Epithelial–Mesenchymal Transitions during Neural Crest and Somite Development
Abstract
:1. Introduction
2. EMT of Neural Crest Cells
2.1. NT–Somite Interactions Underlie NC EMT in the Trunk
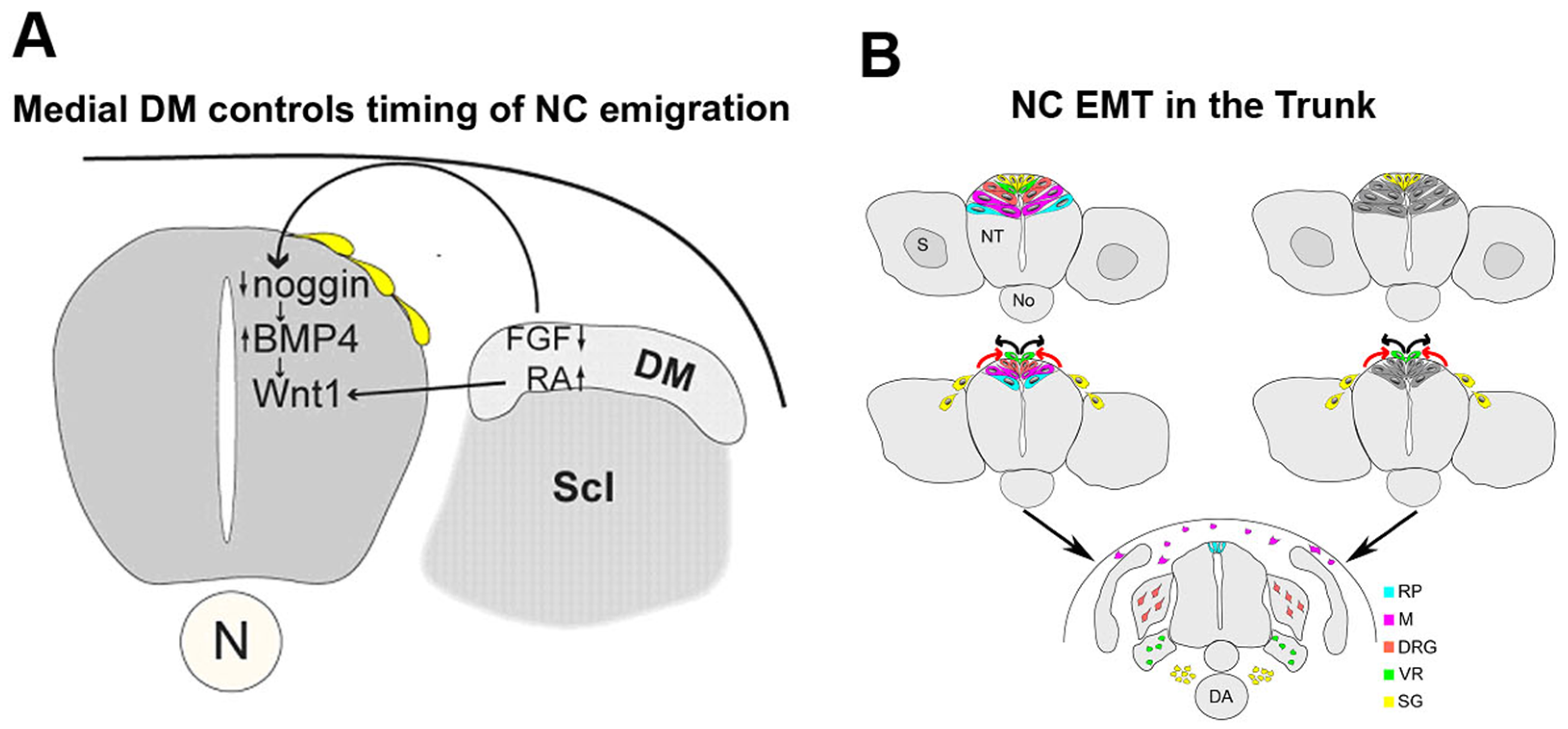
2.2. Mechanisms of Trunk NC EMT that Operate Downstream of BMP Signaling
2.3. Sequential EMT of NC Cells Is Associated with the Colonization of Peripheral Targets and with Fate Restrictions
3. Cell Rearrangements during Somite Development
3.1. Somite Epithelialization—A Mesenchymal-to-Epithelial Transition (MET)
3.2. Somitic EMT: The Sclerotome and Its Subdomains
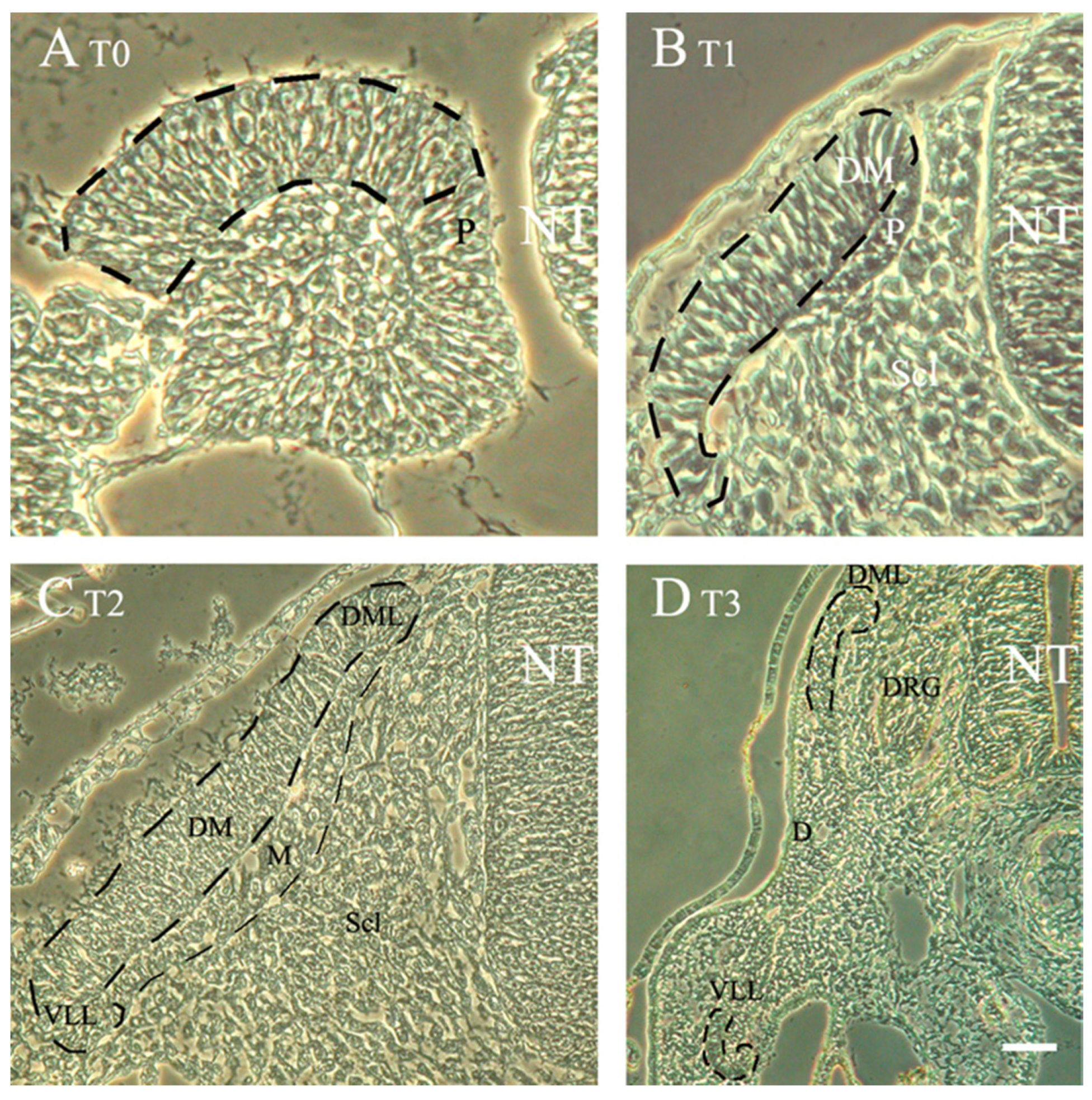
3.3. The Dermomyotome and Its Derivatives
4. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Savagner, P. Leaving the neighborhood: Molecular mechanisms involved during epithelial-mesenchymal transition. BioEssays 2004, 23, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M.; Kalcheim, C. The Neural Crest, 2nd ed.; Cambridge University Press: New York, NY, USA, 1999. [Google Scholar]
- Bronner-Fraser, M. Segregation of cell lineage in the neural crest. Curr. Opin. Genet. Dev. 1993, 3, 641–647. [Google Scholar] [CrossRef]
- Bronner, M.E. Formation and migration of neural crest cells in the vertebrate embryo. Histochem. Cell Biol. 2012, 138, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Weston, J.A. The migration and differentiation of neural crest cells. Adv. Morphog. 1970, 8, 41–114. [Google Scholar] [PubMed]
- Weston, J.A.; Thiery, J.P. Pentimento: Neural crest and the origin of mesectoderm. Dev. Biol. 2015, 401, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.D.; Piatkowska, A.M. Multiple roles of timing in somite formation. Semin. Cell Dev. Biol. 2015, 42, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Kalcheim, C.; Goldstein, R. Segmentation of sensory and sympathetic ganglia: Interaction between neural crest and somite cells. J. Physiol. 1991, 85, 110–117. [Google Scholar]
- Goldstein, R.S.; Kalcheim, C. Determination of epithelial half-somites in skeletal morphogenesis. Development 1992, 116, 441–445. [Google Scholar] [PubMed]
- Hubaud, A.; Pourquie, O. Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 2014, 15, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, B.J.; Maier, J.A.; Mohiuddin, Y.S.; Powers, R.; Lo, Y.; Guimaraes-Camboa, N.; Evans, S.M.; Harfe, B.D. Avian intervertebral disc arises from rostral sclerotome and lacks a nucleus pulposus: Implications for evolution of the vertebrate disc. Dev. Dyn. 2012, 241, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.J.; Zhi, Q.X.; Schmidt, C.; Wilting, J.; Brand-Saberi, B.; Christ, B. Sclerotomal origin of the ribs. Development 2000, 127, 527–532. [Google Scholar] [PubMed]
- Christ, B.; Scaal, M. Formation and differentiation of avian somite derivatives. Adv. Exp. Med. Biol. 2008, 638, 1–41. [Google Scholar] [PubMed]
- Applebaum, M.; Ben-Yair, R.; Kalcheim, C. Segregation of striated and smooth muscle lineages by a notch-dependent regulatory network. BMC Biol. 2014, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yair, R.; Kalcheim, C. Notch and bone morphogenetic protein differentially act on dermomyotome cells to generate endothelium, smooth, and striated muscle. J. Cell Biol. 2008, 180, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.C.; Erickson, C.A. Interkinetic nuclear migration: A mysterious process in search of a function. Dev. Growth Differ. 2012, 54, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Burstyn-Cohen, T.; Kalcheim, C. Association between the cell cycle and neural crest delamination through specific regulation of G1/S transition. Dev. Cell 2002, 3, 383–395. [Google Scholar] [CrossRef]
- Ben-Yair, R.; Kahane, N.; Kalcheim, C. Coherent development of dermomyotome and dermis from the entire mediolateral extent of the dorsal somite. Development 2003, 130, 4325–4336. [Google Scholar] [CrossRef] [PubMed]
- Krispin, S.; Nitzan, E.; Kassem, Y.; Kalcheim, C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development 2010, 137, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Theveneau, E.; Mayor, R. Collective cell migration of the cephalic neural crest: The art of integrating information. Genesis 2011, 49, 164–176. [Google Scholar] [CrossRef]
- Strobl-Mazzulla, P.H.; Bronner, M.E. A PHD12-Snail2 repressive complex epigenetically mediates neural crest epithelial-to-mesenchymal transition. J. Cell Biol. 2012, 198, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Theveneau, E.; Mayor, R. Neural crest delamination and migration: From epithelium-to-mesenchyme transition to collective cell migration. Dev. Biol. 2012, 366, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yair, R.; Kalcheim, C. Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development 2005, 132, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Delfini, M.C.; de la Celle, M.; Gros, J.; Serralbo, O.; Marics, I.; Seux, M.; Scaal, M.; Marcelle, C. The timing of emergence of muscle progenitors is controlled by an FGF/ERK/SNAIL1 pathway. Dev. Biol. 2009, 15, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ordahl, C.P.; Berdougo, E.; Venters, S.J.; Denetclaw, W.F., Jr. The dermomyotome dorsomedial lip drives growth and morphogenesis of both the primary myotome and dermomyotome epithelium. Development 2001, 128, 1731–1744. [Google Scholar] [PubMed]
- Cinnamon, Y.; Kahane, N.; Bachelet, I.; Kalcheim, C. The sub-lip domain—A distinct pathway for myotome precursors that demonstrate rostral-caudal migration. Development 2001, 128, 341–351. [Google Scholar] [PubMed]
- Yip, J.W. Migratory pathways of sympathetic ganglioblasts and other neural crest derivatives in chick embryos. J. Neurosci. 1986, 6, 3465–3473. [Google Scholar] [PubMed]
- Teillet, M.A.; Kalcheim, C.; le Douarin, N.M. Formation of the dorsal root ganglia in the avian embryo: Segmental origin and migratory behavior of neural crest progenitor cells. Dev. Biol. 1987, 120, 329–347. [Google Scholar] [CrossRef]
- Buckingham, M.; Bajard, L.; Chang, T.; Daubas, P.; Hadchouel, J.; Meilhac, S.; Montarras, D.; Rocancourt, D.; Relaix, F. The formation of skeletal muscle: From somite to limb. J. Anat. 2003, 202, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Kalcheim, C.; Ben-Yair, R. Cell rearrangements during development of the somite and its derivatives. Curr. Opin. Genet. Dev. 2005, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kalcheim, C.; Cinnamon, Y.; Kahane, N. Myotome formation: A multistage process. Cell Tissue Res. 1999, 296, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Gros, J.; Scaal, M.; Marcelle, C. A two-step mechanism for myotome formation in chick. Dev. Cell 2004, 6, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yair, R.; Kahane, N.; Kalcheim, C. LGN-dependent orientation of cell divisions in the dermomyotome controls lineage segregation into muscle and dermis. Development 2011, 138, 4155–4166. [Google Scholar] [CrossRef] [PubMed]
- Halperin-Barlev, O.; Kalcheim, C. Sclerotome-derived Slit1 drives directional migration and differentiation of Robo2-expressing pioneer myoblasts. Development 2011, 138, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Cortes, F.; Daggett, D.; Bryson-Richardson, R.J.; Neyt, C.; Maule, J.; Gautier, P.; Hollway, G.E.; Keenan, D.; Currie, P. Cadherin-mediated differential cell adhesion controls slow muscle cell migration in the developing zebrafish myotome. Dev. Cell 2003, 5, 865–876. [Google Scholar] [CrossRef]
- Nitzan, E.; Kalcheim, C. Neural crest and somitic mesoderm as paradigms to investigate cell fate decisions during development. Dev. Growth Differ. 2013, 55, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Loring, J.F.; Erickson, C.A. Neural crest cell migratory pathways in the trunk of the chick embryo. Dev. Biol. 1987, 121, 220–236. [Google Scholar] [CrossRef]
- Debby-Brafman, A.; Burstyn-Cohen, T.; Klar, A.; Kalcheim, C. F-spondin is expressed in somite regions avoided by neural crest cells and mediates the inhibition of distinct somitic domains to neural crest migration. Neuron 1999, 22, 475–488. [Google Scholar] [CrossRef]
- Kalcheim, C.; Teillet, M.A. Consequences of somite manipulation on the pattern of dorsal root ganglion development. Development 1989, 106, 85–93. [Google Scholar] [PubMed]
- Newgreen, D.F.; Erickson, C.A. The migration of neural crest cells. Int. Rev. Cytol. 1986, 103, 89–145. [Google Scholar] [PubMed]
- Rogers, C.D.; Saxena, A.; Bronner, M.E. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest emt. J. Cell Biol. 2013, 203, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Duband, J.L. Diversity in the molecular and cellular strategies of epithelium-to-mesenchyme transitions: Insights from the neural crest. Cell Adh. Migr. 2010, 4, 458–482. [Google Scholar] [CrossRef] [PubMed]
- Duband, J.L.; Dady, A.; Fleury, V. Resolving time and space constraints during neural crest formation and delamination. Curr. Top. Dev. Biol. 2015, 111, 27–67. [Google Scholar] [PubMed]
- Burstyn-Cohen, T.; Stanleigh, J.; Sela-Donenfeld, D.; Kalcheim, C. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development 2004, 131, 5327–5339. [Google Scholar] [CrossRef] [PubMed]
- Sela-Donenfeld, D.; Kalcheim, C. Regulation of the onset of neural crest migration by coordinated activity of BMP4 and noggin in the dorsal neural tube. Development 1999, 126, 4749–4762. [Google Scholar] [PubMed]
- Sela-Donenfeld, D.; Kalcheim, C. Inhibition of noggin expression in the dorsal neural tube by somitogenesis: A mechanism for coordinating the timing of neural crest emigration. Development 2000, 127, 4845–4854. [Google Scholar] [PubMed]
- Martinez-Morales, P.L.; Diez-del-Corral, R.; Olivera-Martinez, I.; Quiroga, A.C.; Das, R.M.; Barbas, J.A.; Storey, K.G.; Morales, A.V. FGF and retinoic acid activity gradients control the timing of neural crest cell emigration in the trunk. J. Cell Biol. 2011, 194, 489–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theveneau, E.; Duband, J.L.; Altabef, M. Ets-1 confers cranial features on neural crest delamination. PLoS ONE 2007, 2, e1142. [Google Scholar] [CrossRef] [PubMed]
- Shoval, I.; Ludwig, A.; Kalcheim, C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development 2007, 134, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, J.D.; Erickson, C.A. The neural crest epithelial-mesenchymal transition in 4D: A “tail” of multiple non-obligatory cellular mechanisms. Development 2009, 136, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Coles, E.G.; Taneyhill, L.A.; Bronner-Fraser, M. A critical role for cadherin6b in regulating avian neural crest emigration. Dev. Biol. 2007, 312, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Takeichi, M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 1998, 125, 2963–2971. [Google Scholar] [PubMed]
- Park, K.S.; Gumbiner, B.M. Cadherin 6B induces BMP signaling and de-epithelialization during the epithelial mesenchymal transition of the neural crest. Development 2010, 137, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Schiffmacher, A.T.; Padmanabhan, R.; Jhingory, S.; Taneyhill, L.A. Cadherin-6B is proteolytically processed during epithelial-to-mesenchymal transitions of the cranial neural crest. Mol. Biol. Cell 2014, 25, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Gumbiner, B.M. Cadherin-6B stimulates an epithelial mesenchymal transition and the delamination of cells from the neural ectoderm via LIMK/cofilin mediated non-canonical BMP receptor signaling. Dev. Biol. 2012, 366, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Clay, M.R.; Halloran, M.C. Cadherin 6 promotes neural crest cell detachment via F-actin regulation and influences active Rho distribution during epithelial-to-mesenchymal transition. Development 2014, 141, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Braga, V.M.; Machesky, L.M.; Hall, A.; Hotchin, N.A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 1997, 137, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Braga, V.M. Small gtpases and regulation of cadherin dependent cell-cell adhesion. Mol. Pathol. 1999, 52, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, L.; Symons, M. Role of Rho family gtpases in epithelial morphogenesis. Genes Dev. 2002, 16, 1032–1054. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Melendez, J.; Baumann, J.M.; Leslie, J.R.; Chauhan, B.K.; Nemkul, N.; Lang, R.A.; Kuan, C.Y.; Zheng, Y.; Yoshida, Y. Loss of RhoA in neural progenitor cells causes the disruption of adherens junctions and hyperproliferation. Proc. Natl. Acad. Sci. USA 2011, 108, 7607–7612. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Jessell, T.M. A role for RhoB in the delamination of neural crest cells from the dorsal neural tube. Development 1998, 125, 5055–5067. [Google Scholar] [PubMed]
- Clay, M.R.; Halloran, M.C. Rho activation is apically restricted by Arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition. Development 2013, 140, 3198–3209. [Google Scholar] [CrossRef] [PubMed]
- Groysman, M.; Shoval, I.; Kalcheim, C. A negative modulatory role for Rho and Rho-associated kinase signaling in delamination of neural crest cells. Neural Dev. 2008, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Fort, P.; Theveneau, E. Pleiotrhopic: Rho pathways are essential for all stages of neural crest development. Small GTPases 2014, 5, e27975. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Rho GTPases and the actin cytoskeleton. Science 1999, 279, 509–514. [Google Scholar] [CrossRef]
- Kraynov, V.S.; Chamberlain, C.; Bokoch, G.M.; Schwartz, M.A.; Slabaugh, S.; Hahn, K.M. Localized Rac activation dynamics visualized in living cells. Science 2000, 290, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Theveneau, E.; Marchant, L.; Kuriyama, S.; Gull, M.; Moepps, B.; Parsons, M.; Mayor, R. Collective chemotaxis requires contact-dependent cell polarity. Dev. Cell 2010, 19, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.K.; Lou, M.; Zheng, Y.; Lang, R.A. Balanced Rac1 and RhoA activities regulate cell shape and drive invagination morphogenesis in epithelia. Proc. Natl. Acad. Sci. USA 2011, 108, 18289–18294. [Google Scholar] [CrossRef] [PubMed]
- Broders-Bondon, F.; Chesneau, A.; Romero-Oliva, F.; Mazabraud, A.; Mayor, R.; Thiery, J.P. Regulation of Xsnail2 expression by Rho gtpases. Dev. Dyn. 2007, 236, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Fontaine, C.; Matthews, H.K.; Kuriyama, S.; Moreno, M.; Dunn, G.A.; Parsons, M.; Stern, C.D.; Mayor, R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 2008, 456, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Marchant, L.; Carmona-Fontaine, C.; Kuriyama, S.; Larrain, J.; Holt, M.R.; Parsons, M.; Mayor, R. Directional migration of neural crest cells in vivo is regulated by syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 2008, 135, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- De Calisto, J.; Araya, C.; Marchant, L.; Riaz, C.F.; Mayor, R. Essential role of non-canonical Wnt signalling in neural crest migration. Development 2005, 132, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Shoval, I.; Kalcheim, C. Antagonistic activities of Rho and Rac GTPases underlie the transition from neural crest delamination to migration. Dev. Dyn. 2012, 241, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Krispin, S.; Nitzan, E.; Kalcheim, C. The dorsal neural tube: A dynamic setting for cell fate decisions. Dev. Neurobiol. 2010, 70, 796–812. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.C.; Fukatsu, K.; Morrison, J.; McLennan, R.; Bronner, M.E.; Kulesa, P.M. Evidence for dynamic rearrangements but lack of fate or position restrictions in premigratory avian trunk neural crest. Development 2013, 140, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, E.; Krispin, S.; Pfaltzgraff, E.R.; Klar, A.; Labosky, P.; Kalcheim, C. A dynamic code of dorsal neural tube genes regulates the segregation between neurogenic and melanogenic neural crest cells. Development 2013, 140, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Burgess, R.; Cserjesi, P.; Ligon, K.L.; Olson, E.N. Paraxis: A basic helix-loop-helix protein expressed in paraxial mesoderm and developing somites. Dev. Biol. 1995, 168, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Dubrulle, J.; Pourquié, O. Coupling segmentation to axis formation. Development 2004, 131, 5783–5793. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Kuroda, S.; Katagiri, Y.T.; Kaibuchi, K.; Takahashi, Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev. Cell 2004, 7, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Stoeckelhuber, M.; McKinnell, I.; Putz, R.; Christ, B.; Patel, K. Wnt 6 regulates the epithelialisation process of the segmental plate mesoderm leading to somite formation. Dev. Biol. 2004, 271, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Rowton, M.; Ramos, P.; Anderson, D.M.; Rhee, J.M.; Cunliffe, H.E.; Rawls, A. Regulation of mesenchymal-to-epithelial transition by paraxis during somitogenesis. Dev. Dyn. 2013, 242, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.S.; Sanchez, S.S. Paraxis is required for somite morphogenesis and differentiation in Xenopus laevis. Dev. Dyn. 2015, 244, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Brand-Saberi, B.; Christ, B. Evolution and development of distinct cell lineages derived from somites. Curr. Top. Dev. Biol. 2000, 48, 1–42. [Google Scholar] [PubMed]
- Christ, B.; Huang, R.; Scaal, M. Formation and differentiation of the avian sclerotome. Anat. Embryol. 2004, 208, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Teillet, M.A.; le Douarin, N.M. Consequences of neural tube and notochord excision on the development of the peripheral nervous system in the chick embryo. Dev. Biol. 1983, 98, 192–211. [Google Scholar] [CrossRef]
- Barnes, G.L.; Alexander, P.G.; Hsu, C.W.; Mariani, B.D.; Tuan, R.S. Cloning and characterization of chicken Paraxis: A regulator of paraxial mesoderm development and somite formation. Dev. Biol. 1997, 189, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Burgess, R.; Rawls, A.; Brown, D.; Bradley, A.; Olson, E.N. Requirement of the Paraxis gene for somite formation and musculoskeletal patterning. Nature 1996, 384, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Hrabe-de-Angelis, M.; McIntyre, J., II; Gossler, A. Maintenance of somite borders in mice requires the Delta homologue Dii1. Nature 1997, 386, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.; Wilm, B.; Sakai, N.; Imai, K.; Maas, R.; Balling, R. Pax1 and Pax9 synergistically regulate vertebral column development. Development 1999, 126, 5399–5408. [Google Scholar] [PubMed]
- Tribioli, C.; Lufkin, T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development 1999, 126, 5699–5711. [Google Scholar] [PubMed]
- Scaal, M.; Fuchtbauer, E.M.; Brand-Saberi, B. Cdermo-1 expression indicates a role in avian skin development. Anat. Embryol. 2001, 203, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cinnamon, Y.; Ben-Yair, R.; Kalcheim, C. Differential effects of N-cadherin-mediated adhesion on the development of myotomal waves. Development 2006, 133, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Sagar; Prols, F.; Wiegreffe, C.; Scaal, M. Communication between distant epithelial cells by filopodia-like protrusions during embryonic development. Development 2015, 142, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Brill, G.; Kahane, N.; Carmeli, C.; von Schack, D.; Barde, Y.-A.; Kalcheim, C. Epithelial-mesenchymal conversion of dermatome progenitors requires neural tube-derived signals: Characterization of the role of neurotrophin-3. Development 1995, 121, 2583–2594. [Google Scholar] [PubMed]
- Hornik, C.; Krishan, K.; Yusuf, F.; Scaal, M.; Brand-Saberi, B. Cdermo-1 misexpression induces dense dermis, feathers, and scales. Dev. Biol. 2005, 277, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Vasyutina, E.; Birchmeier, C. The development of migrating muscle precursor cells. Anat. Embryol. 2006, 211 (Suppl. 1), 37–41. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Abou-Rebyeh, F.; Brohmann, H.; Bladt, F.; Sonnenberg-Riethmacher, E.; Yamaai, T.; Lumsden, A.; Brand-Saberi, B.; Birchmeier, C. The role of SF/HGF and c-Met in the development of skeletal muscle. Development 1999, 126, 1621–1629. [Google Scholar] [PubMed]
- Brand-Saberi, B.; Muller, T.S.; Wilting, J.; Christ, B.; Birchmeier, C. Scatter factor/hepatocyte growth factor (SF/HGF) induces emigration of myogenic cells at interlimb level in vivo. Dev. Biol. 1996, 179, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Morosan-Puopolo, G.; Balakrishnan-Renuka, A.; Yusuf, F.; Chen, J.; Dai, F.; Zoidl, G.; Ludtke, T.H.; Kispert, A.; Theiss, C.; Abdelsabour-Khalaf, M.; et al. Wnt11 is required for oriented migration of dermogenic progenitor cells from the dorsomedial lip of the avian dermomyotome. PLoS ONE 2014, 9, e92679. [Google Scholar] [CrossRef] [PubMed]
- Kahane, N.; Ribes, V.; Kicheva, A.; Briscoe, J.; Kalcheim, C. The transition from differentiation to growth during dermomyotome-derived myogenesis depends on temporally restricted hedgehog signaling. Development 2013, 140, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalcheim, C. Epithelial–Mesenchymal Transitions during Neural Crest and Somite Development. J. Clin. Med. 2016, 5, 1. https://doi.org/10.3390/jcm5010001
Kalcheim C. Epithelial–Mesenchymal Transitions during Neural Crest and Somite Development. Journal of Clinical Medicine. 2016; 5(1):1. https://doi.org/10.3390/jcm5010001
Chicago/Turabian StyleKalcheim, Chaya. 2016. "Epithelial–Mesenchymal Transitions during Neural Crest and Somite Development" Journal of Clinical Medicine 5, no. 1: 1. https://doi.org/10.3390/jcm5010001
APA StyleKalcheim, C. (2016). Epithelial–Mesenchymal Transitions during Neural Crest and Somite Development. Journal of Clinical Medicine, 5(1), 1. https://doi.org/10.3390/jcm5010001




