Malignant Cardiac Tamponade from Non-Small Cell Lung Cancer: Case Series from the Era of Molecular Targeted Therapy
Abstract
:1. Introduction
| Case | Age | Sex | NSCLC histology | Driver mutation | Smoking history | Presentation of cardiac tamponade in relation to NSCLC diagnosis | Initial intervention | Recurrence of cardiac tamponade and subsequent intervention | Pericardial fluid cytology | Performance status after intervention | Cancer therapy prior | Cancer therapy after | Survival after cardiac tamponade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | Adenocarcinoma | Wild type for EGFR, ALK | Smoker 25 pack years | Presentation at diagnosis | Surgical subxiphoid pericardia-peritoneal window | No | Adenocarcinoma | ECOG 1 | None | Carboplatin and gemcitabine 4 cycles | 6 months |
| 2 | 65 | F | Adenocarcinoma | EGFR mutation exon 21 L858R | Never | 2 years after stage IIA NSCLC | Surgical subxiphoid pericardia-peritoneal window | No | Adenocarcinoma | ECOG 2 | Left upper lobectomy | Erlotinib | Alive at 15 months |
| 3 | 49 | M | Large cell neuroendocrine carcinoma | Unknown | Smoker 20 pack years | Presentation at diagnosis | Surgical subxiphoid pericardial-peritoneal window | No | No malignant cells | ECOG 1 | None | Concurrent chemoradiation to mediastinum, carboplatin and etoposide 6 cycles, prophylactic cranial irradiation | Alive at 17 months |
| 4 | 49 | F | Adenocarcinoma | Wild type for EGFR, ALK | Never | 4 months after stage IV NSCLC | Surgical subxiphoid pericardial-peritoneal window | Yes, 2 months after, thoracoscopic pericardial-pleural window | Adenocarcinoma | ECOG 1 | Carboplatin and gemcitabine 4 cycles | Nanoparticle albumin bound paclitaxel 2 cycles | 3 months |
| 5 | 48 | M | Adenocarcinoma | Wild type for EGFR, ALK | Never | 10 months after stage IV NSCLC | Surgical subxiphoid pericardial-peritoneal window | Yes, 3 weeks after, thoracotomy pericardial-pleural window | Adenocarcinoma | ECOG 1 | Carboplatin and pemetrexed 5 cycles | Erlotinib | 3 months |
| 6 | 70 | F | Adenocarcinoma | Wild type for EGFR, ALK | Never | 2 months after stage IV NSCLC | Pericardiocentesis and percutaneous drain | Yes, 1 month after, repeat pericardiocentesis and percutaneous drain, surgical subxiphoid pericardial-peritoneal window | Adenocarcinoma | ECOG 4 | Carboplatin and gemcitabine 1 cycle | None | 2 months |
| 7 | 62 | M | Large cell carcinoma | Unknown | Ex-smoker, 40 pack years | 4 months after stage IIIB NSCLC | Pericardiocentesis and percutaneous drain | No | Atypical cells | ECOG 4 | Cisplatin and etoposide 1 cycle | None | 9 days |
| 8 | 55 | F | Adenocarcinoma | Wild type for EGFR, ALK | Ex-smoker, 30 pack years | 2 years after stage IV NSCLC | Surgical subxiphoid pericardial window | No | Adenocarcinoma | ECOG 3 | Carboplatin and gemcitabine 6 cycles, pemetrexed 3 cycles, radiotherapy to axillary lymph nodes, erlotinib 3 months, paclitaxel 5 months | None | 7 months |
2. Case 1
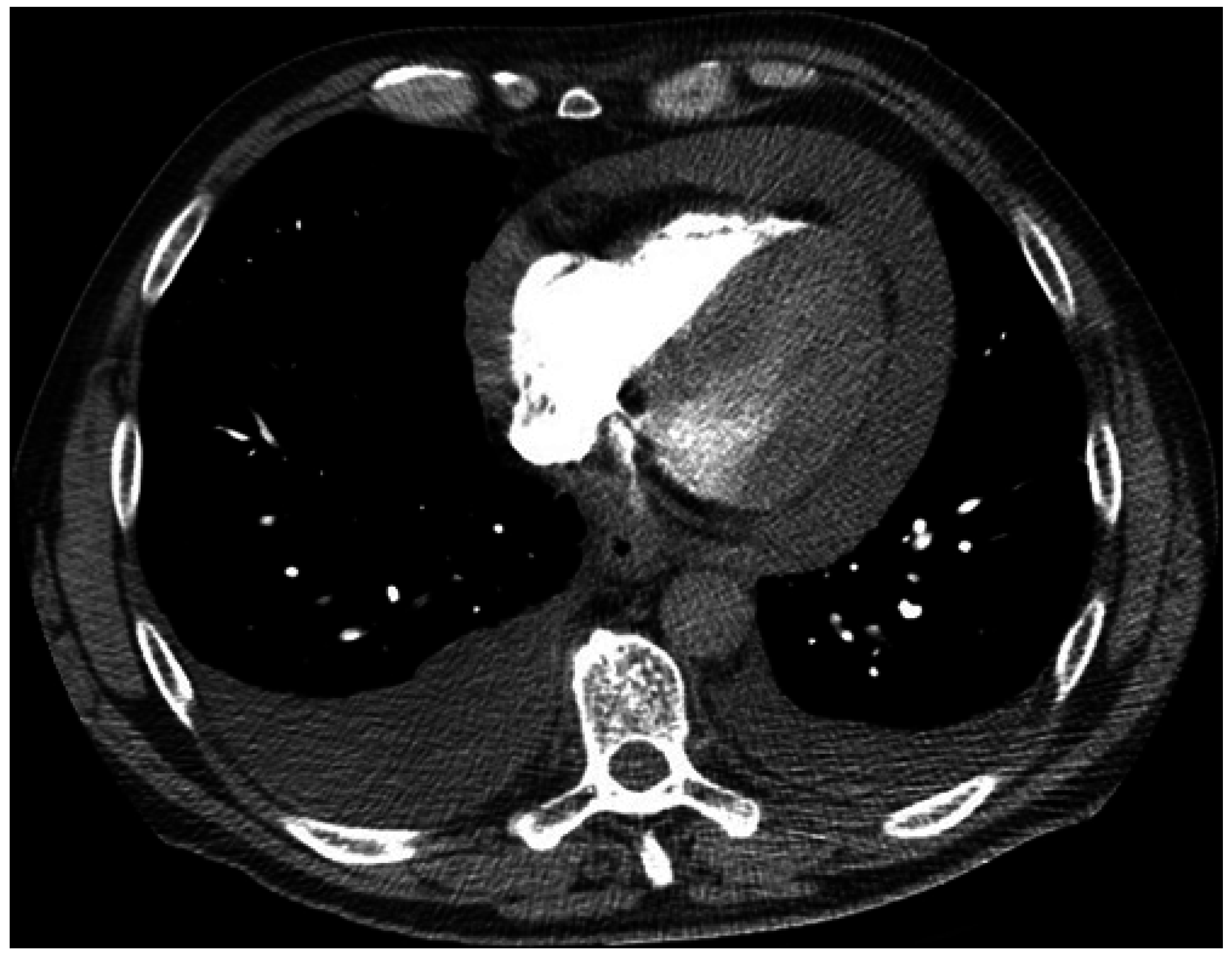
3. Case 2

4. Case 3
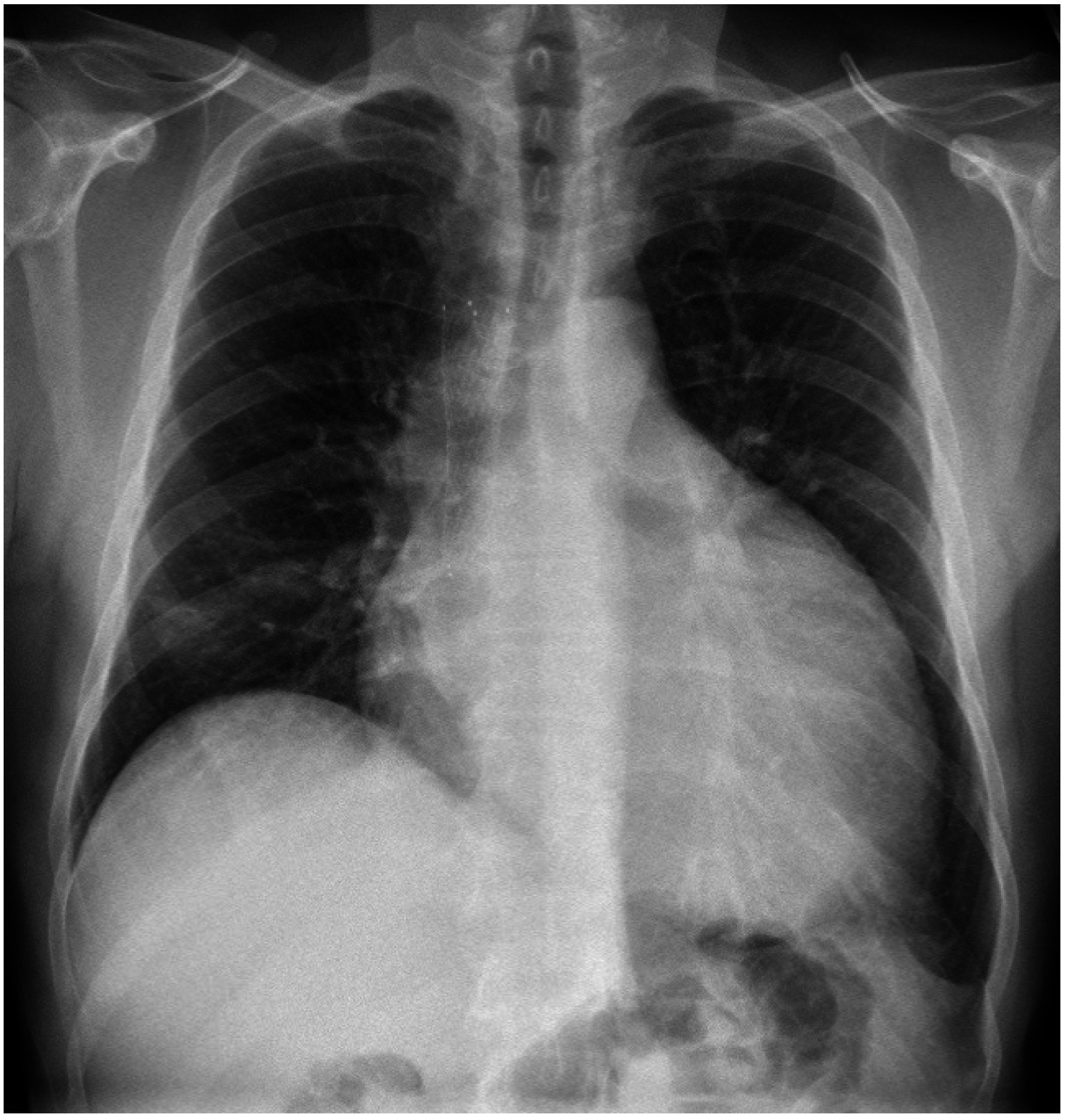
5. Discussion
6. Conclusions
Author Contributions
Practice Points
- Cardiac tamponade complicating malignant pericardial effusion is an understudied oncological emergency.
- The most common underlying cause is lung cancer, predominantly through regional lymphatic drainage.
- Prognosis is generally poor after malignant cardiac tamponade but longer term survival is possible in some patients after successful systemic therapy such as molecular targeted therapy.
- Surgical pericardial window may provide durable palliation in suitably fit patients and should be considered in clinical practice.
- More research is required to guide evidence based personalized treatment for this understudied oncological emergency.
Conflicts of Interest
References
- Fraser, R.S.; Viloria, J.B.; Wang, N.S. Cardiac tamponade as a presentation of extracardiac malignancy. Cancer 1980, 45, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Klatt, E.C.; Heitz, D.R. Cardiac metastases. Cancer 1990, 65, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Dickens, P.; Chan, A.C. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch. Pathol. Lab. Med. 1993, 117, 1027–1031. [Google Scholar] [PubMed]
- Gornik, H.L.; Gerhard-Herman, M.; Beckman, J.A. Abnormal cytology predicts poor prognosis in cancer patients with pericardial effusion. J. Clin. Oncol. 2005, 23, 5211–5216. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Matsubara, O.; Yoshimura, N.; Kasuga, T.; Akagawa, S.; Aoki, N. Cardiac metastasis of lung cancer. A study of metastatic pathways and clinical manifestations. Cancer 1992, 70, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Yang, K.Y.; Chao, J.Y.; Liu, J.M.; Perng, R.P.; Yen, S.H. Prognostic role of pericardial fluid cytology in cardiac tamponade associated with non-small cell lung cancer. Chest 2000, 118, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.A.; Paz, I.B.; Smith, D.; Carter, N.; Grannis, F.W., Jr. Prognostic factors in the surgical management of pericardial effusion in the patient with concurrent malignancy. Chest 2004, 125, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Shinkai, T.; Yamakido, M.; Saijo, N. Cardiac tamponade caused by primary lung cancer and the management of pericardial effusion. Cancer 1993, 71, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A. Malignant pericardial effusion. Curr. Opin. Oncol. 1997, 9, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Wu, Y.L.; Zhang, L. A small step towards personalized medicine for non-small cell lung cancer. Discov. Med. 2009, 8, 227–231. [Google Scholar] [PubMed]
- Varlotto, J.M.; Medford-Davis, L.N.; Recht, A.; Flickinger, J.C.; Schaefer, E.; Zander, D.S.; DeCamp, M.M. Should large cell neuroendocrine lung carcinoma be classified and treated as a small cell lung cancer or with other large cell carcinomas? J. Thorac. Oncol. 2011, 6, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Moriya, T.; Takiguchi, Y.; Tabeta, H.; Watanabe, R.; Kimura, H.; Nagao, K.; Kuriyama, T. Controlling malignant pericardial effusion by intrapericardial carboplatin administration in patients with primary non-small-cell lung cancer. Br. J. Cancer 2000, 83, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Kunitoh, H.; Tamura, T.; Shibata, T.; Imai, M.; Nishiwaki, Y.; Nishio, M.; Yokoyama, A.; Watanabe, K.; Noda, K.; Saijo, N. A randomised trial of intrapericardial bleomycin for malignant pericardial effusion with lung cancer (JCOG9811). Br. J. Cancer 2009, 100, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Sosinska-Mielcarek, K.; Senkus-Konefka, E.; Jassem, J.; Kulczycka, J.; Jendrzejewski, J.; Jaskiewicz, K. Cardiac involvement at presentation of non-small-cell lung cancer. J. Clin. Oncol. 2008, 26, 1010–1011. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Crump, M.; Goss, P.E.; Dancey, J.; Shepherd, F.A. Prospective comparison of the sclerosing agents doxycycline and bleomycin for the primary management of malignant pericardial effusion and cardiac tamponade. J. Clin. Oncol. 1996, 14, 3141–3147. [Google Scholar] [PubMed]
- DeCamp, M.M., Jr.; Mentzer, S.J.; Swanson, S.J.; Sugarbaker, D.J. Malignant effusive disease of the pleura and pericardium. Chest 1997, 112 (Suppl. S4), 291–295. [Google Scholar] [CrossRef]
- Celik, S.; Celik, M.; Aydemir, B.; Tanrikulu, H.; Okay, T.; Tanrikulu, N. Surgical properties and survival of a pericardial window via left minithoracotomy for benign and malignant pericardial tamponade in cancer patients. World J. Surg. Oncol. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Bischiniotis, T.S.; Lafaras, C.T.; Platogiannis, D.N.; Moldovan, L.; Barbetakis, N.G.; Katseas, G.P. Intrapericardial cisplatin administration after pericardiocentesis in patients with lung adenocarcinoma and malignant cardiac tamponade. Hell. J. Cardiol. 2005, 46, 324–329. [Google Scholar]
- Maruyama, R.; Yokoyama, H.; Seto, T.; Nagashima, S.; Kashiwabara, K.; Araki, J.; Semba, H.; Ichinose, Y. Catheter drainage followed by the instillation of bleomycin to manage malignant pericardial effusion in non-small cell lung cancer: A multi-institutional phase II trial. J. Thorac. Oncol. 2007, 2, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Lestuzzi, C.; Bearz, A.; Lafaras, C.; Gralec, R.; Cervesato, E.; Tomkowski, W.; DeBiasio, M.; Viel, E.; Bishiniotis, T.; Platogiannis, D.N.; et al. Neoplastic pericardial disease in lung cancer: Impact on outcomes of different treatment strategies. A multicenter study. Lung Cancer 2011, 72, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Lestuzzi, C.; Cervesato, E.; Lafaras, C.; Celik, S.; Dequanter, D.; Tomkowski, W.; de Biasio, M.; Moreo, A.; Piotti, P.; Imazio, M. Which is the best approach for neoplastic pericardial effusion? A retrospective analysis of 264 cases. Eur. Heart J. 2013, 34 (Suppl. S1), 820–821. [Google Scholar] [CrossRef]
- Celik, S.; Lestuzzi, C.; Cervesato, E.; Dequanter, D.; Piotti, P.; de Biasio, M.; Imazio, M. Systemic chemotherapy in combination with pericardial window has better outcomes in malignant pericardial effusions. J. Thorac. Cardiovasc. Surgery 2014, 148, 2288–2293. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.T.; Pearson, A.; Pavlakis, N.; Bell, D.; Lee, A.; Chan, D.; Harden, M.; Mathur, M.; Marshman, D.; Brady, P.; et al. Malignant Cardiac Tamponade from Non-Small Cell Lung Cancer: Case Series from the Era of Molecular Targeted Therapy. J. Clin. Med. 2015, 4, 75-84. https://doi.org/10.3390/jcm4010075
Li BT, Pearson A, Pavlakis N, Bell D, Lee A, Chan D, Harden M, Mathur M, Marshman D, Brady P, et al. Malignant Cardiac Tamponade from Non-Small Cell Lung Cancer: Case Series from the Era of Molecular Targeted Therapy. Journal of Clinical Medicine. 2015; 4(1):75-84. https://doi.org/10.3390/jcm4010075
Chicago/Turabian StyleLi, Bob T., Antonia Pearson, Nick Pavlakis, David Bell, Adrian Lee, David Chan, Michael Harden, Manu Mathur, David Marshman, Peter Brady, and et al. 2015. "Malignant Cardiac Tamponade from Non-Small Cell Lung Cancer: Case Series from the Era of Molecular Targeted Therapy" Journal of Clinical Medicine 4, no. 1: 75-84. https://doi.org/10.3390/jcm4010075
APA StyleLi, B. T., Pearson, A., Pavlakis, N., Bell, D., Lee, A., Chan, D., Harden, M., Mathur, M., Marshman, D., Brady, P., & Clarke, S. (2015). Malignant Cardiac Tamponade from Non-Small Cell Lung Cancer: Case Series from the Era of Molecular Targeted Therapy. Journal of Clinical Medicine, 4(1), 75-84. https://doi.org/10.3390/jcm4010075






