Diabetic Nephropathy and CKD—Analysis of Individual Patient Serum Creatinine Trajectories: A Forgotten Diagnostic Methodology for Diabetic CKD Prognostication and Prediction
Abstract
:1. Introduction
1.1. Diabetic Nephropathy
1.2. Creatinine-Metabolism, Chemical Structure, and Excretion in the Urine
2. Main Review
2.1. Serum Creatinine Trajectories in Diabetic Nephropathy
i. Acute Kidney Injury (AKI): the Rainbow Spectrum of Renal Outcomes in AKI in CKD Patients
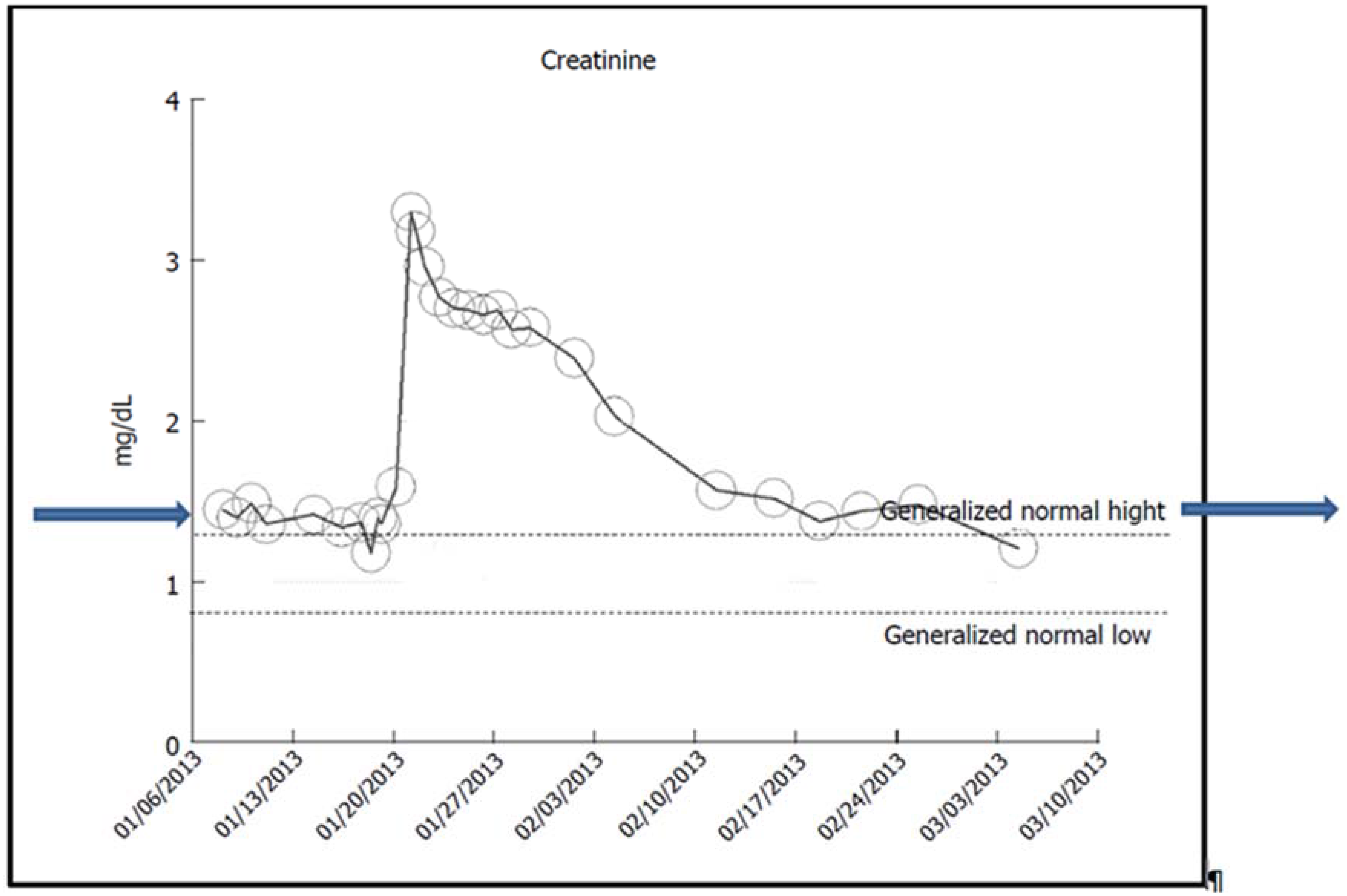
a. Rapid and Full Recovery of Renal Function Following AKI on CKD
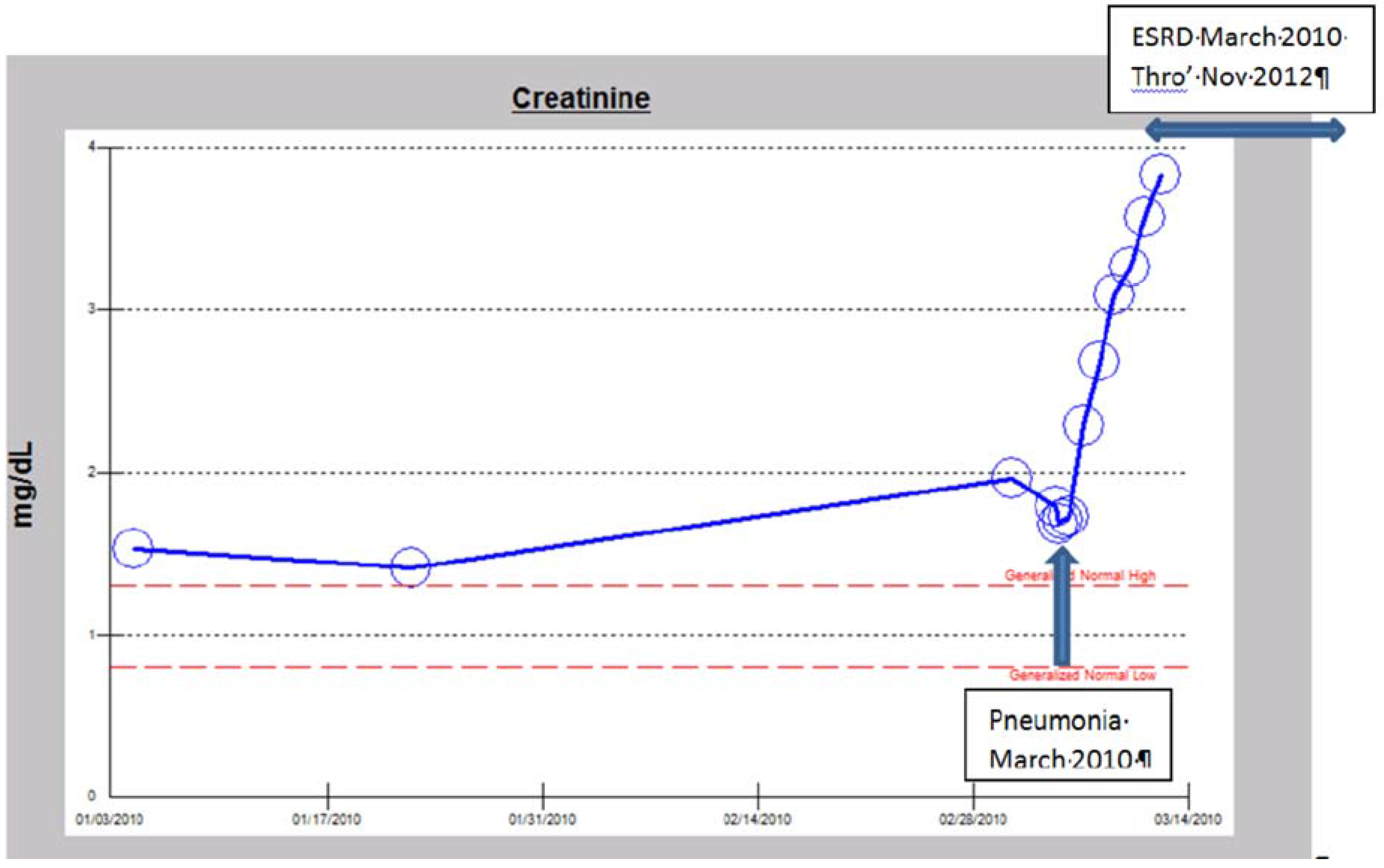
b. The Syndrome of Rapid Onset ESRD (SORO-ESRD) Following AKI on CKD in a Diabetic Patient
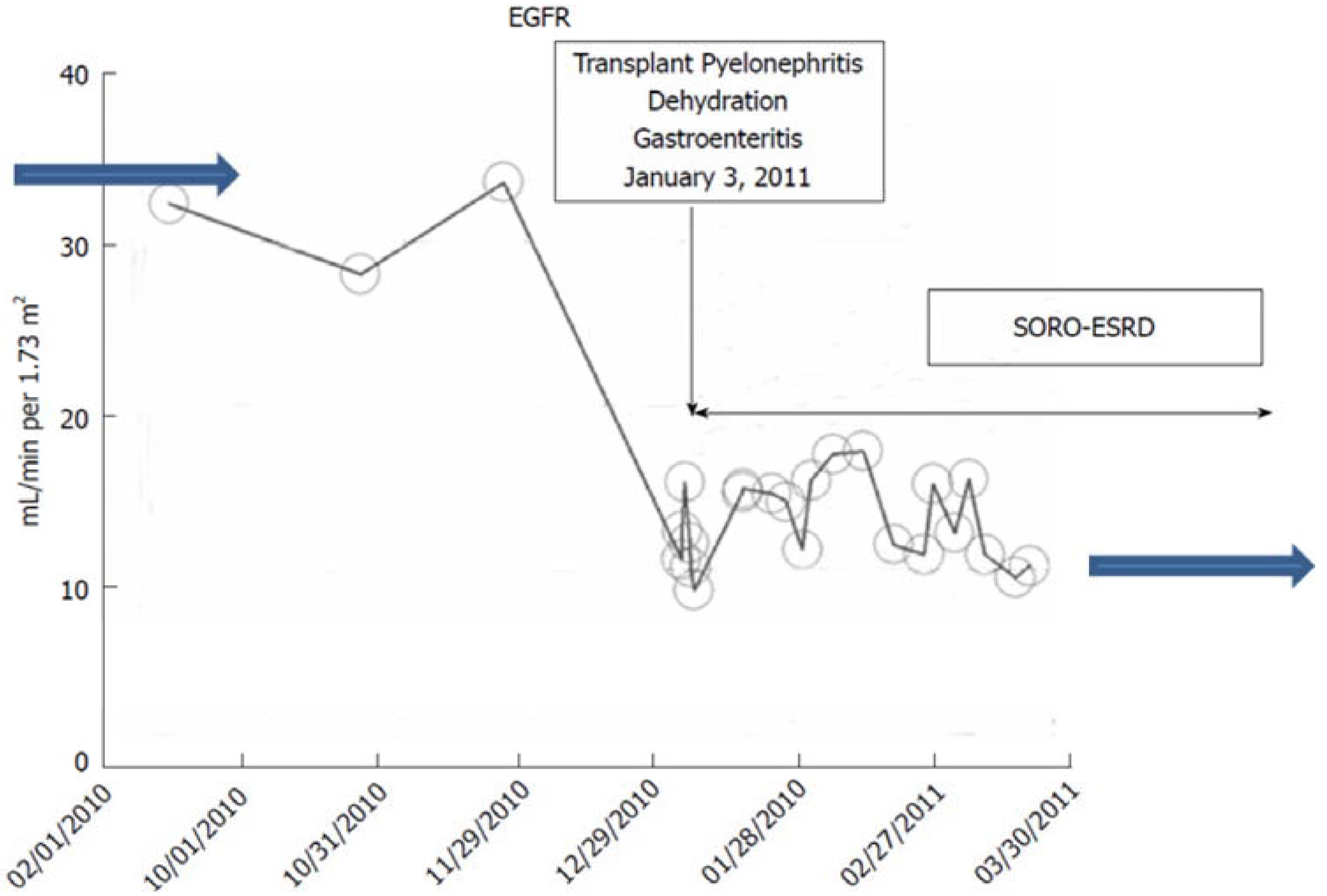
c. The Syndrome of Rapid Onset ESRD (SORO-ESRD) Following AKI on CKD in a Kidney Transplant Recipient
ii. The NKF KDOQI CKD Staging Paradigm revisited: CKD Prediction is an Inexact Science: the Novel Concept of CKD “Progressors” and CKD “Nonprogessors”.
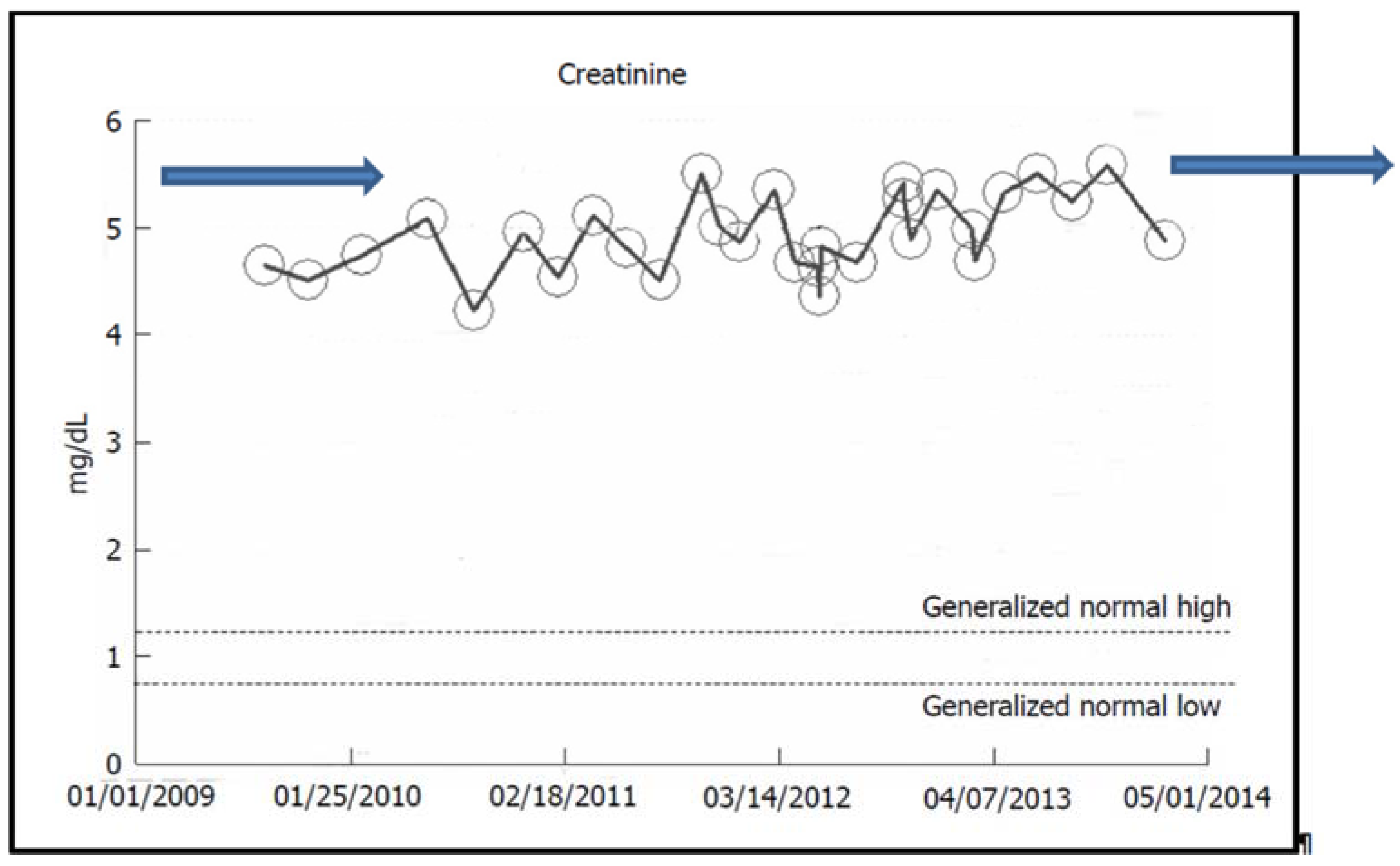
2.2. Stable CKD V over Eight Years in a Now-79-Year-Old Caucasian Hypertensive Diabetic Male
iii. Predictable Pattern of CKD to ESRD Progression or the So-Called “Classic” Pattern of CKD to ESRD Progression
2.3. “Classic” Pattern of CKD-ESRD Progression in a Caucasian Hypertensive Diabetic Male
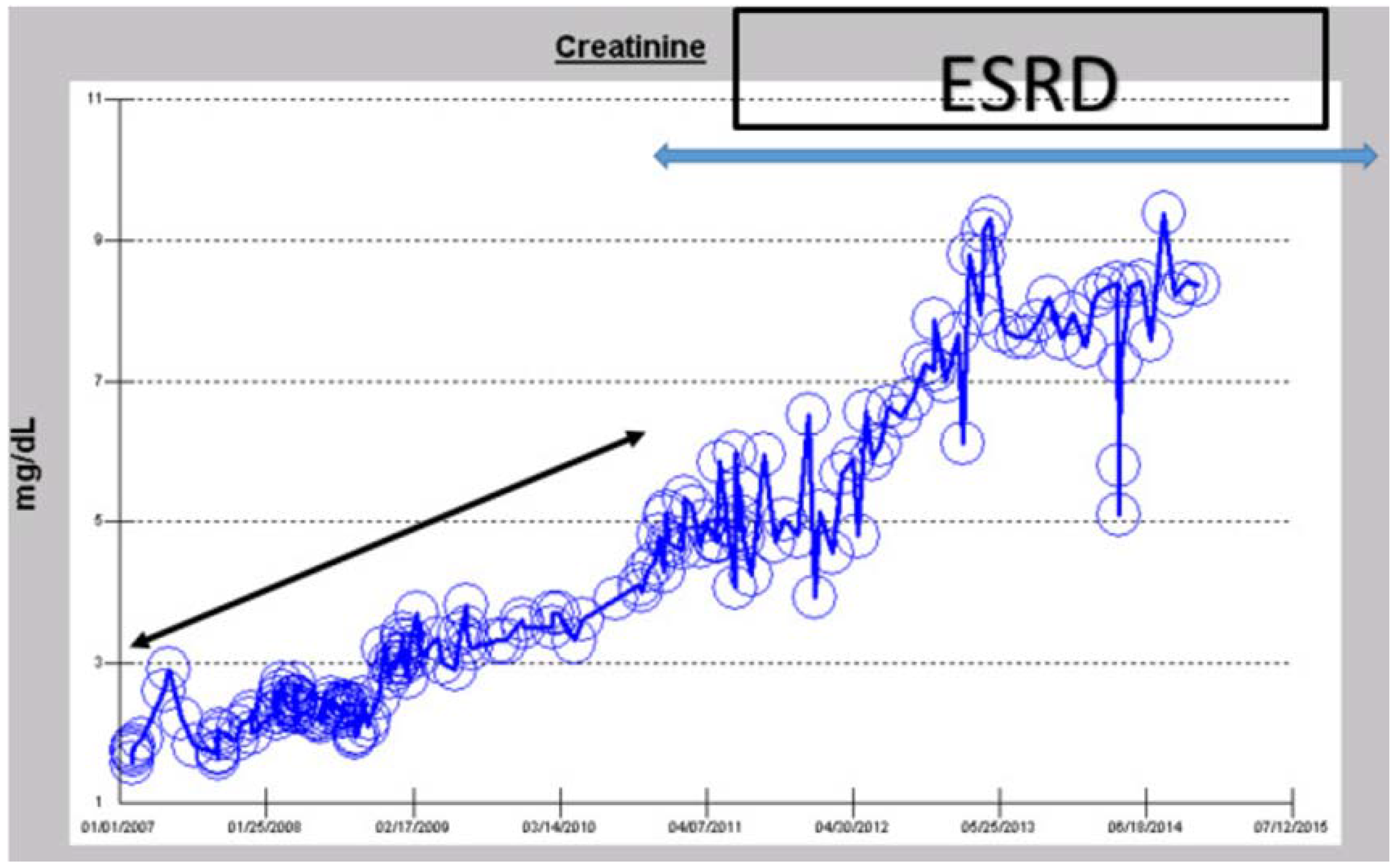
iv. The Syndrome of Rapid Onset ESRD in a Diabetic Following AKI Complicating an Elective Cardiothoracic Procedure
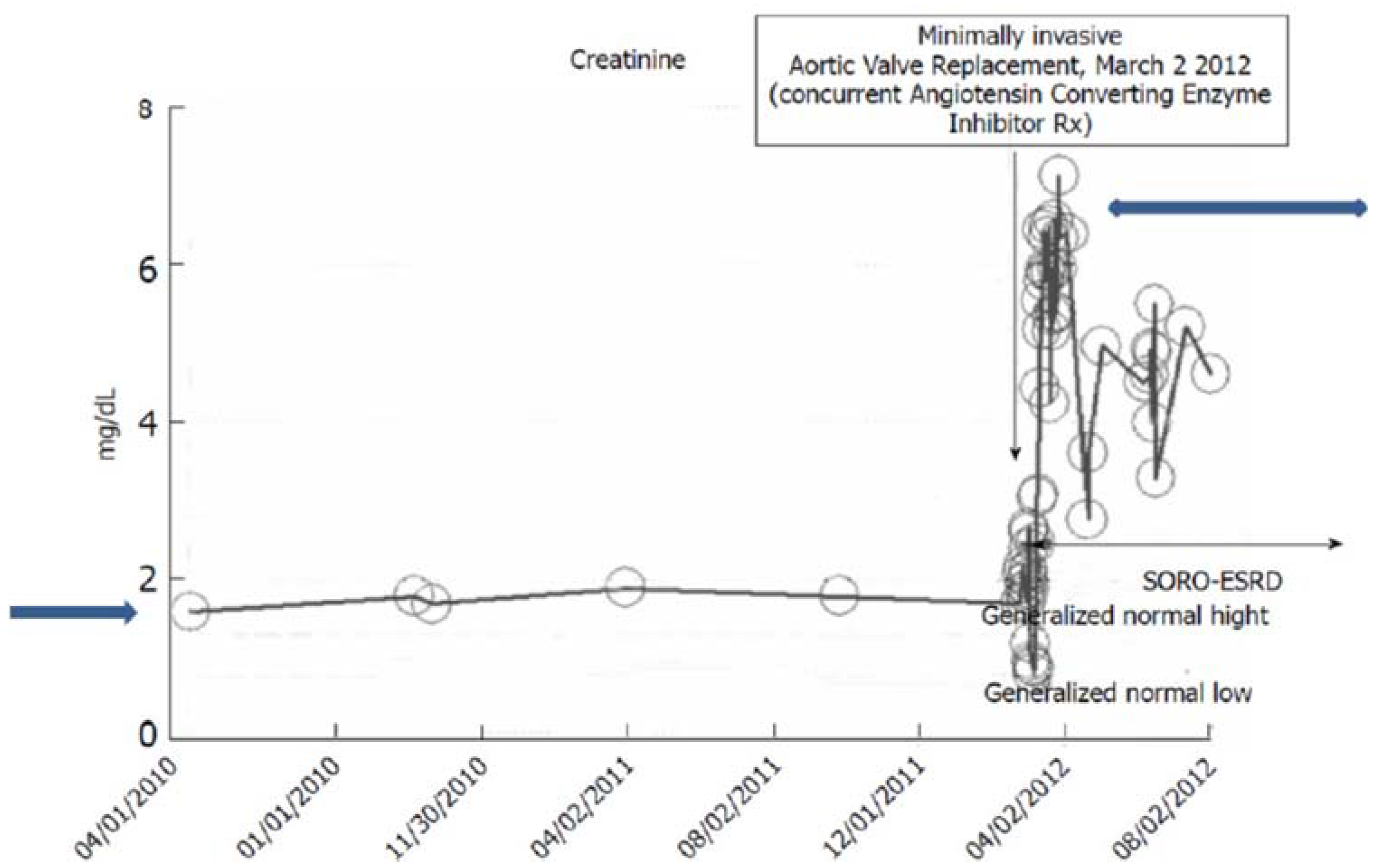
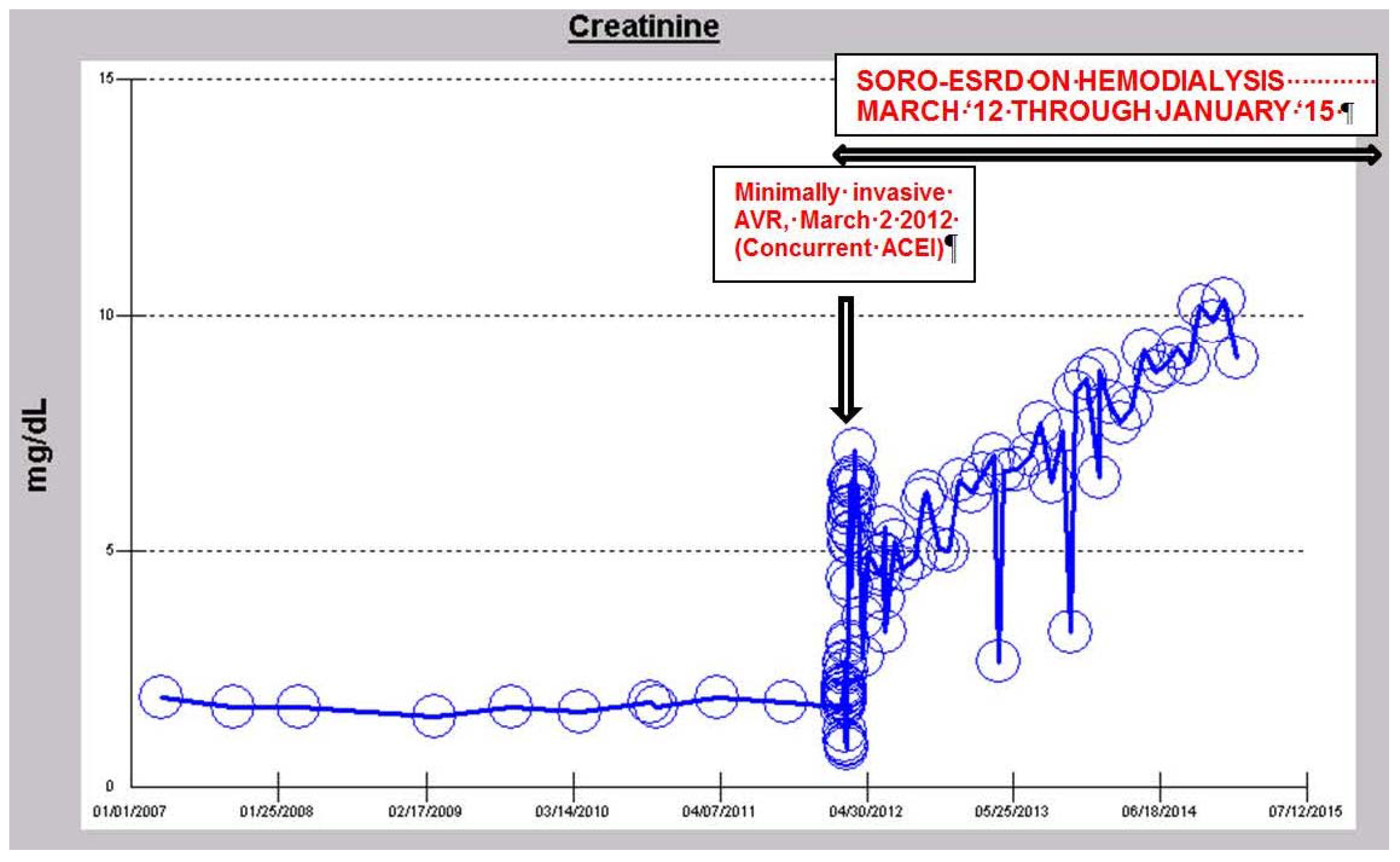
v. Late Onset End Stage Renal Failure from Angiotensin Blockade (LORFFAB)
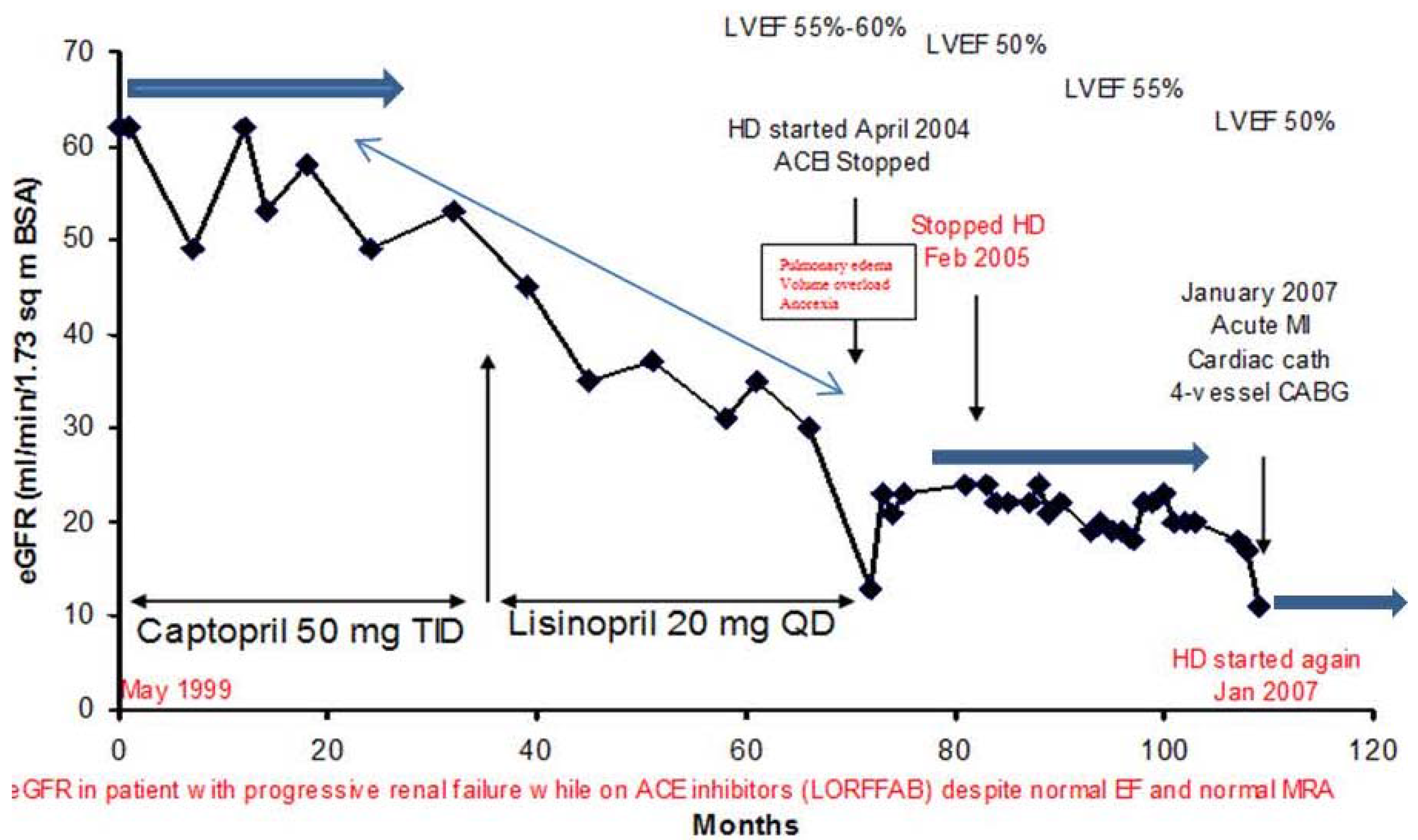
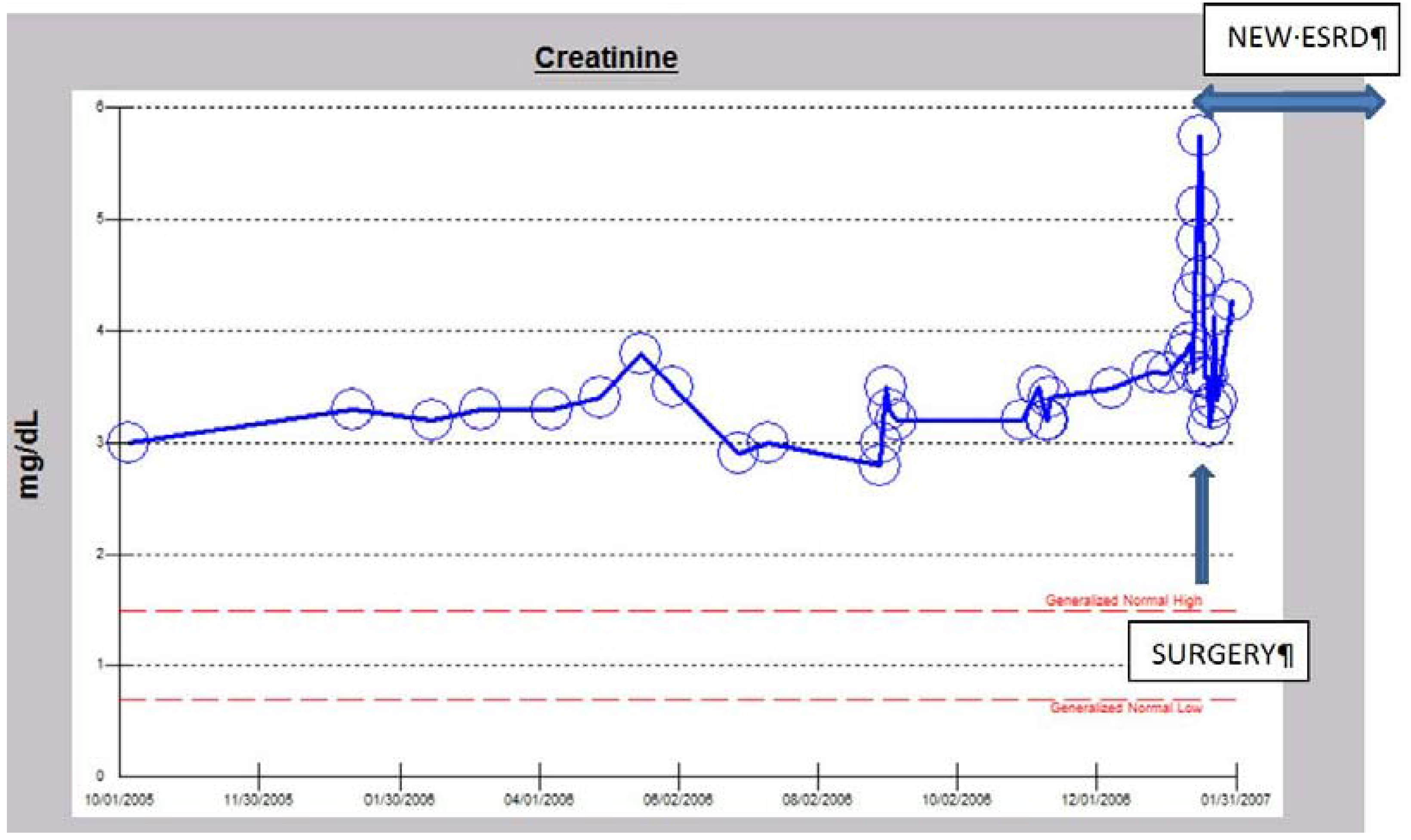
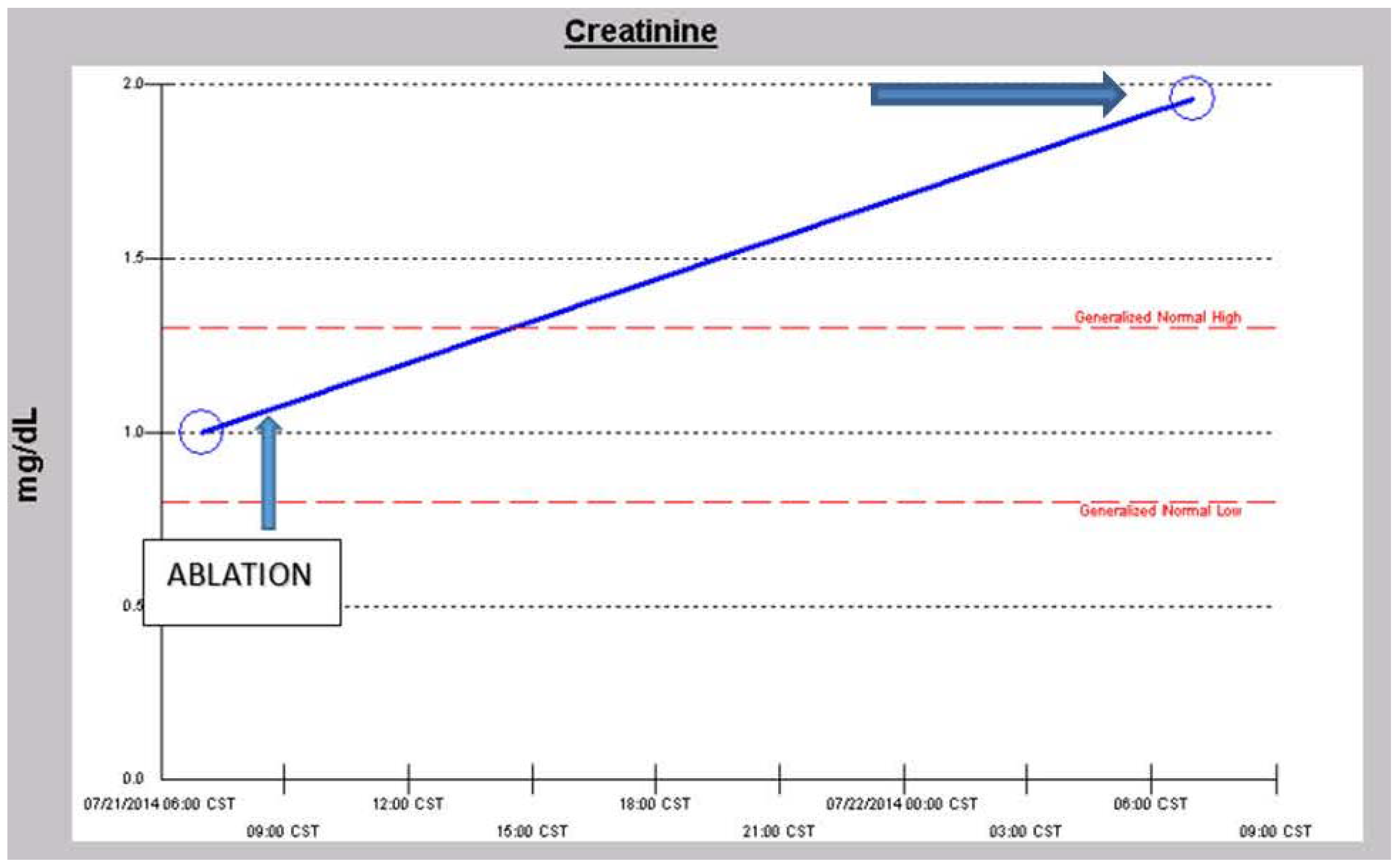
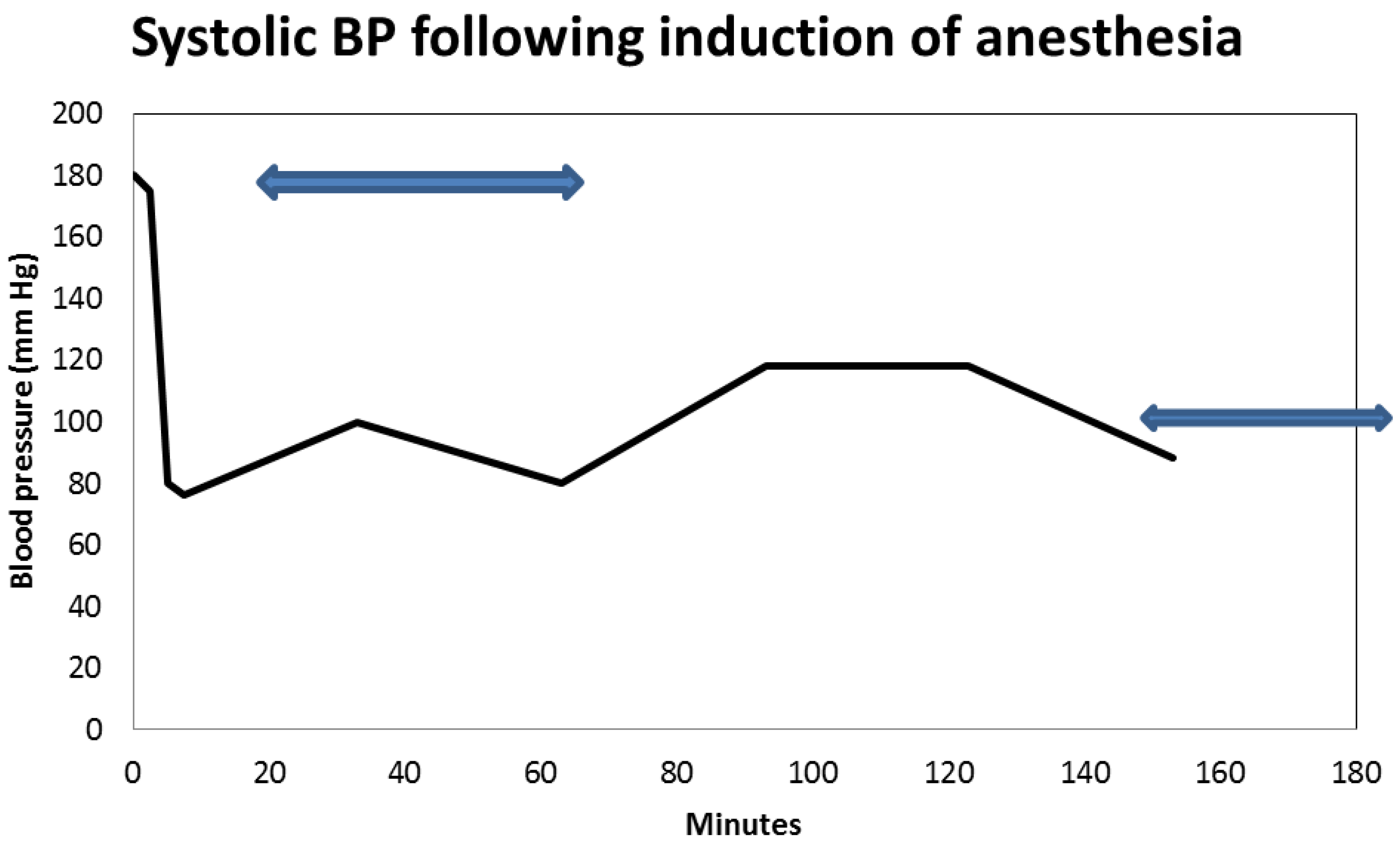
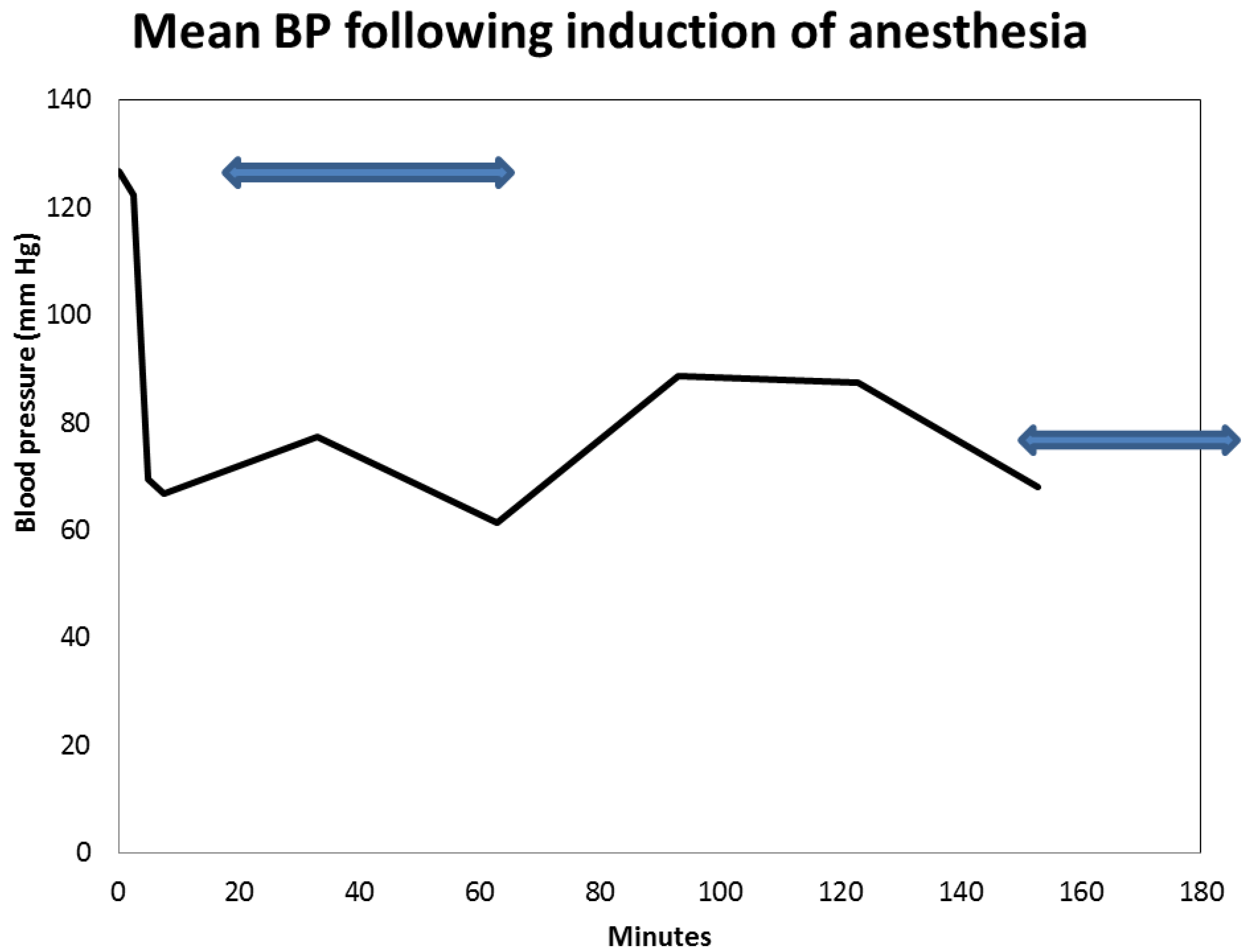
vi. Peri-Operative AKI in Diabetic Nephropathy and the Association with Intra-Operative Hypotension While Concurrently on Angiotensin Inhibition
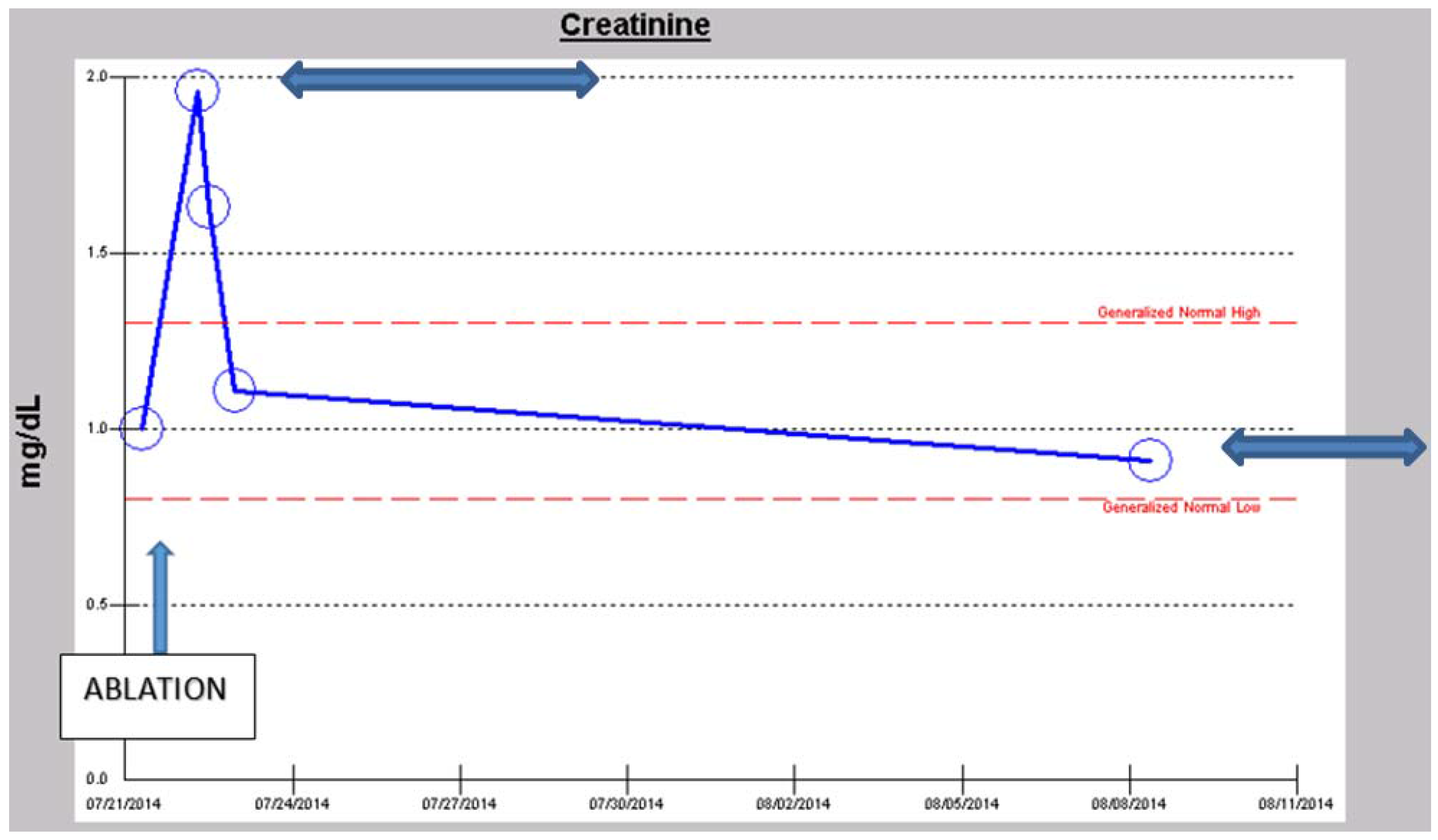
3. Conclusions
3.1. A Need for More Preventative Renal Medicine–Renoprevention Revisited: The Introduction of the New Innovative CKD Express © IT Software Application
3.2. GFR Loss in Diabetic Patients without or Independent of Albuminuria
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Boer, I.H.; Rue, T.C.; Cleary, P.A.; Lachin, J.M.; Molitch, M.E.; Steffes, M.W.; Sun, W.; Zinman, B.; Brunzell, J.D.; White, N.H.; et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: An analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch. Intern. Med. 2011, 171, 412–420. [Google Scholar]
- Perkins, B.A.; Ficociello, L.H.; Roshan, B.; Warram, J.H.; Krolewski, A.S. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010, 77, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Caramori, M.L.; Fioretto, P.; Mauer, M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: An indicator of more advanced glomerular lesions. Diabetes 2003, 52, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- MacIsaac, R.J.; Panagiotopoulos, S.; McNeil, K.J.; Smith, T.J.; Tsalamandris, C.; Hao, H.; Matthews, P.G.; Thomas, M.C.; Power, D.A.; Jerums, G. Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes Care 2006, 29, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A. Causes of renal failure in patients with type 2 diabetes mellitus. JAMA 2003, 290, 1855–1856. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, C.; Allen, T.J.; Gilbert, R.E.; Sinha, A.; Panagiotopoulos, S.; Cooper, M.E.; Jerums, G. Progressive decline in renal function in diabetic patients with and without albuminuria. Diabetes 1994, 43, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.H. Clinical Chemistry; John Wiley and Sons: New York, NY, USA, 1989; pp. 58–62. [Google Scholar]
- Levey, A.S. Measurement of renal function in chronic renal disease. Kidney Int. 1990, 38, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Ponte, B.; Felipe, C.; Muriel, A.; Tenorio, M.T.; Liano, F. Long-term functional evolution after an acute kidney injury: A 10-year study. Nephrol. Dial. Transplant. 2008, 23, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Wald, R.; Quinn, R.R.; Luo, J.; Li, P.; Li, P.; Scales, D.C.; Mamdani, M.M.; Ray, J.G. University of Toronto Acute Kidney Injury Research Group: Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009, 302, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A.; Achebe, N.J. Acute Kidney Injury on Chronic Kidney Disease-The Rainbow Syndrome of Too Many Colors: A Mayo Clinic Health System Case Series Report. In ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics; Onuigbo, M.A.C., Ed.; NOVA Publishers: New York, NY, USA, 2013; Volume 1, pp. 91–108. [Google Scholar]
- Onuigbo, M.A. Syndrome of rapid-onset end-stage renal disease: A new unrecognized pattern of CKD progression to ESRD. Ren. Fail. 2010, 32, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.; Agbasi, N.; Oguejiofor, O.; Okocha, E.; Aneke, C.; Odenigbo, C. Serum Creatinine Trajectories in Kidney Disease. In Biomarkers in Disease: Methods, Discoveries and Applications; Preedy, V., Ed.; Springer Publishers: London, UK, 2015. [Google Scholar]
- Onuigbo, M.A.; Achebe, N.J.; Musso, C.G. The syndrome of rapid onset ESRD in the last 100 consecutive incident Northwestern Wisconsin Mayo Clinic chronic hemodialysis patients, 2010–2011: Results of the analysis of individual patient-level serum creatinine trajectories to ESRD-Can there be a link with angiotensin inhibition and renal senescence in older CKD patients? In ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics; Onuigbo, M.A.C., Ed.; NOVA Publishers: New York, NY, USA, 2013; Volume 1, pp. 109–125. [Google Scholar]
- Onuigbo, M.A.; Onuigbo, N.T.; Musso, C.G. Syndrome of rapid onset end stage renal disease in incident Mayo Clinic chronic hemodialysis patient. Indian J. Nephrol. 2014, 24, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A. Syndrome of rapid-onset end-stage renal disease in two consecutive renal transplant recipients. Indian J. Nephrol. 2013, 23, 222–225. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.L.; Chien, K.L.; Lin, S.L.; Chen, Y.M.; Tsai, T.J.; Wu, K.D. Outcomes of stage 3–5 chronic kidney disease before endstage renal disease at a single center in Taiwan. Nephron. Clin. Pract. 2008, 109, c109–c118. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.; Agbasi, N. Chronic kidney disease prediction is an inexact science: The concept of “progressors” and “nonprogressors”. World J. Nephrol. 2014, 3, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Ballardie, F.W.; Gartside, S.; Mallick, N.P. Computer prediction of the need for dialysis and transplantation using calculated creatinine clearance. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 1328–1331. [Google Scholar] [CrossRef]
- Levin, A.; Djurdjev, O.; Beaulieu, M.; Er, L. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am. J. Kidney Dis. 2008, 52, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.D.; Baek, C.H.; Kim, J.S.; Kim, S.M.; Kim, J.H.; Kim, S.B. Does stage III chronic kidney disease always progress to endstage renal disease? A ten-year follow-up study. Scand. J. Urol. Nephrol. 2012, 46, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Walser, M.; Drew, H.H.; LaFrance, N.D. Reciprocal creatinine slopes often give erroneous estimates of progression of chronic renal failure. Kidney Int. 1989, 27 (Suppl.), S81–S85. [Google Scholar]
- Sikaneta, T.; Abdolell, M.; Taskapan, H.; Roscoe, J.; Fung, J.; Nagai, G.; Ting, R.H.; Ng, P.; Wu, G.; Oreopoulos, D.; et al. Variability in CKD stage in outpatients followed in two large renal clinics. Int. Urol. Nephrol. 2012, 44, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.V.; Levey, A.S. Spontaneous changes in the rate of decline in reciprocal serum creatinine: Errors in predicting the progression of renal disease from extrapolation of the slope. J. Am. Soc. Nephrol. 1992, 2, 1186–1191. [Google Scholar] [PubMed]
- Onuigbo, M.A. The natural history of chronic kidney disease revisited—A 72-month Mayo Health System Hypertension Clinic practice-based research network prospective report on end-stage renal disease and death rates in 100 high-risk chronic kidney disease patients: A call for circumspection. Adv. Perit. Dial. 2009, 25, 85–88. [Google Scholar] [PubMed]
- Onuigbo, M.A. The CKD enigma with misleading statistics and myths about CKD, and conflicting ESRD and death rates in the literature: Results of a 2008 U.S. population-based cross-sectional CKD outcomes analysis. Ren. Fail. 2013, 35, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A.; Onuigbo, C.; Onuigbo, V.; Onuigbo, M.; Onuigbo, N.; Joshi, M.; Egbuniwe, O. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: The introduction of the new “CKD express©” IT software program. In ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics; Onuigbo, M.A.C., Ed.; NOVA Publishers: New York, NY, USA, 2013; Volume 1, pp. 41–56. [Google Scholar]
- Onuigbo, M.A.; Onuigbo, N.T. The Syndrome of Rapid Onset End-Stage Renal Disease (SOROESRD)—A New Mayo Clinic Dialysis Services Experience, January 2010–February 2011. In Hemodialysis: When, How, Why; Di Iorio, B., Heidland, A., Onuigbo, M., Ronco, C., Eds.; NOVA Publishers: New York, NY, USA, 2012; pp. 443–485. [Google Scholar]
- Rutherford, W.E.; Blondin, J.; Miller, J.P.; Greenwalt, A.S.; Vavra, J.D. Chronic progressive renal disease: Rate of change of serum creatinine concentration. Kidney Int. 1977, 11, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A.; Onuigbo, N.T. Late onset renal failure from angiotensin blockade (LORFFAB): A prospective thirty-month Mayo Health System clinic experience. Med. Sci. Monit. 2005, 11, CR462–CR469. [Google Scholar] [PubMed]
- Onuigbo, M.A.; Onuigbo, N.T. Late-onset renal failure from angiotensin blockade (LORFFAB) in 100 CKD patients. Int. Urol. Nephrol. 2008, 40, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A.; Onuigbo, N.T. Late onset azotemia from RAAS blockade in CKD patients with normal renal arteries and no precipitating risk factors. Ren. Fail. 2008, 30, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A.; Achebe, N.J. Late Onset Renal Failure From Angiotensin Blockade (LORFFAB)-The Results of a Mayo Clinic Health System Angiotensin Inhibition Withdrawal Study: A Clarion Call For More Preventative Nephrology, Also Called Renoprevention. In ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics; Onuigbo, M.A.C., Ed.; NOVA Publishers: New York, NY, USA, 2013; Volume 1, pp. 75–90. [Google Scholar]
- Onuigbo, M.; Onuigbo, N. (Eds.) Chronic Kidney Disease and RAAS Blockade: A New View of Renoprotection; Lambert Academic Publishing GmbH 11 and Co. KG.: London, UK, 2011.
- Nielson, E.; Hennrikus, E.; Lehman, E.; Mets, B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J. Hosp. Med. 2014, 9, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A. Preoperative angiotensin axis blockade therapy, intraoperative hypotension, and the risks of postoperative acute kidney injury. J. Hosp. Med. 2014, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A. Renoprevention: A new concept for reengineering nephrology care—An economic impact and patient outcome analysis of two hypothetical patient management paradigms in the CCU. Ren. Fail. 2013, 35, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A. Renoprevention revisited--The impact of preemptive withdrawal of RAAS blockade prior to iodinated contrast exposure in older CKD patients: results of a new meta-analysis. Cardiology 2015, 130, 25–26. [Google Scholar] [CrossRef]
- Hunsicker, L.G.; Adler, S.; Caggiula, A.; England, B.K.; Greene, T.; Kusek, J.W.; Rogers, N.L.; Teschan, P.E. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997, 51, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gadegbeku, C.; Lipkowitz, M.S.; Rostand, S.; Lewis, J.; Wright, J.T.; Appel, L.J.; Greene, T.; Gassman, J.; Astor, B.C. Kidney function can improve in patients with hypertensive CKD. J. Am. Soc. Nephrol. 2012, 23, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Astor, B.C.; Lewis, J.; Hu, B.; Appel, L.J.; Lipkowitz, M.S.; Toto, R.D.; Wang, X.; Wright, J.T.; Greene, T.H. Longitudinal progression trajectory of GFR among patients with CKD. Am. J. Kidney Dis. 2012, 59, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Taskapan, H.; Tam, P.; Au, V.; Chow, S.; Fung, J.; Nagai, G.; Roscoe, J.; Ng, P.; Sikaneta, T.; Ting, R.; et al. Improvement in eGFR in patients with chronic kidney disease attending a nephrology clinic. Int. Urol. Nephrol. 2008, 40, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Weis, L.; Metzger, M.; Haymann, J.P.; Thervet, E.; Flamant, M.; Vrtovsnik, F.; Gauci, C.; Houillier, P.; Froissart, M.; Letavernier, E.; et al. Renal function can improve at any stage of chronic kidney disease. PLoS ONE 2013, 8, e81835. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.A. CKD Express ©—A New IT-Software Proposed for a Paradigm Change in CKD Care. Open Med. Inform. J. 2012, 6, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, M.; Agbasi, N. Medicare and the Escalating Costs of US ESRD Care Compared with the United Kingdom National Health Service: A Case Study of Reengineering Current US CDC Hepatitis B Virus Testing Protocols among Hemodialysis Patients to Achieve Cost Savings—The Use of a New IT Software Program, The CKD Express ©, to Achieve Cost-effective CKD Care for the US Medicare. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; NOVA Publishers: New York, NY, USA, 2014; Volume 76, pp. 21–38. [Google Scholar]
- Onuigbo, M.; Agbasi, N. Escalating Cost of Pre-dialysis Chronic Kidney Disease Care in the US: The Introduction of an Innovative Chronic Kidney Disease Care Paradigm Using “The CKD Express © IT Software Program”—A New Recommended Tool for Medicare and the Affordable Care Act. Open Med. Inform. J. 2015, in press. [Google Scholar]
- Middleton, R.J.; Foley, R.N.; Hegarty, J.; Cheung, C.M.; McElduff, P.; Gibson, J.M.; Kalra, P.A.; O’Donoghue, D.J.; New, J.P. The unrecognized prevalence of chronic kidney disease in diabetes. Nephrol. Dial. Transplant. 2006, 21, 88–92. [Google Scholar] [CrossRef] [PubMed]
- New, J.P.; Middleton, R.J.; Klebe, B.; Farmer, C.K.; de Lusignan, S.; Stevens, P.E.; O’Donoghue, D.J. Assessing the prevalence, monitoring and management of chronic kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet. Med. 2007, 24, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Komatsu, Y.; Mifune, M.; Antoku, S.; Ishida, H.; Takeuchi, Y.; Togane, M. The estimated GFR, but not the stage of diabetic nephropathy graded by the urinary albumin excretion, is associated with the carotid intima-media thickness in patients with type 2 diabetes mellitus: A cross-sectional study. Cardiovasc. Diabetol. 2010, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Halimi, J.M. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes MeTable 2012, 38, 291–297. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onuigbo, M.A.C.; Agbasi, N. Diabetic Nephropathy and CKD—Analysis of Individual Patient Serum Creatinine Trajectories: A Forgotten Diagnostic Methodology for Diabetic CKD Prognostication and Prediction. J. Clin. Med. 2015, 4, 1348-1368. https://doi.org/10.3390/jcm4071348
Onuigbo MAC, Agbasi N. Diabetic Nephropathy and CKD—Analysis of Individual Patient Serum Creatinine Trajectories: A Forgotten Diagnostic Methodology for Diabetic CKD Prognostication and Prediction. Journal of Clinical Medicine. 2015; 4(7):1348-1368. https://doi.org/10.3390/jcm4071348
Chicago/Turabian StyleOnuigbo, Macaulay Amechi Chukwukadibia, and Nneoma Agbasi. 2015. "Diabetic Nephropathy and CKD—Analysis of Individual Patient Serum Creatinine Trajectories: A Forgotten Diagnostic Methodology for Diabetic CKD Prognostication and Prediction" Journal of Clinical Medicine 4, no. 7: 1348-1368. https://doi.org/10.3390/jcm4071348
APA StyleOnuigbo, M. A. C., & Agbasi, N. (2015). Diabetic Nephropathy and CKD—Analysis of Individual Patient Serum Creatinine Trajectories: A Forgotten Diagnostic Methodology for Diabetic CKD Prognostication and Prediction. Journal of Clinical Medicine, 4(7), 1348-1368. https://doi.org/10.3390/jcm4071348





