Molecular Imaging of Angiogenesis and Vascular Remodeling in Cardiovascular Pathology
Abstract
:1. Introduction
2. Imaging Technology
3. Role of Molecular Imaging
3.1. Imaging of VEGF Pathway
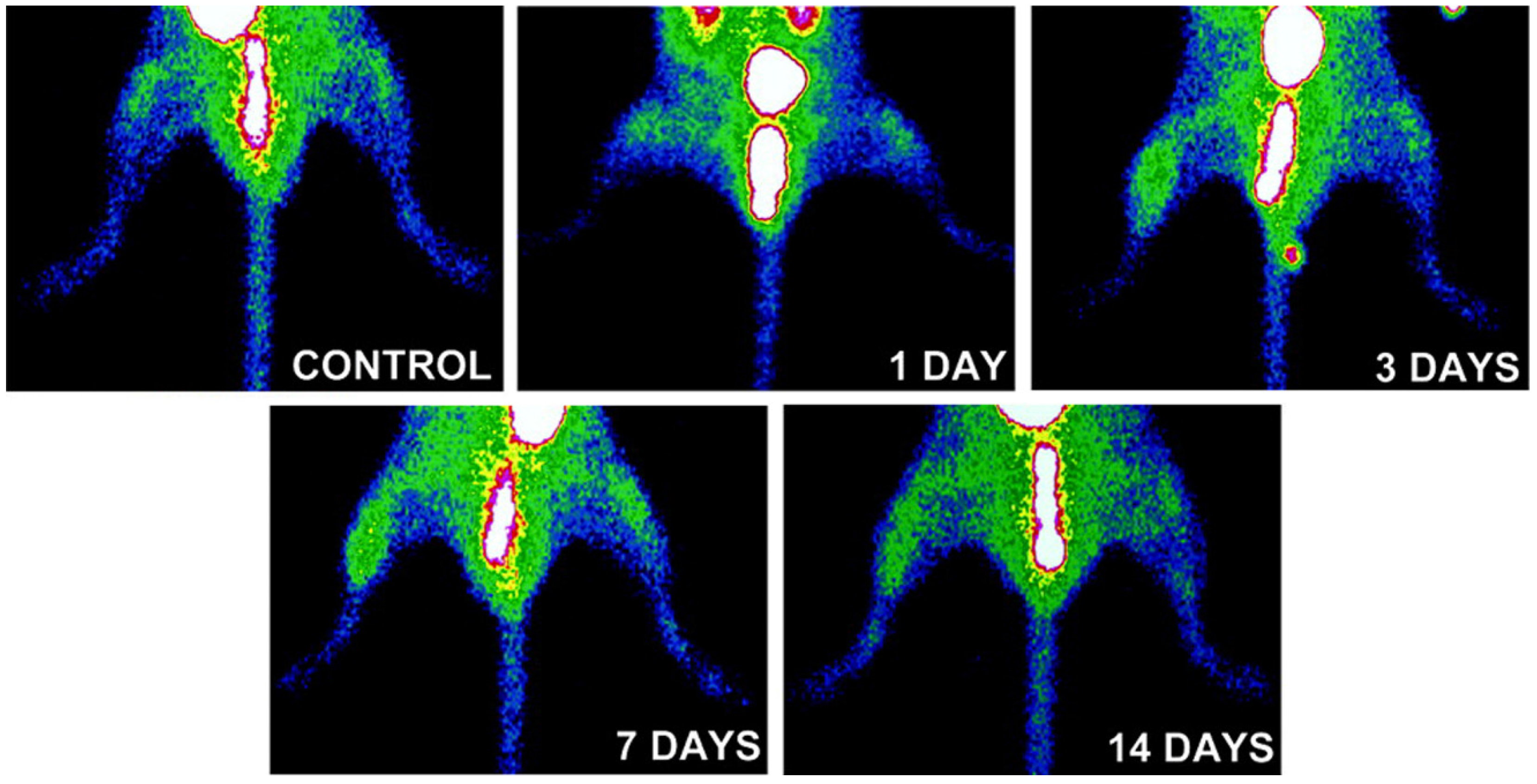
3.2. Imaging of αvβ3 Integrin
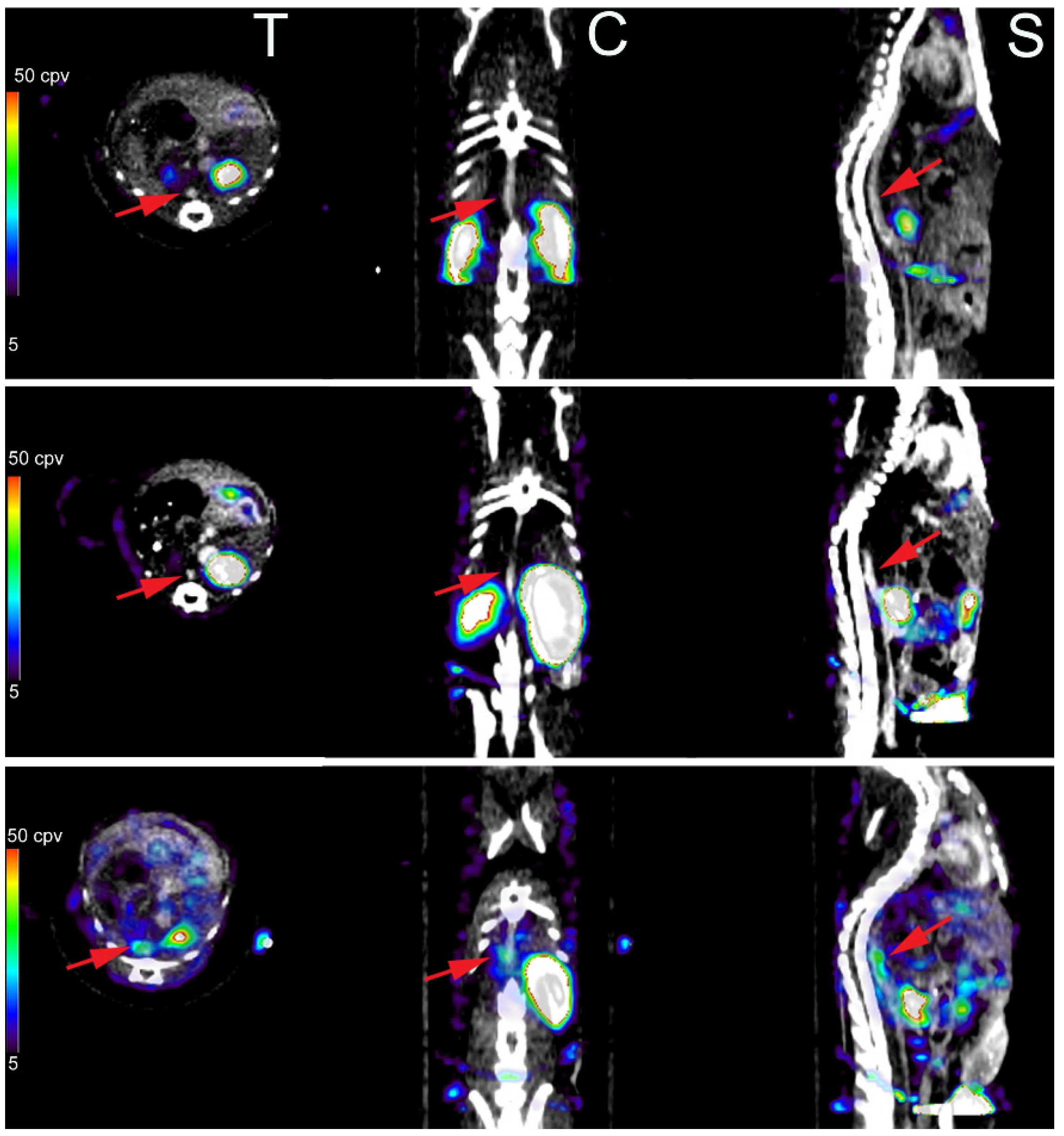
3.3. Imaging of Protease Activation
3.4. Imaging of Other Targets
4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| CAVD | calcific aortic valve disease |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| SPECT | single photon emission tomography |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| RGD | arginine-glycine-aspartate |
| TIMP | tissue inhibitor of matrix metalloproteinases |
| ICAM-1 | intercellular adhesion molecule-1 |
References
- Simons, M. Angiogenesis: Where do we stand now? Circulation 2005, 111, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, G.H.; Dzau, V.J. The emerging concept of vascular remodeling. N. Engl. J. Med. 1994, 330, 1431–1438. [Google Scholar] [PubMed]
- Mankoff, D.A. A definition of molecular imaging. J. Nucl. Med. 2007, 48, 18–21. [Google Scholar]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tung, C.H.; Mahmood, U.; Ntziachristos, V.; Gyurko, R.; Fishman, M.C.; Huang, P.L.; Weissleder, R. In vivo imaging of proteolytic activity in atherosclerosis. Circulation 2002, 105, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, F.A.; Kim, D.E.; Quinti, L.; Tung, C.H.; Aikawa, E.; Pande, A.N.; Kohler, R.H.; Shi, G.P.; Libby, P.; Weissleder, R. Optical visualization of cathepsin k activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation 2007, 115, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.M.; Morawski, A.M.; Caruthers, S.D.; Fuhrhop, R.W.; Zhang, H.; Williams, T.A.; Allen, J.S.; Lacy, E.K.; Robertson, J.D.; Lanza, G.M.; et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with αvβ3-integrin-targeted nanoparticles. Circulation 2003, 108, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Leong-Poi, H.; Christiansen, J.; Klibanov, A.L.; Kaul, S.; Lindner, J.R. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to αv-integrins. Circulation 2003, 107, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart. J. 2013, 34, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Porcel, M.; Cai, W.; Gheysens, O.; Willmann, J.K.; Chen, K.; Wang, H.; Chen, I.Y.; He, L.; Wu, J.C.; Li, Z.B.; et al. Imaging of VEGF receptor in a rat myocardial infarction model using pet. J. Nucl. Med. 2008, 49, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Chen, K.; Wang, H.; Paulmurugan, R.; Rollins, M.; Cai, W.; Wang, D.S.; Chen, I.Y.; Gheysens, O.; Rodriguez-Porcel, M.; et al. Monitoring of the biological response to murine hindlimb ischemia with 64Cu-labeled vascular endothelial growth factor-121 positron emission tomography. Circulation 2008, 117, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Silva, T.; Yarovinsky, T.; Manes, T.D.; Tavakoli, S.; Nie, L.; Tellides, G.; Pober, J.S.; Bender, J.R.; Sadeghi, M.M. VEGF blockade inhibits lymphocyte recruitment and ameliorates immune-mediated vascular remodeling. Circ. Res. 2010, 107, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Chathery, Y.; Wu, Y.; Rathore, N.; Tong, R.K.; Peale, F.; Bagri, A.; Tessier-Lavigne, M.; Koch, A.W.; Watts, R.J. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J. Biol. Chem. 2007, 282, 24049–24056. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Bauters, C.; Pastore, C.; Kearney, M.; Rossow, S.; Bunting, S.; Ferrara, N.; Symes, J.F.; Isner, J.M. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation 1995, 91, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Egashira, K.; Hiasa, K.; Zhao, Q.; Kitamoto, S.; Ishibashi, M.; Usui, M.; Inoue, S.; Yonemitsu, Y.; Sueishi, K.; et al. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation 2004, 110, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Razavian, M.; Tavakoli, S.; Nie, L.; Tellides, G.; Backer, J.M.; Backer, M.V.; Bender, J.R.; Sadeghi, M.M. Molecular imaging of vascular endothelial growth factor receptors in graft arteriosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Nagengast, W.B.; Hooge, M.N.; van Straten, E.M.; Kruijff, S.; Brouwers, A.H.; den Dunnen, W.F.; de Jong, J.R.; Hollema, H.; Dierckx, R.A.; Mulder, N.H.; et al. VEGF-spect with 111In-bevacizumab in stage III/IV melanoma patients. Eur. J. Cancer 2011, 47, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Oosting, S.F.; Brouwers, A.H.; van Es, S.C.; Nagengast, W.B.; Oude Munnink, T.H.; Lub-de Hooge, M.N.; Hollema, H.; de Jong, J.R.; de Jong, I.J.; de Haas, S.; et al. 89Zr-bevacizumab pet visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J. Nucl. Med. 2015, 56, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Zeebregts, C.J.; van Scheltinga, A.G.T.; Lub-de Hooge, M.N.; van Dam, G.M.; Glaudemans, A.W.; Dierckx, R.A.; Tio, R.A.; Suurmeijer, A.J.; Boersma, H.H.; et al. Feasibility of vascular endothelial growth factor imaging in human atherosclerotic plaque using 89Zr-bevacizumab positron emission tomography. Mol. Imaging 2013, 12, 235–243. [Google Scholar] [PubMed]
- Brooks, P.C.; Clark, R.A.; Cheresh, D.A. Requirement of vascular integrin αvβ3 for angiogenesis. Science 1994, 264, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002, 2, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. αv integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 2011, 1, a006478. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated mgmt promoter (centric eortc 26071–22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet. Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef]
- Cox, D.; Brennan, M.; Moran, N. Integrins as therapeutic targets: Lessons and opportunities. Nat. Rev. Drug Discov. 2010, 9, 804–820. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W. Ligand binding to integrins. J. Biol. Chem. 2000, 275, 21785–21788. [Google Scholar] [CrossRef] [PubMed]
- Kenny, L.M.; Coombes, R.C.; Oulie, I.; Contractor, K.B.; Miller, M.; Spinks, T.J.; McParland, B.; Cohen, P.S.; Hui, A.M.; Palmieri, C.; et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J. Nucl. Med. 2008, 49, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical application of radiolabeled RGD peptides for pet imaging of integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.J.; Haubner, R.; Goebel, M.; Luderschmidt, S.; Spilker, M.E.; Wester, H.J.; Weber, W.A.; Schwaiger, M. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18f-galacto-rgd in cancer patients. J. Nucl. Med. 2005, 46, 1333–1341. [Google Scholar] [PubMed]
- Battle, M.R.; Goggi, J.L.; Allen, L.; Barnett, J.; Morrison, M.S. Monitoring tumor response to antiangiogenic sunitinib therapy with 18F-fluciclatide, an 18F-labeled αvβ3-integrin and αvβ5-integrin imaging agent. J. Nucl. Med. 2011, 52, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Uemura, H.; Sano, F.; Terao, H.; Nagashima, Y.; Yamanaka, S.; Shizukuishi, K.; Tateishi, U.; Kubota, Y.; Inoue, T. The potential of FDG-PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann. Nucl. Med. 2011, 25, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Guo, N.; Pan, D.; Yu, C.; Weng, Y.; Luo, S.; Ding, H.; Xu, Y.; Wang, L.; Lang, L.; et al. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J. Nucl. Med. 2013, 54, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Dearling, J.L.; Barnes, J.W.; Panigrahy, D.; Zimmerman, R.E.; Fahey, F.; Treves, S.T.; Morrison, M.S.; Kieran, M.W.; Packard, A.B. Specific uptake of 99mTc-NC100692, an αvβ3-targeted imaging probe, in subcutaneous and orthotopic tumors. Nucl. Med. Biol. 2013, 40, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.M.; Krassilnikova, S.; Zhang, J.; Gharaei, A.A.; Fassaei, H.R.; Esmailzadeh, L.; Kooshkabadi, A.; Edwards, S.; Yalamanchili, P.; Harris, T.D.; et al. Detection of injury-induced vascular remodeling by targeting activated αvβ3 integrin in vivo. Circulation 2004, 110, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Meoli, D.F.; Sadeghi, M.M.; Krassilnikova, S.; Bourke, B.N.; Giordano, F.J.; Dione, D.P.; Su, H.; Edwards, D.S.; Liu, S.; Harris, T.D.; et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J. Clin. Investig. 2004, 113, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Bengel, F.M.; Seidl, S.; Watzlowik, P.; Kessler, H.; Hegenloh, R.; Reder, S.; Nekolla, S.G.; Wester, H.J.; Schwaiger, M. Assessment of αvβ3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc. Res 2008, 78, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, I.; Notni, J.; Pohle, K.; Rudelius, M.; Farrell, E.; Nekolla, S.G.; Henriksen, G.; Neubauer, S.; Kessler, H.; Wester, H.J.; et al. Comparison of cyclic RGD peptides for αvβ3 integrin detection in a rat model of myocardial infarction. EJNMMI Res. 2013, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Dimastromatteo, J.; Riou, L.M.; Ahmadi, M.; Pons, G.; Pellegrini, E.; Broisat, A.; Sancey, L.; Gavrilina, T.; Boturyn, D.; Dumy, P.; et al. In vivo molecular imaging of myocardial angiogenesis using the αvβ3 integrin-targeted tracer 99mTc-RAFT-RGD. J. Nucl. Cardiol. 2010, 17, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, L.; Kusmic, C.; Panetta, D.; Arosio, D.; Petroni, D.; Matteucci, M.; Salvadori, P.A.; Casagrande, C.; L’Abbate, A.; Manzoni, L. MicroPET/Ct imaging of αvβ3 integrin via a novel 68Ga-NOTA-RGD peptidomimetic conjugate in rat myocardial infarction. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Dobrucki, L.W.; Sadeghi, M.M.; Zhang, J.; Bourke, B.N.; Cavaliere, P.; Song, J.; Chow, C.; Jahanshad, N.; van Royen, N.; et al. Noninvasive imaging of angiogenesis with a 99mTc-labeled peptide targeted at αvβ3 integrin after murine hindlimb ischemia. Circulation 2005, 111, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Razavian, M.; Marfatia, R.; Mongue-Din, H.; Tavakoli, S.; Sinusas, A.J.; Zhang, J.; Nie, L.; Sadeghi, M.M. Integrin-targeted imaging of inflammation in vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2820–2826. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.M.; Caruthers, S.D.; Zhang, H.; Williams, T.A.; Wickline, S.A.; Lanza, G.M. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc. Imaging 2008, 1, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, I.; Saraste, A.; Weidl, E.; Poethko, T.; Weber, A.W.; Nekolla, S.G.; Leppanen, P.; Yla-Herttuala, S.; Holzlwimmer, G.; Walch, A.; et al. Evaluation of αvβ3 integrin-targeted positron emission tomography tracer 18F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ. Cardiovasc. Imaging 2009, 2, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Waldeck, J.; Hager, F.; Holtke, C.; Lanckohr, C.; von Wallbrunn, A.; Torsello, G.; Heindel, W.; Theilmeier, G.; Schafers, M.; Bremer, C. Fluorescence reflectance imaging of macrophage-rich atherosclerotic plaques using an αvβ3 integrin-targeted fluorochrome. J. Nucl. Med. 2008, 49, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Mirfeizi, L.; Zeebregts, C.J.; Westra, J.; de Haas, H.J.; Glaudemans, A.W.; Koole, M.; Luurtsema, G.; Tio, R.A.; Dierckx, R.A.; et al. Feasibility of [18F]-RGD for ex vivo imaging of atherosclerosis in detection of αvβ3 integrin expression. J. Nucl. Cardiol. 2015, 22, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, E.; Aikawa, M.; Libby, P.; Figueiredo, J.L.; Rusanescu, G.; Iwamoto, Y.; Fukuda, D.; Kohler, R.H.; Shi, G.P.; Jaffer, F.A.; et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin s deficiency in chronic renal disease. Circulation 2009, 119, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, J.O.; Aikawa, M.; Tung, C.H.; Aikawa, E.; Kim, D.E.; Ntziachristos, V.; Weissleder, R.; Libby, P. Inflammation in atherosclerosis: Visualizing matrix metalloproteinase action in macrophages in vivo. Circulation 2006, 114, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Vucic, E.; Cornily, J.C.; Sharma, R.; Amirbekian, V.; Blackwell, F.; Lancelot, E.; Corot, C.; Fuster, V.; Galis, Z.S.; et al. Monitoring of arterial wall remodelling in atherosclerotic rabbits with a magnetic resonance imaging contrast agent binding to matrix metalloproteinases. Eur. Heart J. 2011, 32, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, T.; Lancelot, E.; Hyafil, F.; Rienzo, M.; Deux, F.; Lemaitre, M.; Duquesnoy, S.; Garot, J.; Roques, B.P.; Michel, J.B.; et al. Molecular and cellular targets of the mri contrast agent P947 for atherosclerosis imaging. Mol. Pharm. 2012, 9, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, L.; Razavian, M.; Ahmed, M.; Dobrucki, L.W.; Asadi, A.; Edwards, D.S.; Azure, M.; Sinusas, A.J.; Sadeghi, M.M. Molecular imaging of activated matrix metalloproteinases in vascular remodeling. Circulation 2008, 118, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Razavian, M.; Zhang, J.; Nie, L.; Marfatia, R.; Dobrucki, L.W.; Sinusas, A.J.; Robinson, S.; Edwards, D.S.; Sadeghi, M.M. Matrix metalloproteinase activation predicts amelioration of remodeling after dietary modification in injured arteries. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Razavian, M.; Zhang, J.; Nie, L.; Tavakoli, S.; Razavian, N.; Dobrucki, L.W.; Sinusas, A.J.; Edwards, D.S.; Azure, M.; Sadeghi, M.M. Molecular imaging of matrix metalloproteinase activation to predict murine aneurysm expansion in vivo. J. Nucl. Med. 2010, 51, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Razavian, M.; Nie, L.; Zhang, J.; Jung, J.J.; Ye, Y.; de Roo, M.; Hilgerink, K.; Liu, C.; Robinson, S.P.; et al. Imaging vessel wall biology to predict outcome in abdominal aortic aneurysm. Circ. Cardiovasc. Imaging 2015, 8, e002471. [Google Scholar] [CrossRef] [PubMed]
- Razavian, M.; Tavakoli, S.; Zhang, J.; Nie, L.; Dobrucki, L.W.; Sinusas, A.J.; Azure, M.; Robinson, S.; Sadeghi, M.M. Atherosclerosis plaque heterogeneity and response to therapy detected by in vivo molecular imaging of matrix metalloproteinase activation. J. Nucl. Med. 2011, 52, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Razavian, M.; Nie, L.; Challa, A.; Zhang, J.; Golestani, R.; Jung, J.J.; Robinson, S.; Sadeghi, M.M. Lipid lowering and imaging protease activation in atherosclerosis. J. Nucl. Cardiol. 2014, 21, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Yutzey, K.E.; Demer, L.L.; Body, S.C.; Huggins, G.S.; Towler, D.A.; Giachelli, C.M.; Hofmann-Bowman, M.A.; Mortlock, D.P.; Rogers, M.B.; Sadeghi, M.M.; et al. Calcific aortic valve disease: A consensus summary from the alliance of investigators on calcific aortic valve disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Edep, M.E.; Shirani, J.; Wolf, P.; Brown, D.L. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc. Pathol. 2000, 9, 281–286. [Google Scholar] [CrossRef]
- Kaden, J.J.; Vocke, D.C.; Fischer, C.S.; Grobholz, R.; Brueckmann, M.; Vahl, C.F.; Hagl, S.; Haase, K.K.; Dempfle, C.E.; Borggrefe, M. Expression and activity of matrix metalloproteinase-2 in calcific aortic stenosis. Z. Kardiol. 2004, 93, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Satta, J.; Oiva, J.; Salo, T.; Eriksen, H.; Ohtonen, P.; Biancari, F.; Juvonen, T.S.; Soini, Y. Evidence for an altered balance between matrix metalloproteinase-9 and its inhibitors in calcific aortic stenosis. Ann. Thorac. Surg. 2003, 76, 681–688. [Google Scholar] [CrossRef]
- Bosse, Y.; Miqdad, A.; Fournier, D.; Pepin, A.; Pibarot, P.; Mathieu, P. Refining molecular pathways leading to calcific aortic valve stenosis by studying gene expression profile of normal and calcified stenotic human aortic valves. Circ. Cardiovasc. Genet. 2009, 2, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.J.; Razavian, M.; Challa, A.A.; Nie, L.; Golestani, R.; Zhang, J.; Ye, Y.; Russell, K.S.; Robinson, S.P.; Heistad, D.D.; et al. Multimodality and molecular imaging of matrix metalloproteinase activation in calcific aortic valve disease. J. Nucl. Med. 2015, 56, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.M. (18)f-fdg pet and vascular inflammation: Time to refine the paradigm? J. Nucl. Cardiol. 2015, 22, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Vashist, A.; Sadeghi, M.M. Molecular imaging of plaque vulnerability. J. Nucl. Cardiol. 2014, 21, 1112–1128; quiz 1129. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Sadeghi, M.M. Emergence of molecular imaging of aortic aneurysm: Implications for risk stratification and management. J. Nucl. Cardiol. 2014, 21, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Toczek, J.; Meadows, J.L.; Sadeghi, M.M. Novel molecular imaging approaches to abdominal aortic aneurysm risk stratification. Circ. Cardiovasc. Imaging 2016, 9, e003023. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golestani, R.; Jung, J.-J.; Sadeghi, M.M. Molecular Imaging of Angiogenesis and Vascular Remodeling in Cardiovascular Pathology. J. Clin. Med. 2016, 5, 57. https://doi.org/10.3390/jcm5060057
Golestani R, Jung J-J, Sadeghi MM. Molecular Imaging of Angiogenesis and Vascular Remodeling in Cardiovascular Pathology. Journal of Clinical Medicine. 2016; 5(6):57. https://doi.org/10.3390/jcm5060057
Chicago/Turabian StyleGolestani, Reza, Jae-Joon Jung, and Mehran M. Sadeghi. 2016. "Molecular Imaging of Angiogenesis and Vascular Remodeling in Cardiovascular Pathology" Journal of Clinical Medicine 5, no. 6: 57. https://doi.org/10.3390/jcm5060057
APA StyleGolestani, R., Jung, J.-J., & Sadeghi, M. M. (2016). Molecular Imaging of Angiogenesis and Vascular Remodeling in Cardiovascular Pathology. Journal of Clinical Medicine, 5(6), 57. https://doi.org/10.3390/jcm5060057




