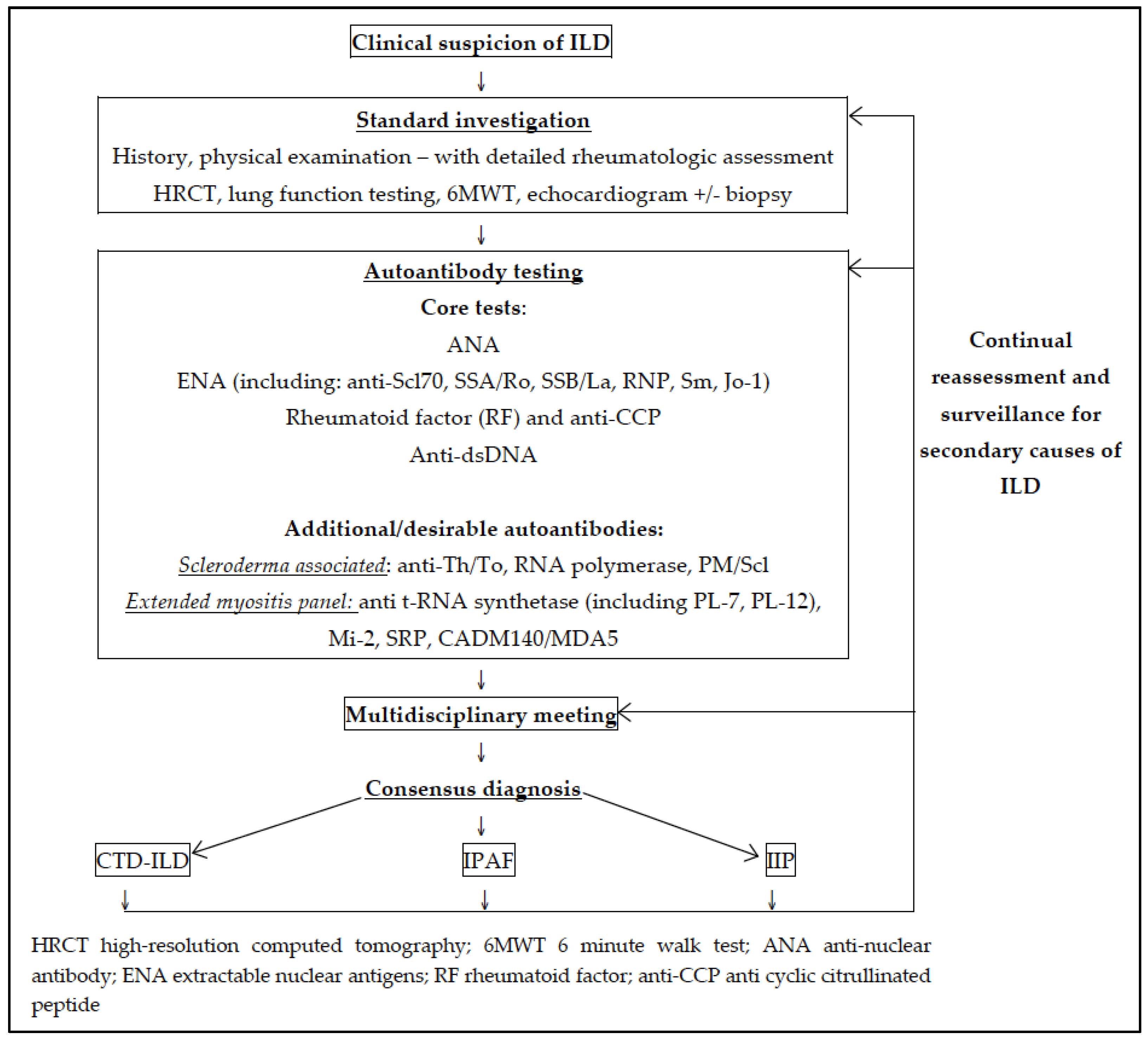
2.4. Role of Autoantibodies in the Diagnosis of CTD-ILD
3. CTD Associated Autoantibodies
3.1. Antinuclear Antibodies (ANA) and Antibodies Associated with Systemic-Sclerosis
3.1.1. Anti-topoisomerase I antibodies (ATA; anti-Scl70)
3.1.2. Anti-Centromere Antibodies (ACA)
3.1.3. Anti-RNA Polymerase (RNA pol) Antibodies
3.1.4. Other SSc-Associated Autoantibodies
Anti Th/To Antibodies
Anti-PM/Scl Antibodies
Antibodies to Small Nuclear Ribonucleoprotein (Anti-U3, anti-U1 RNP)
Anti-Histone Antibodies
Other
3.2. Rheumatoid Factor (RF) and Anti-Citrullinated Cyclic Peptide Antibodies (anti-CCP)
3.3. Myositis Autoantibodies (Including tRNA Synthetase Antibodies)
3.3.1. Anti t-RNA Synthetase Antibodies
3.3.2. Anti CADM140/MDA5 Antibodies
3.3.3. Anti-Mi2 Antibodies
3.3.4. Anti-SRP Antibody
3.4. Anti-SSA/Ro60, Anti-Ro52 and Anti SSB/La Antibodies
3.5. Anti-dsDNA and Anti-Sm Antibodies
4. Autoantibodies and Interstitial Pneumonia with Autoimmune Features
5. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, D.S.; Park, I.N.; Jang, S.J.; Kitaichi, M.; Nicholson, A.G.; Colby, T.V. Prognosis of fibrotic interstitial pneumonia: Idiopathic versus collagen vascular disease-related subtypes. Am. J. Respir. Crit. Care Med. 2007, 175, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.J.; Chartrand, S.; Fischer, A. Current approach to connective tissue disease-associated interstitial lung disease. Curr. Opin. Pulm. Med. 2014, 20, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Strek, M.E. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest 2013, 143, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; du Bois, R. Interstitial lung disease in connective tissue disorders. Lancet 2012, 380, 689–698. [Google Scholar] [CrossRef]
- Tzelepis, G.E.; Toya, S.P.; Moutsopoulos, H.M. Occult connective tissue diseases mimicking idiopathic interstitial pneumonias. Eur. Respir. J. 2008, 31, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mittoo, S.; Gelber, A.C.; Christopher-Stine, L.; Horton, M.R.; Lechtzin, N.; Danoff, S.K. Ascertainment of collagen vascular disease in patients presenting with interstitial lung disease. Respir. Med. 2009, 103, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Castelino, F.V.; Varga, J. Interstitial lung disease in connective tissue diseases: Evolving concepts of pathogenesis and management. Arthritis Res. Ther. 2010, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Corte, T.J.; Copley, S.J.; Desai, S.R.; Zappala, C.J.; Hansell, D.M.; Nicholson, A.G.; Colby, T.V.; Renzoni, E.; Maher, T.M.; Wells, A.U. Significance of connective tissue disease features in idiopathic interstitial pneumonia. Eur. Respir. J. 2012, 39, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Castelino, F.V.; Goldberg, H.; Dellaripa, P.F. The impact of rheumatological evaluation in the management of patients with interstitial lung disease. Rheumatology 2011, 50, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Sulli, A.; Smith, V. How to perform and interpret capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2013, 27, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Pizzorni, C.; Secchi, M.E.; Sulli, A. Capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2008, 22, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Pizzorni, C.; Sulli, A.; Smith, V. Early Diagnostic and Predictive Value of Capillaroscopy in Systemic Sclerosis. Curr. Rheumatol. Rev. 2013, 9, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Cakmakci Karadogan, D.; Balkarli, A.; Onal, O.; Altinisik, G.; Cobankara, V. The role of nailfold capillaroscopy in interstitial lung diseases—Can it differentiate idiopathic cases from collagen tissue disease associated interstitial lung diseases? Tuberkuloz Toraks 2015, 63, 22–30. [Google Scholar] [CrossRef]
- Assayag, D.; Elicker, B.M.; Urbania, T.H.; Colby, T.V.; Kang, B.H.; Ryu, J.H.; King, T.E.; Collard, H.R.; Kim, D.S.; Lee, J.S. Rheumatoid arthritis-associated interstitial lung disease: Radiologic identification of usual interstitial pneumonia pattern. Radiology 2014, 270, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, S.J.; Groshong, S.; Brown, K.K.; Lynch, D.A. Nonspecific Interstitial Pneumonia: Radiologic, Clinical, and Pathologic Considerations. Radio Gr. 2009, 29, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, J.; Grimberg, A.; Thompson, B.M.; Antunes, V.B.; Jasinowodolinski, D.; Meirelles, G.S.P. Thoracic Manifestations of Collagen Vascular Diseases. Radio Gr. 2012, 32, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, J.; Arora, D.; Kanne, J.P.; Henry, T.S.; Godwin, J.D. Imaging of Pulmonary Manifestations of Connective Tissue Diseases. Radiol. Clin. N. Am. 2016, 54, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V. Significance of connective tissue diseases features in pulmonary fibrosis. Eur. Respir. Rev. 2013, 22, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.S.; Little, B.P.; Veeraraghavan, S.; Bhalla, S.; Elicker, B.M. The Spectrum of Interstitial Lung Disease in Connective Tissue Disease. J. Thorac. Imaging 2016, 31, 65–77. [Google Scholar] [CrossRef] [PubMed]
- De Lauretis, A.; Veeraraghavan, S.; Renzoni, E. Review series: Aspects of interstitial lung disease: Connective tissue disease-associated interstitial lung disease: How does it differ from IPF? How should the clinical approach differ? Chronic Respir. Dis. 2011, 8, 53–82. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V. Idiopathic interstitial pneumonias with connective tissue diseases features: A review. Respirology 2016, 21, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.J.; Chung, J.H.; Cosgrove, G.P.; Demoruelle, M.K.; Fernandez-Perez, E.R.; Fischer, A.; Frankel, S.K.; Hobbs, S.B.; Huie, T.J.; Ketzer, J.; et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 2016, 47, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Richeldi, L. Cross-disciplinary collaboration in connective tissue disease-related lung disease. Semin. Respir. Crit. Care Med. 2014, 35, 159–165. [Google Scholar] [PubMed]
- De Lauretis, A.; Sestini, P.; Pantelidis, P.; Hoyles, R.; Hansell, D.M.; Goh, N.S.; Zappala, C.J.; Visca, D.; Maher, T.M.; Denton, C.P.; et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J. Rheumatol. 2013, 40, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Colby, T.V.; Travis, W.D.; Toews, G.B.; Mumford, J.; Murray, S.; Thannickal, V.J.; Kazerooni, E.A.; Gross, B.H.; Lynch, J.P., 3rd; et al. Fibroblastic foci in usual interstitial pneumonia: Idiopathic versus collagen vascular disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Dalurzo, M.; Panse, P.; Parish, J.; Leslie, K. Usual interstitial pneumonia-pattern fibrosis in surgical lung biopsies. Clinical, radiological and histopathological clues to aetiology. J. Clin. Pathol. 2013, 66, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Do, K.H.; Kim, M.Y.; Jang, S.J.; Colby, T.V.; Kim, D.S. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest 2009, 136, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.; Vummidi, D.; Khanna, D. Management of connective tissue diseases associated interstitial lung disease: A review of the published literature. Curr. Opin. Rheumatol. 2016, 28, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D.; Conte, C.; Owens, G.R.; Medsger, T.A., Jr. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994, 37, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.J.; Patel, A.S.; Hatabu, H.; Nishino, M.; Wu, G.; Osorio, J.C.; Golzarri, M.F.; Traslosheros, A.; Chu, S.G.; Frits, M.L.; et al. Detection of Rheumatoid Arthritis-Interstitial Lung Disease Is Enhanced by Serum Biomarkers. Am. J. Respir. Crit. Care Med. 2015, 191, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Vikgren, J.; Boijsen, M.; Tylen, U.; Jorfeldt, L.; Tornling, G.; Lundberg, I.E. Interstitial lung disease in polymyositis and dermatomyositis: Longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum. 2008, 59, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Connors, G.R.; Christopher-Stine, L.; Oddis, C.V.; Danoff, S.K. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years? Chest 2010, 138, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Roca, F.; Dominique, S.; Schmidt, J.; Smail, A.; Duhaut, P.; Levesque, H.; Marie, I. Interstitial lung disease in primary Sjogren’s syndrome. Autoimmun. Rev. 1984, 46, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Park, J.K.; Roh, J.H.; Song, J.W.; Lee, C.K.; Kim, M.; Jang, S.J.; Colby, T.V.; Kim, D.S. Clinical significance of serum autoantibodies in idiopathic interstitial pneumonia. J. Korean Med. Sci. 2013, 28, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Ohtsuka, Y.; Tanimura, K.; Kusaka, H.; Munakata, M.; Kawakami, Y.; Ogasawara, H. Can interstitial pneumonia as the sole presentation of collagen vascular diseases be differentiated from idiopathic interstitial pneumonia? Respiration 1995, 62, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.F.; Solomon, D.H. The American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: Anti-DNA antibody tests. Arthritis Care Res. 2002, 47, 546–555. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Cottin, V.; du Bois, R.M.; Selman, M.; Kimura, T.; Bailes, Z.; Schlenker-Herceg, R.; Stowasser, S.; Brown, K.K. Nintedanib in patients with idiopathic pulmonary fibrosis: Combined evidence from the tomorrow and inpulsis((r)) trials. Respir. Med. 2016, 113, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G. Idiopathic pulmonary fibrosis: Guidelines for diagnosis and clinical management have advanced from consensus-based in 2000 to evidence-based in 2011. Eur. Respir. J. 2011, 37, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Arita, M.; Morita, M.; Ikeo, S.; Ito, A.; Tokioka, F.; Noyama, M.; Misaki, K.; Notohara, K.; Ishida, T. Interstitial lung disease in clinically amyopathic dermatomyositis with and without anti-MDA-5 antibody: To lump or split? BMC Pulm. Med. 2015, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, L.-S.; Wei, Y.-R.; Du, S.-S.; Du, Y.-K.; He, X.; Li, N.; Zhou, Y.; Li, Q.-H.; Su, Y.-L.; et al. Clinical characteristics of connective tissue disease-associated interstitial lung disease in 1044 chinese patients. Chest 2016, 149, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D.; Medsger, T.A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum. Dis. 2007, 66, 940–944. [Google Scholar] [CrossRef] [PubMed]
- ATS/ERS. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2002, 165, 277–304. [Google Scholar]
- Solomon, D.H.; Kavanaugh, A.J.; Schur, P.H. Evidence-based guidelines for the use of immunologic tests: Antinuclear antibody testing. Arthritis Rheum. 2002, 47, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Feltkamp, T.E.; Smolen, J.S.; Butcher, B.; Dawkins, R.; Fritzler, M.J.; Gordon, T.; Hardin, J.A.; Kalden, J.R.; Lahita, R.G.; et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997, 40, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Kavanaugh, A.J.; Schur, P.H. American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: Antinuclear antibody testing. Arthritis Care Res. 2002, 47, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D. Autoantibodies in systemic sclerosis. Semin. Arthritis Rheum. 2005, 35, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Walker, U.A.; Tyndall, A.; Czirjak, L.; Denton, C.; Farge-Bancel, D.; Kowal-Bielecka, O.; Muller-Ladner, U.; Bocelli-Tyndall, C.; Matucci-Cerinic, M. Clinical risk assessment of organ manifestations in systemic sclerosis: A report from the EULAR Scleroderma Trials And Research group database. Ann. Rheum. Dis. 2007, 66, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Nihtyanova, S.I.; Denton, C.P. Autoantibodies as predictive tools in systemic sclerosis. Nat. Rev. Rheumatol. 2010, 6, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.; Dieudé, M.; Senécal, J.-L. Predictive value of antinuclear autoantibodies: The lessons of the systemic sclerosis autoantibodies. Autoimmun. Rev. 2008, 7, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.T.; Reveille, J.D. The clinical relevance of autoantibodies in scleroderma. Arthritis Res. Ther. 2003, 5, 80. [Google Scholar] [PubMed]
- Mehra, S.; Walker, J.; Patterson, K.; Fritzler, M.J. Autoantibodies in systemic sclerosis. Autoimmun. Rev. 2013, 12, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Elicha Gussin, H.A.; Ignat, G.P.; Varga, J.; Teodorescu, M. Anti-topoisomerase I (Anti–Scl-70) antibodies in patients with systemic lupus erythematosus. Arthritis Rheum. 2001, 44, 376–383. [Google Scholar] [CrossRef]
- Reveille, J.D.; Solomon, D.H. The American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: Anticentromere, Scl-70, and nucleolar antibodies. Arthritis Care Res. 2003, 49, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Ihn, H.; Yamane, K.; Kubo, M.; Tamaki, K. The prevalence and clinical significance of anti-U1 RNA antibodies in patients with systemic sclerosis. J. Investig. Dermatol. 2003, 120, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hesselstrand, R.; Scheja, A.; Shen, G.Q.; Wiik, A.; Akesson, A. The association of antinuclear antibodies with organ involvement and survival in systemic sclerosis. Rheumatology 2003, 42, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.; Domsic, R.T.; Lucas, M.; Fertig, N.; Medsger, T.A., Jr. A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum. 2012, 64, 2986–2994. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, E.J.; Reveille, J.D. Autoantibodies in systemic sclerosis and fibrosing syndromes: Clinical indications and relevance. Curr. Opin. Rheumatol. 2004, 16, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y. Autoantibody profiles in systemic sclerosis: Predictive value for clinical evaluation and prognosis. J. Dermatol. 2010, 37, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Kuwana, M.; Ogasawara, T.; Ajmani, A.K.; Langdon, J.J.; Kimpel, D.; Wang, J.; Reeves, W.H. Association of autoantibodies to topoisomerase I and the phosphorylated (IIO) form of RNA polymerase II in Japanese scleroderma patients. J. Immunol. 1994, 153, 5838–5848. [Google Scholar] [PubMed]
- Kuwana, M.; Okano, Y.; Pandey, J.P.; Silver, R.M.; Fertig, N.; Medsger, T.A., Jr. Enzyme-linked immunosorbent assay for detection of anti-RNA polymerase III antibody: Analytical accuracy and clinical associations in systemic sclerosis. Arthritis Rheum. 2005, 52, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Bunn, C.C.; Denton, C.P.; Shi-Wen, X.; Knight, C.; Black, C.M. Anti-RNA polymerases and other autoantibody specificities in systemic sclerosis. Br. J. Rheumatol. 1998, 37, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nikpour, M.; Hissaria, P.; Byron, J.; Sahhar, J.; Micallef, M.; Paspaliaris, W.; Roddy, J.; Nash, P.; Sturgess, A.; Proudman, S.; et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: A cross-sectional analysis of data from an Australian cohort. Arthritis Res. Ther. 2011, 13, R211. [Google Scholar] [CrossRef] [PubMed]
- Mitri, G.M.; Lucas, M.; Fertig, N.; Steen, V.D.; Medsger, T.A. A comparison between anti-Th/To- and anticentromere antibody-positive systemic sclerosis patients with limited cutaneous involvement. Arthritis Rheum. 2003, 48, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Raijmakers, R. Novel aspects of autoantibodies to the PM/Scl complex: Clinical, genetic and diagnostic insights. Autoimmun. Rev. 2007, 6, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Hanke, K.; Bruckner, C.S.; Dahnrich, C.; Huscher, D.; Komorowski, L.; Meyer, W.; Janssen, A.; Backhaus, M.; Becker, M.; Kill, A.; et al. Antibodies against PM/Scl-75 and PM/Scl-100 are independent markers for different subsets of systemic sclerosis patients. Arthritis Res. Ther. 2009, 11, R22. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Fritzler, M.J. The changing landscape of the clinical value of the PM/Scl autoantibody system. Arthritis Res. Ther. 2009, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Fertig, N.; Domsic, R.T.; Rodriguez-Reyna, T.; Kuwana, M.; Lucas, M.; Medsger, T.A.; Feghali-Bostwick, C.A. Anti–U11/U12 RNP antibodies in systemic sclerosis: A new serologic marker associated with pulmonary fibrosis. Arthritis Care Res. 2009, 61, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Lucas, M.; Fertig, N.; Oddis, C.V.; Medsger, T.A. Anti–U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum. 2009, 60, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Reveille, J.D.; Fischbach, M.; McNearney, T.; Friedman, A.W.; Aguilar, M.B.; Lisse, J.; Fritzler, M.J.; Ahn, C.; Arnett, F.C. Systemic sclerosis in 3 US ethnic groups: A comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin. Arthritis Rheum. 2001, 30, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Tormey, V.J.; Bunn, C.C.; Denton, C.P.; Black, C.M. Anti-fibrillarin antibodies in systemic sclerosis. Rheumatology 2001, 40, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Sato, S.; Kikuchi, K.; Takehara, K. Antigen specificity of antihistone antibodies in systemic sclerosis. Ann. Rheum. Dis. 1998, 57, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Ihn, H.; Kikuchi, K.; Takehara, K. Antihistone antibodies in systemic sclerosis. Association with pulmonary fibrosis. Arthritis Rheum. 1994, 37, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Morozzi, G.; Bellisai, F.; Fineschi, I.; Scaccia, F.; Pucci, G.; Simpatico, A.; Tampoia, M.; Chialà, A.; Lapadula, G.; Galeazzi, M. Prevalence of anti-histone antibodies, their clinical significance and correlation with other autoantibodies in a cohort of Italian scleroderma patients. Autoimmun. Highlights 2011, 2, 29–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ihn, H.; Sato, S.; Fujimoto, M.; Igarashi, A.; Yazawa, N.; Kubo, M.; Kikuchi, K.; Takehara, K.; Tamaki, K. Characterization of autoantibodies to endothelial cells in systemic sclerosis (SSc): Association with pulmonary fibrosis. Clin. Exp. Immunol. 2000, 119, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Ogawa, F.; Iwata, Y.; Komura, K.; Hara, T.; Muroi, E.; Bae, S.J.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; et al. Autoantibody against activating transcription factor-2 in patients with systemic sclerosis. Clin. Exp. Rheumatol. 2009, 27, 751–757. [Google Scholar] [PubMed]
- Iwata, Y.; Ogawa, F.; Komura, K.; Muroi, E.; Hara, T.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; Tomita, Y.; Sato, S. Autoantibody against peroxiredoxin I, an antioxidant enzyme, in patients with systemic sclerosis: possible association with oxidative stress. Rheumatology 2007, 46, 790–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewandowska, K.; Ciurzynski, M.; Gorska, E.; Bienias, P.; Irzyk, K.; Siwicka, M.; Zycinska, K.; Pruszczyk, P.; Demkow, U. Antiendothelial cells antibodies in patients with systemic sclerosis in relation to pulmonary hypertension and lung fibrosis. Adv. Exp. Med. Biol. 2013, 756, 147–153. [Google Scholar] [PubMed]
- Bongartz, T.; Nannini, C.; Medina-Velasquez, Y.F.; Achenbach, S.J.; Crowson, C.S.; Ryu, J.H.; Vassallo, R.; Gabriel, S.E.; Matteson, E.L. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2010, 62, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Koduri, G.; Batley, M.; Kulinskaya, E.; Gough, A.; Norton, S.; Dixey, J. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology 2007, 46, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Assayag, D.; Lubin, M.; Lee, J.S.; King, T.E.; Collard, H.R.; Ryerson, C.J. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology 2014, 19, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Collins, B.F.; Ho, L.A.; Raghu, G. Rheumatoid arthritis-associated lung disease. Eur. Respir. Rev. 2015, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.A.; Saravanan, V.; Nisar, M.; Arthanari, S.; Woodhead, F.A.; Price-Forbes, A.N.; Dawson, J.; Sathi, N.; Ahmad, Y.; Koduri, G.; et al. Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics—A large multicentre UK study. Rheumatology 2014, 53, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Bridges, S.L. Update on autoantibodies in rheumatoid arthritis. Curr. Rheumatol. Rep. 2004, 6, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mahou, M.; López-Longo, F.J.; Sánchez-Ramón, S.; Estecha, A.; García-Segovia, A.; Rodríguez-Molina, J.J.; Carreño, L.; Fernández-Cruz, E. Association of anti-cyclic citrullinated peptide and anti-Sa/citrullinated vimentin autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2006, 55, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Kaptanoglu, E.; Zahir Bakici, M.; Sezer, H.; Elden, H.; Hizmetli, S. Diagnostic value of autoantibodies against citrullinated peptide antigens in rheumatoid arthritis: Comparison of different commercial kits. Turkish J. Rheumatol. 2011, 26, 13. [Google Scholar] [CrossRef]
- Whiting, P.F.; Smidt, N.; Sterne, J.A.; Harbord, R.; Burton, A.; Burke, M.; Beynon, R.; Ben-Shlomo, Y.; Axford, J.; Dieppe, P. Systematic review: Accuracy of anti-citrullinated Peptide antibodies for diagnosing rheumatoid arthritis. Ann. Intern. Med. 2010, 152, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Malmström, V.; Lundberg, K.; Padyukov, L.; Alfredsson, L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin. Immunol. 2011, 23, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Baka, Z.; Buzas, E.; Nagy, G. Rheumatoid arthritis and smoking: Putting the pieces together. Arthritis Res. Ther. 2009, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Munoz, A.D.; Ponce-Guarneros, M.; Gamez-Nava, J.I.; Olivas-Flores, E.M.; Mejia, M.; Juarez-Contreras, P.; Martinez-Garcia, E.A.; Corona-Sanchez, E.G.; Rodriguez-Hernandez, T.M.; Vazquez-del Mercado, M.; et al. Anti-Cyclic Citrullinated Peptide Antibodies and Severity of Interstitial Lung Disease in Women with Rheumatoid Arthritis. J. Immunol. Res. 2015, 2015, 151626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reynisdottir, G.; Karimi, R.; Joshua, V.; Olsen, H.; Hensvold, A.H.; Harju, A.; Engström, M.; Grunewald, J.; Nyren, S.; Eklund, A.; et al. Structural Changes and Antibody Enrichment in the Lungs Are Early Features of Anti-Citrullinated Protein Antibody-Positive Rheumatoid Arthritis. Arthritis Rheumatol. 2014, 66, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Danoff, S.K.; Sokolove, J.; Wagner, C.A.; Winchester, R.; Pappas, D.A.; Siegelman, S.; Connors, G.; Robinson, W.H.; Bathon, J.M. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann. Rheum. Dis. 2014, 73, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liang, D.; Zhao, L.; Li, Y.; Liu, W.; Ren, Y.; Li, Y.; Zeng, X.; Zhang, F.; Tang, F.; et al. Anti-Cyclic Citrullinated Peptide Antibody Is Associated with Interstitial Lung Disease in Patients with Rheumatoid Arthritis. PLoS ONE 2014, 9, e92449. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Solomon, J.J.; du Bois, R.M.; Deane, K.D.; Olson, A.L.; Fernandez-Perez, E.R.; Huie, T.J.; Stevens, A.D.; Gill, M.B.; Rabinovitch, A.M.; et al. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir. Med. 2012, 106, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Gizinski, A.M.; Mascolo, M.; Loucks, J.L.; Kervitsky, A.; Meehan, R.T.; Brown, K.K.; Holers, V.M.; Deane, K.D. Rheumatoid arthritis (RA)-specific autoantibodies in patients with interstitial lung disease and absence of clinically apparent articular RA. Clin. Rheumatol. 2009, 28, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Lega, J.C.; Reynaud, Q.; Belot, A.; Fabien, N.; Durieu, I.; Cottin, V. Idiopathic inflammatory myopathies and the lung. Eur. Respir. Rev. 2015, 24, 216–238. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Miller, F.W.; Fritzler, M.J. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: A comprehensive review. Autoimmun. Rev. 2014, 13, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Hervier, B.; Uzunhan, Y.; Hachulla, E.; Benveniste, O.; Nunes, H.; Delaval, P.; Musset, L.; Dubucquoi, S.; Wallaert, B.; Hamidou, M. Antisynthetase syndrome positive for anti-threonyl-tRNA synthetase (anti-PL7) antibodies. Eur. Respir. J. 2011, 37, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Swigris, J.J.; Bois, R.D.; Lynch, D.; Downey, G.P.; Cosgrove, G.P.; Frankel, S.K.; Fernandez-Perez, E.; Gillis, J.; Brown, K.K. Anti-Synthetase Syndrome in ANA and anti-JO-1 Negative Patients Presenting with Idiopathic Interstitial Pneumonia. Respir. Med. 2009, 103, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Hohlfeld, R. Polymyositis and dermatomyositis. Lancet 2003, 362, 971–982. [Google Scholar] [CrossRef]
- Targoff, I.N. Humoral Immunity in Polymyositis/Dermatomyositis. J. Investig. Dermatol. 1993, 100, S116–S123. [Google Scholar] [CrossRef]
- Cruellas, M.G.P.; dos Santos Trindade Viana, V.; Levy-Neto, M.; de Souza, F.H.C.; Shinjo, S.K. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics 2013, 68, 909–914. [Google Scholar] [CrossRef]
- Hirakata, M.; Katsuki, Y.; Sato, S. Immunologic tests: Anti-PL 7 antibodies, anti-PL-12 antibodies, and other anti-aminoacyl tRNA synthetase antibodies. Nihon rinsho. Jpn. J. Clin. Med. 2005, 63, 508–511. [Google Scholar]
- Ghirardello, A.; Borella, E.; Beggio, M.; Franceschini, F.; Fredi, M.; Doria, A. Myositis autoantibodies and clinical phenotypes. Auto-Immunity Highlights 2014, 5, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kuwana, M.; Fujita, T.; Suzuki, Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod. Rheumatol. 2013, 23, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Hirakata, M.; Kuwana, M.; Suwa, A.; Inada, S.; Mimori, T.; Nishikawa, T.; Oddis, C.V.; Ikeda, Y. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005, 52, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Takahashi, K.; Yamaguchi, Y.; Inasaka, M.; Kuwana, M.; Ikezawa, Z. Analysis of dermatomyositis-specific autoantibodies and clinical characteristics in Japanese patients. J. Dermatol. 2011, 38, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, R.D. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J. Am. Acad. Dermatol. 2002, 46, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, F.; Cavazzana, I. Anti-Ro/SSA and La/SSB antibodies. Autoimmunity 2005, 38, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.; Pope, J.; Mahler, M.; Tatibouet, S.; Steele, R.; Baron, M.; Fritzler, M.J. Clinical significance of antibodies to Ro52/TRIM21 in systemic sclerosis. Arthritis Res. Ther. 2012, 14, R50. [Google Scholar] [CrossRef] [PubMed]
- Mierau, R.; Moinzadeh, P.; Riemekasten, G.; Melchers, I.; Meurer, M.; Reichenberger, F.; Buslau, M.; Worm, M.; Blank, N.; Hein, R.; et al. Frequency of disease-associated and other nuclear autoantibodies in patients of the German Network for Systemic Scleroderma: Correlation with characteristic clinical features. Arthritis Res. Ther. 2011, 13, R172. [Google Scholar] [CrossRef] [PubMed]
- Catoggio, L.J.; Skinner, R.P.; Smith, G.; Maddison, P.J. Systemic lupus erythematosus in the elderly: Clinical and serological characteristics. J. Rheumatol. 1984, 11, 175. [Google Scholar] [PubMed]
- Hochberg, M.C.; Boyd, R.E.; Ahearn, J.M.; Arnett, F.C.; Bias, W.B.; Provost, T.T.; Stevens, M.B. Systemic lupus erythematosus: A review of clinico-laboratory features and immunogenetic markers in 150 patients with emphasis on demographic subsets. Medicine 1985, 64, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2007, 57, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Parambil, J.G.; Myers, J.L.; Lindell, R.M.; Matteson, E.L.; Ryu, J.H. Interstitial lung disease in primary Sjogren syndrome. Chest 2006, 130, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- La Corte, R.; Lo Mo Naco, A.; Locaputo, A.; Dolzani, F.; Trotta, F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006, 39, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Vancsa, A.; Csipo, I.; Nemeth, J.; Devenyi, K.; Gergely, L.; Danko, K. Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol. Int. 2009, 29, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Pego-Reigosa, J.M.; Medeiros, D.A.; Isenberg, D.A. Respiratory manifestations of systemic lupus erythematosus: Old and new concepts. Best Pract. Res. Clin. Rheumatol. 2009, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Cheema, G.S.; Quismorio, F.P., Jr. Interstitial lung disease in systemic lupus erythematosus. Curr. Opin. Pulm. Med. 2000, 6, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Weinrib, L.; Sharma, O.P.; Quismorio, F.P., Jr. A long-term study of interstitial lung disease in systemic lupus erythematosus. Semin. Arthritis Rheum. 1990, 20, 48–56. [Google Scholar] [CrossRef]
- Cervera, R.; Khamashta, M.A.; Font, J.; Sebastiani, G.D.; Gil, A.; Lavilla, P.; Domenech, I.; Aydintug, A.O.; Jedryka-Goral, A.; de Ramon, E.; et al. Systemic lupus erythematosus: Clinical and immunologic patterns of disease expression in a cohort of 1000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine 1993, 72, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Huang, Y.; Deng, W.; Tang, L.; Weng, W.; Zhang, X. Clinical and serologic correlations and autoantibody clusters in systemic lupus erythematosus: A retrospective review of 917 patients in South China. Medicine 2010, 89, 62–67. [Google Scholar] [CrossRef] [PubMed]
- To, C.H.; Mok, C.C.; Tang, S.S.; Ying, S.K.; Wong, R.W.; Lau, C.S. Prognostically distinct clinical patterns of systemic lupus erythematosus identified by cluster analysis. Lupus 2009, 18, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Frodlund, M.; Dahlström, Ö.; Kastbom, A.; Skogh, T.; Sjöwall, C. Associations between antinuclear antibody staining patterns and clinical features of systemic lupus erythematosus: Analysis of a regional Swedish register. BMJ Open 2013, 3, e003608. [Google Scholar] [CrossRef] [PubMed]
- Kinder, B.W.; Collard, H.R.; Koth, L.; Daikh, D.I.; Wolters, P.J.; Elicker, B.; Jones, K.D.; King, T.E., Jr. Idiopathic nonspecific interstitial pneumonia: Lung manifestation of undifferentiated connective tissue disease? Am. J. Respir. Crit. Care Med. 2007, 176, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Noth, I.; Strek, M.E. Autoimmune-featured interstitial lung disease: A distinct entity. Chest 2011, 140, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; West, S.G.; Swigris, J.J.; Brown, K.K.; du Bois, R.M. Connective tissue disease-associated interstitial lung disease: A call for clarification. Chest 2010, 138, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Assayag, D.; Kim, E.J.; Elicker, B.M.; Jones, K.D.; Golden, J.A.; King, T.E., Jr.; Koth, L.L.; Shum, A.K.; Wolters, P.J.; Collard, H.R.; et al. Survival in interstitial pneumonia with features of autoimmune disease: A comparison of proposed criteria. Respir. Med. 2015, 109, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, E.J.; Lynch, K.L.; Elicker, B.; Ryerson, C.J.; Katsumoto, T.R.; Shum, A.K.; Wolters, P.J.; Cerri, S.; Richeldi, L.; et al. Prevalence and clinical significant of circulating autoantibodies in idiopathic pulmonary fibrosis. Respir. Med. 2013, 107, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, S.; Swigris, J.J.; Stanchev, L.; Lee, J.S.; Brown, K.K.; Fischer, A. Clinical features and natural history of interstitial pneumonia with autoimmune features: A single center experience. Respir. Med. 2016, 119, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Oldham, J.M.; Adegunsoye, A.; Valenzi, E.; Lee, C.; Witt, L.; Chen, L.; Husain, A.N.; Montner, S.; Chung, J.H.; Cottin, V.; et al. Characterisation of patients with interstitial pneumonia with autoimmune features. Eur. Respir. J. 2016, 47, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Hagiwara, E.; Kitamura, H.; Yamanaka, Y.; Ikeda, S.; Sekine, A.; Baba, T.; Iso, S.; Okudela, K.; Iwasawa, T.; et al. Clinical features of idiopathic interstitial pneumonia with systemic sclerosis-related autoantibody in comparison with interstitial pneumonia with systemic sclerosis. PLoS ONE 2016, 11, e0161908. [Google Scholar] [CrossRef] [PubMed]
- Takato, H.; Waseda, Y.; Watanabe, S.; Inuzuka, K.; Katayama, N.; Ichikawa, Y.; Yasui, M.; Fujimura, M. Pulmonary manifestations of anti-ARS antibody positive interstitial pneumonia—With or without PM/DM. Respir. Med. 2013, 107, 128–133. [Google Scholar] [CrossRef] [PubMed]
| CTD | Prevalence of ILD | Radiological/Histopathological Pattern |
|---|---|---|
| SSc | 40–75% with clinically significant disease (at least moderate impairment on pulmonary function) [11,31,32] Up to 70% with detectable interstitial changes on HRCT [31] | Most common: NSIP Other: UIP |
| RA | Detectable on HRCT: 30–60% Clinically evident 10–30% [33] | Most common: UIP Other: NSIP, OP, LIP |
| IIM | 30–50% [34,35] | Most common: NSIP Other: UIP, OP, DAD |
| SLE | 3–11% chronic diffuse interstitial disease [36] Up to 30% with detectable interstitial changes on HRCT Need to distinguish from acute pneumonitis (1–10%) and alveolar haemorrhage (rare) | Most common: NSIP Other: LIP, OP, UIP |
| SS | 10–30% [31] Need to exclude pulmonary lymphoma | Most common: NSIP Other: LIP, OP, UIP |
| MCTD | 20–85% [31] | Common: NSIP Other: UIP |
| Autoantibody | Associated CTD(s) |
|---|---|
| Antinuclear antibody (ANA; ≥1:320) | SSc, SLE, Sjögren’s, PM/DM |
| Systemic sclerosis associated | |
| Anti-topoisomerase (ATA/anti-Scl70) | SSc (diffuse) |
| Anti-centromere | SSc (limited) |
| Anti-RNA polymerase (RNA-pol) | SSc |
| Anti-Th/To | SSc |
| Anti-PM/Scl-75/100 | SSc-myositis overlap, SLE, Sjögren’s |
| Anti-U3 ribonucleoprotein (anti-U3 RNP) | SSc |
| Anti-U1 ribonucleoprotein (anti-RNP or anti-U1 RNP) | SSc-overlap, MCTD |
| Anti-U11/U12 ribonucleoprotein (anti-U11/U12 RNP) | SSc |
| Rheumatoid arthritis associated | |
| Rheumatoid factor (≥60 IU/mL) | RA, Sjögren’s, SLE |
| Anti-cyclic citrullinated peptide (anti CCP) | RA |
| Myositis associated | |
| Anti-synthetase (Jo-1, PL-7, PL-12, EJ, OJ, KS) | PM/DM (anti-synthetase syndrome) |
| Anti-Mi2 | PM/DM |
| Anti-CADM140 (anti-MDA5) | Clinically amyopathic DM |
| Overlap syndromes | |
| Anti-Ku | SSc, SSc-PM overlap, SLE, myositis, |
| Anti SS-A/Ro, anti SS-B/La | Sjögren’s, SLE, Sjögren’s/SLE overlap, SSc, RA, DM |
| Systemic lupus erythematosus associated | |
| Anti ds-DNA | SLE |
| Anti-Smith | SLE |
| Autoantibody | Clinical Associations |
|---|---|
| Myositis specific autoantibodies | |
| Anti- tRNA synthetases (Jo-1, PL-7, PL-12, EJ, OJ, KS, Ha, Zo) | PM, DM, anti-synthetase syndrome |
| Anti-Mi-2 | “Classic DM”; lung-sparing |
| Anti- CADM140 (MDA5) | Clinically amyopathic DM ILD; poor prognosis |
| Anti-SRP | Severe necrotising myopathy; association with ILD not described |
| Myositis associated antibodies | |
| Anti-Ro/SSA | PM/Sjögren’s overlap; severe ILD |
| Anti-PM/Scl | PM/Scleroderma overlap; severe ILD |
| Anti-Ku | PM/Scleroderma overlap; severe ILD |
| Anti-U1RNP | PM/SLE overlap; ILD |
Patient must have:
|
| ||
| A. Clinical domain | B. Serological Domain | C. Morphological domain |
|
| 1. Suggestive radiology patterns by HRCT
|
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jee, A.S.; Adelstein, S.; Bleasel, J.; Keir, G.J.; Nguyen, M.; Sahhar, J.; Youssef, P.; Corte, T.J. Role of Autoantibodies in the Diagnosis of Connective-Tissue Disease ILD (CTD-ILD) and Interstitial Pneumonia with Autoimmune Features (IPAF). J. Clin. Med. 2017, 6, 51. https://doi.org/10.3390/jcm6050051
Jee AS, Adelstein S, Bleasel J, Keir GJ, Nguyen M, Sahhar J, Youssef P, Corte TJ. Role of Autoantibodies in the Diagnosis of Connective-Tissue Disease ILD (CTD-ILD) and Interstitial Pneumonia with Autoimmune Features (IPAF). Journal of Clinical Medicine. 2017; 6(5):51. https://doi.org/10.3390/jcm6050051
Chicago/Turabian StyleJee, Adelle S., Stephen Adelstein, Jane Bleasel, Gregory J. Keir, MaiAnh Nguyen, Joanne Sahhar, Peter Youssef, and Tamera J. Corte. 2017. "Role of Autoantibodies in the Diagnosis of Connective-Tissue Disease ILD (CTD-ILD) and Interstitial Pneumonia with Autoimmune Features (IPAF)" Journal of Clinical Medicine 6, no. 5: 51. https://doi.org/10.3390/jcm6050051
APA StyleJee, A. S., Adelstein, S., Bleasel, J., Keir, G. J., Nguyen, M., Sahhar, J., Youssef, P., & Corte, T. J. (2017). Role of Autoantibodies in the Diagnosis of Connective-Tissue Disease ILD (CTD-ILD) and Interstitial Pneumonia with Autoimmune Features (IPAF). Journal of Clinical Medicine, 6(5), 51. https://doi.org/10.3390/jcm6050051






