The Future Prospects of Immune Therapy in Gastric and Esophageal Adenocarcinoma
Abstract
:1. Introduction
2. Pathways of Immune Targets
2.1. Adoptive Cell Immunity
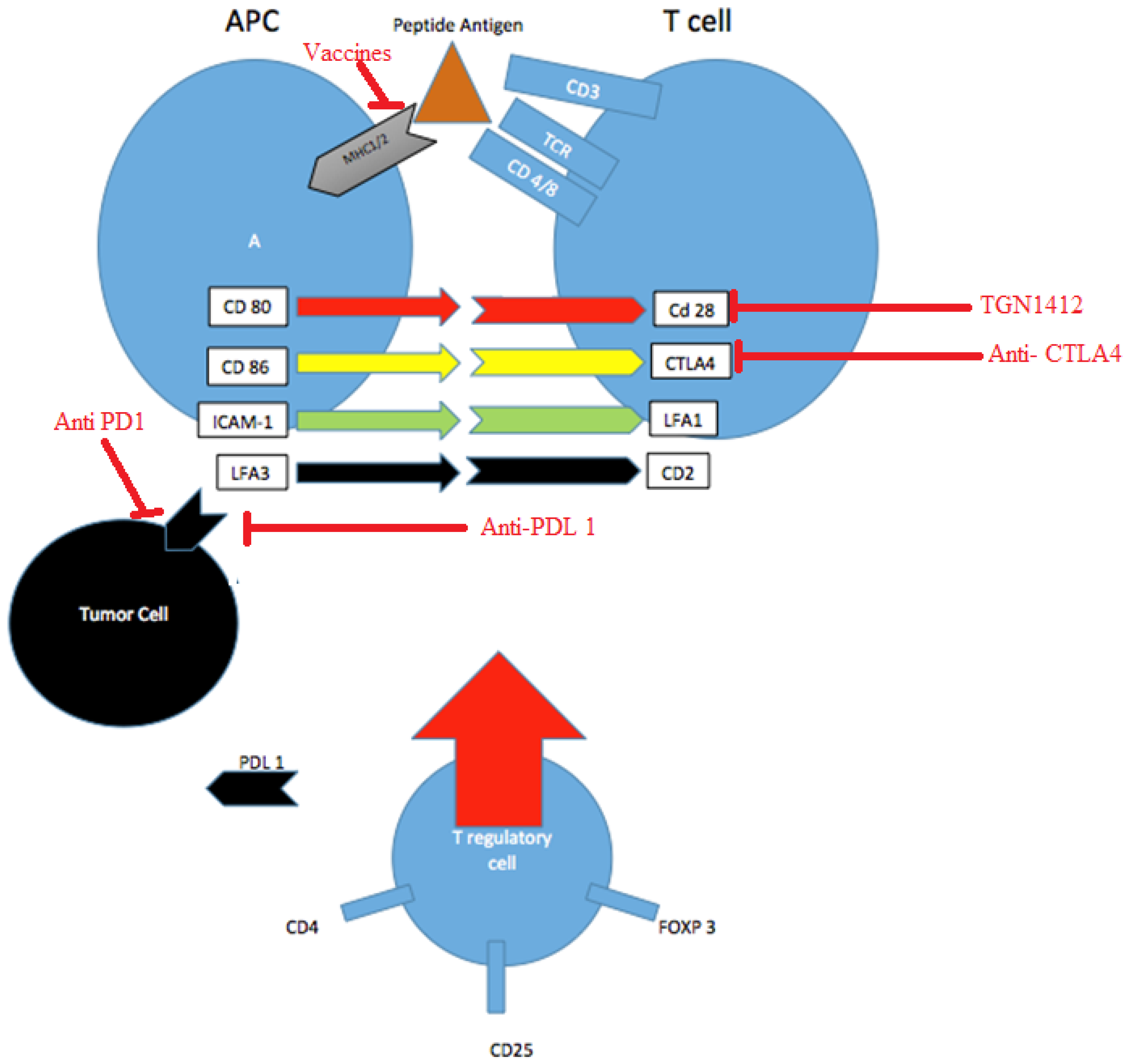
2.2. Antigen Presentation and Vaccine Peptides
2.3. Checkpoint Inhibitors
3. Conclusions
Author Contributions
Conflicts of Interest
References
- Edgren, G.; Adami, H.O.; Weiderpass, E.; Nyren, O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2013, 62, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA: Cancer J. Clin. 2016, 66, 7–30. [Google Scholar]
- Wagner, A.D.; Grothe, W.; Haerting, J.; Kleber, G.; Grothey, A.; Fleig, W.E. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J. Clin. Oncol. 2006, 24, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Park, Y.S.; Hwang, H.S.; Park, H.J.; Ryu, M.H.; Chang, H.M.; Yook, J.H.; Kim, B.S.; Jang, S.J.; Kang, Y.K. Comprehensive analysis of HER2 expression and gene amplification in gastric cancers using immunohistochemistry and in situ hybridization: Which scoring system should we use? Hum. Pathol. 2012, 43, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Koh, Y.W.; Chang, H.M.; Kim, T.H.; Yook, J.H.; Kim, B.S.; Jang, S.J.; Park, Y.S. Evaluation of HER2 protein expression in gastric carcinomas: Comparative analysis of 1414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann. Surg. Oncol. 2011, 18, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Blechacz, B.; Gores, G.J. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology 2008, 48, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, S.; Higuchi, K.; Hioki, O.; Okada, K.; Tsukishiro, T.; Tsuchida, T.; Miyagiwa, M.; Nambu, S.; Yasuyama, T.; Inoue, K.; et al. Induction of allogeneic tumour- and lymphokine-activated lymphocytes against hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1992, 7, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Takahashi, A.; Ichihara, F.; Amemiya, H.; Iizuka, H.; Fujii, H.; Sekikawa, T.; Matsumoto, Y. Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: A randomized trial. Clin. Cancer Res. 2002, 8, 1767–1771. [Google Scholar] [PubMed]
- Katano, M.; Nakamura, M.; Morisaki, T.; Fujimoto, K. Melanoma antigen-encoding gene-1 expression in invasive gastric carcinoma: Correlation with stage of disease. J. Surg. Oncol. 1997, 64, 195–201. [Google Scholar] [CrossRef]
- Fukuyama, T.; Yamazaki, T.; Fujita, T.; Uematsu, T.; Ichiki, Y.; Kaneko, H.; Suzuki, T.; Kobayashi, N. Helicobacter pylori, a carcinogen, induces the expression of melanoma antigen-encoding gene (Mage)-A3, a cancer/testis antigen. Tumour Biol. 2012, 33, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Z.H.; Zhou, J.J.; Chen, R.-F.; Cheng, L.-Z.; Zhou, Q.-B.; Yang, L.-Q. Preparation and antitumor effects of nanovaccines with MAGE-3 peptides in transplanted gastric cancer in mice. Chin. J. Cancer 2010, 29, 359–364. [Google Scholar] [CrossRef] [PubMed]
- A Study of DKN-01 in Combination with Paclitaxel. Available online: https://clinicaltrials.gov/ct2/show/NCT02013154 (accessed on 11 December 2013).
- Deschoolmeester, V.; Baay, M.; Van Marck, E.; Weyler, J.; Vermeulen, P.; Lardon, F.; Vermorken, J.B. Tumor infiltrating lymphocytes: An intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Al-Shibli, K.I.; Donnem, T.; Al-Saad, S.; Persson, M.; Bremnes, R.M.; Busund, L.T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Yaman Suleiman, D.C.; Zibadi, S.; Dalia, S.; Juan, T.H.; Lee, J.K.; Malafa, M.P.; Soliman, H.H.; Kim, R.D. Prognostic value of tumor-infiltrating lymphocytes (TILs) and expression of PDL1 in cholangiocarcinoma. J. Clin. Oncol. 2015, 33, 2015. [Google Scholar]
- Linsley, P.S.; Greene, J.L.; Brady, W.; Bajorath, J.; Ledbetter, J.A.; Peach, R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA4 receptors. Immunity 1994, 1, 793–801. [Google Scholar] [CrossRef]
- Peggs, K.S.; Quezada, S.A.; Chambers, C.A.; Korman, A.J.; Allison, J.P. Blockade of CTLA4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA4 antibodies. J. Exp. Med. 2009, 206, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- An Efficacy Study in Gastric and Gastroesophageal Junction Cancer Comparing Ipilimumab Versus Standard of Care Immediately Following First Line Chemotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT01585987 (accessed on 25 April 2012).
- Ralph, C.; Elkord, E.; Burt, D.J.; O’Dwyer, J.F.; Austin, E.B.; Stern, P.L.; Hawkins, R.E.; Thistlethwaite, F.C. Modulation of lymphocyte regulation for cancer therapy: A phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin. Cancer Res. 2010, 16, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD1 and CTLA4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Duraiswamy, J.; Freeman, G.J.; Coukos, G. Dual blockade of PD1 and CTLA4 combined with tumor vaccine effectively restores T-cell rejection function in tumors—Response. Cancer Res. 2014, 74, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; Meng, Q.; Zhang, Q.X.; Wang, S.D.; Liu, Z.J.; Zhang, X.F. Expression and significance of B7-H1 and its receptor PD1 in human gastric carcinoma. Zhonghua Zhong Liu Za Zhi [Chin. J. Oncol.] 2008, 30, 192–195. [Google Scholar] [PubMed]
- Wu, C.; Zhu, Y.; Jiang, J.; Zhao, J.; Zhang, X.G.; Xu, N. Immunohistochemical localization of programmed death-1 ligand-1 (PDL1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006, 108, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T. Safety and activity of nivolumab monotherapy in advanced and meta-static (A/M) gastric or gastroesophageal junction cancer (GC/GEC): Results from the CheckMate-032 study. In Proceedings of the JCO Abstract Meeting ASCO GI 2015, San Fransisco, CA, USA, 15 January 2015.
- Gillison, M.L.; Blumenschein, G.; Fayette, J. Nivolumab (nivo) vs investigator’s choice (IC) for recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): CheckMate-141. In Proceedings of the Oral Presentation at: AACR Annual Meeting 2016, New Orleans, LA, USA, 16–20 April 2016.
- Muro, K.; Bang, Y.; Shankaran, V.; Geva, R.; Catenacci, D.V.T.; Gupta, S.; Chung, H.C. LBA15A phase 1b study of pembrolizumab (Pembro; MK-3475) in patients (Pts) with advanced gastric cancer. Ann. Oncol. 2014, 25, 1–41. [Google Scholar] [CrossRef]
- Doi, T. Updated results for the advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028 study of pembrolizumab (MK-3475). In Proceedings of the ASCO GI 2015 Meeting, San Fransisco, CA, USA, 2015.
- Piha-Paul, S.A.; Jalal, S.I.; Mai-Dang, H.; Yuan, S.; Koshiji, M.; Csiki, I.; Bennouna, J. Pembrolizumab (MK-3475) for patients (pts) with advanced esophageal carcinoma: Preliminary results from KEYNOTE-028. J. Clin. Oncol. 2015, 33, 2015. [Google Scholar]
- Study of Pembrolizumab (MK-3475) in Previously-Treated Participants with Advanced Carcinoma of the Esophagus or Esophagogastric Junction (MK-3475-180/KEYNOTE-180). Available online: https://clinicaltrials.gov/ct2/show/NCT02559687 (accessed on 23 September 2015).
- Avelumab in First-Line Gastric Cancer (JAVELIN Gastric 100). Available online: https://clinicaltrials.gov/ct2/show/ NCT02625610. (accessed on 4 December 2015).
| Trial | N | Phase | Regimen | Cancer Subtype | OS (Months) | 1 Year OS (%) | ORR (%) |
|---|---|---|---|---|---|---|---|
| Ralph et al. [22] | 18 | II | Tremelimumab | Gastric and Esophageal Adenocarcinoma | 4.8 | - | 5 |
| Le et al. [28] | 59 | I | Nivolumab | Gastric and Gastroesophageal adenocarcinoma | - | 36 | 14 |
| Muro et al. [30] | 39 | Ib | Pembrolizumab | Gastric adenocarcinoma | - | - | 32 in Asian population. 30 in non-Asian population |
| NCT02559687 (ongoing) [33] | 100 (estimated) | II | Pembrolizumab | Esophageal Adenocarcinoma and Squamous Cell Carcinoma | - | - | - |
| NCT01585987 (ongoing) [21] | 114 | II | Ipilimumab | Gastric and Gastroesophageal adenocarcinoma | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaib, W.L.; Nammour, J.P.A.; Gill, H.; Mody, M.; Saba, N.F. The Future Prospects of Immune Therapy in Gastric and Esophageal Adenocarcinoma. J. Clin. Med. 2016, 5, 100. https://doi.org/10.3390/jcm5110100
Shaib WL, Nammour JPA, Gill H, Mody M, Saba NF. The Future Prospects of Immune Therapy in Gastric and Esophageal Adenocarcinoma. Journal of Clinical Medicine. 2016; 5(11):100. https://doi.org/10.3390/jcm5110100
Chicago/Turabian StyleShaib, Walid L., Jean Paul A. Nammour, Harpaul Gill, Mayur Mody, and Nabil F. Saba. 2016. "The Future Prospects of Immune Therapy in Gastric and Esophageal Adenocarcinoma" Journal of Clinical Medicine 5, no. 11: 100. https://doi.org/10.3390/jcm5110100
APA StyleShaib, W. L., Nammour, J. P. A., Gill, H., Mody, M., & Saba, N. F. (2016). The Future Prospects of Immune Therapy in Gastric and Esophageal Adenocarcinoma. Journal of Clinical Medicine, 5(11), 100. https://doi.org/10.3390/jcm5110100






