Clinical Results of Hypomethylating Agents in AML Treatment
Abstract
:1. Introduction
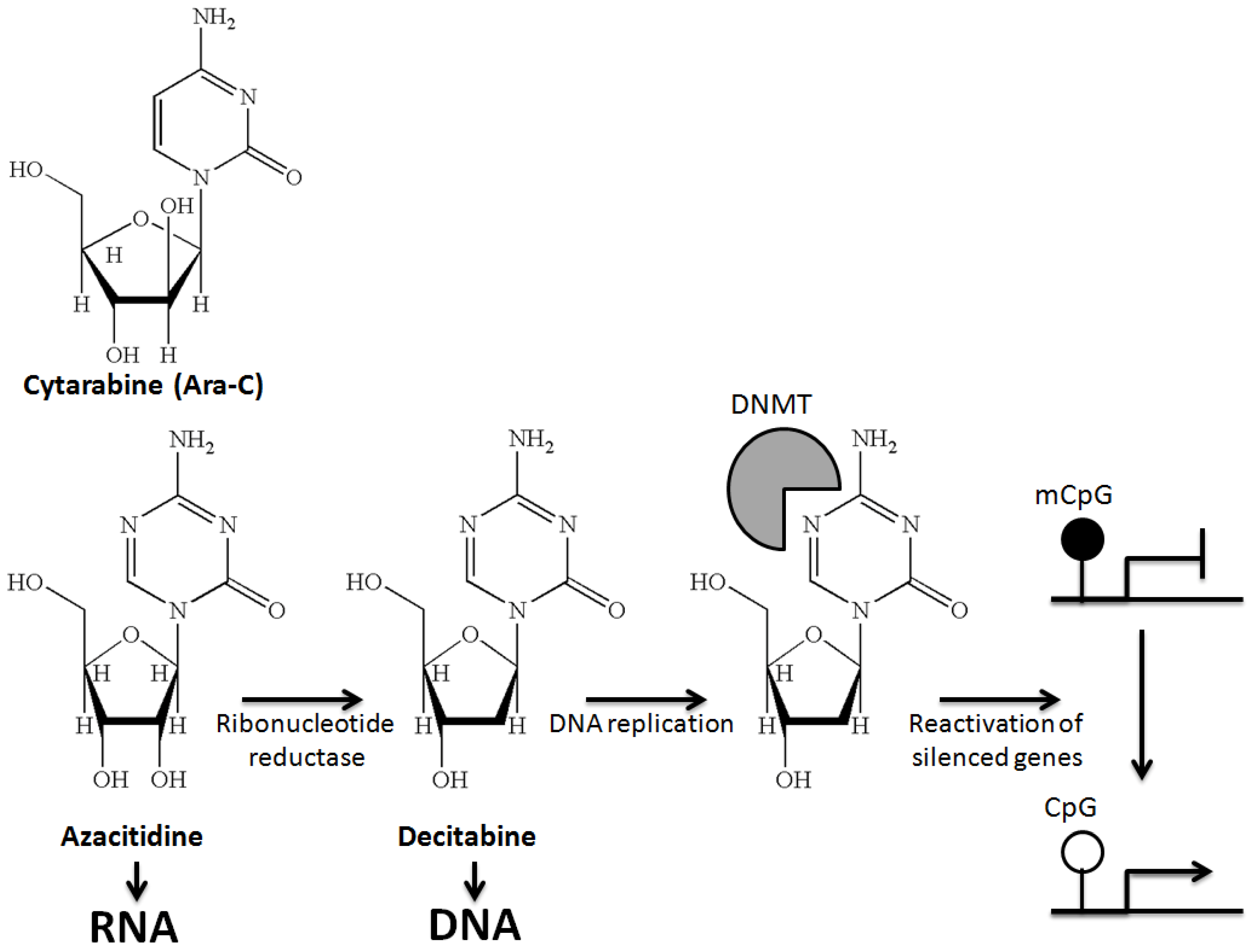
2. DNA Hypomethylating Agents
3. Azacitidine and AML
4. Decitabine and AML
5. Azacitidine and Decitabine in 10-Day Schedules
| Azacitidine (7 Days 75 mg/m2 SC; Every 4 Weeks) | ||||
|---|---|---|---|---|
| Study | Competitors | CR (%) | Median OS | 1/2-Year OS |
| Post hoc analysis CALGB 9221 (AML 20%–30% blasts) [11] | AZA (n = 27) vs. Observation (n = 12) | 7% vs. 0% | 19.3 months vs. NA; Combining CALGB 8421, 8921, 9221: 12.9 months (n = 25; p = NA) | NA |
| Post hoc analysis AZA001 study (AML 20%–30% blasts) [12] | Aza (n = 55) vs. CCR (n = 58) (BSC = 27/LDAC = 20/IC = 11) | 18% vs. 16% | 24.5 months vs. 16 months (p = 0.005) | 50% vs. 16% (p = 0.001) (2-year OS) |
| AML001 study (AML >30% blasts) [13] | Aza (n = 241) vs. CCR (n = 247) (BSC = 45/LDAC = 158/IC = 44) | 20% vs. 22% | 10.4 months vs. 6.5 months (p = 0.08). Analysis censored for subsequent Tx: 12.1 months vs. 6.9 months (p = 0.01) | 46.5% vs. 34.2% (p = NA) (1-year OS) |
| Decitabine (5 Days 20 mg/m2 IV; Every 4 Weeks) | ||||
| DACO-016 (AML >20% blasts; only intermediate and poor risk) [19] | Decit (n = 242) vs. TC (n = 243) (BSC = 28/LDAC = 215) | 15.7% vs. 7.4% | 7.7 months vs. 5.0 months (p = 0.11). Analysis censored for subsequent Tx: 8.5 months vs. 5.3 months (p = 0.04). Unplanned analysis after 446 deaths: 7.7 months vs. 5.0 months (p = 0.04) | NA |
| Azacitidine | |||
|---|---|---|---|
| Study | Dosing | CR (%) | Median OS |
| Post hoc analysis AML001 study (phase 3) (AML 20%–30% blasts) [12] | Aza (n = 55) 7 days 75 mg/m2 SC; every 4 weeks | 18% | 24.5 months |
| AML001 study (phase 3) (AML >30% blasts) [13] | Aza (n = 241) 7 days 75 mg/m2 SC; every 4 weeks | 20% | 10.4 months |
| United States Leukemia Intergroup Trial E1905 (phase 2) [20] | Aza (n = 74) 10 days 50 mg/m2 SC; every 4 weeks | 12% | 18 months |
| Decitabine | |||
| German phase 2 study [14] | Decit (n = 227) 3 days (135 mg/m2 total) | 13% | 5.5 months |
| DACO-016 (phase 3) (AML >20% blasts; only intermediate and poor risk) [19] | Decit (n = 242) 5 days 20 mg/m2 | 15.7% | 7.7 months |
| Ohio State University experience (phase 2) [16] | Decit (n = 53) 10 days 20 mg/m2 | 47% | 12.7 months |
| Cornell University experience (report of retrospective analysis) [17] | Decit (n = 52) 10 days 20 mg/m2 | 40% (after excluding 6 patients who received prior azanucleotide CR = 46%) | 10.5 months |
6. Hypomethylating Agents as Maintenance Therapy
7. DNA Hypomethylating Agent Therapy as a Bridging Strategy to AlloHCT
8. Use of Hypomethylating Agents after AlloHCT
9. Treatment of Older AML Patients
10. Conclusions
Author Contributions
Conflicts of Interest
References
- Gronbaek, K.; Hother, C.; Jones, P.A. Epigenetic changes in cancer. APMIS 2007, 115, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Baylin, S.B. The epigenomics of cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Skrabanek, L.; Li, Y.; Jiemjit, A.; Fandy, T.E.; Paietta, E.; Fernandez, H.; Tallman, M.S.; Greally, J.M.; Carraway, H.; et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood 2009, 114, 3448–3458. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Lugthart, S.; Li, Y.; Erpelinck-Verschueren, C.; Deng, X.; Christos, P.J.; Schifano, E.; Booth, J.; van Putten, W.; Skrabanek, L.; et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell 2010, 17, 13–17. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar]
- Patel, J.P.; Gönen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1079–1089. [Google Scholar] [CrossRef]
- Jüttermann, R.; Li, E.; Jaenisch, R. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc. Natl. Acad. Sci. USA 1994, 91, 11797–11801. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, O.; Agathanggelou, A.; Novitzky-Basso, I.; Siddique, S.; McSkeane, T.; Ryan, G.; Vyas, P.; Cavenagh, J.; Stankovic, T.; Moss, P.; et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood 2010, 116, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellström-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Silverman, L.R.; McKenzie, D.R.; Peterson, B.L.; Holland, J.F.; Backstrom, J.T.; Beach, C.L.; Larson, R.A. Cancer and Leukemia group B Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J. Clin. Oncol. 2006, 20, 3895–3903. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellström-Lindberg, E.; Santini, V.; Gattermann, N.; Germing, U.; Sanz, G.; List, A.F.; Gore, S.; Seymour, J.F.; et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukaemia. J. Clin. Oncol. 2010, 28, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.; Candoni, A.; et al. Results of a phase 3, multicenter, randomized, open-label study of azacitidine vs. conventional care regimens in older patients with newly diagnosed acute myeloid leukemia. In Proceedings of the 19th Congres of the European Hematology Association, Milan, Italy, 12–15 June 2014.
- Lübbert, M.; Rüter, B.H.; Claus, R.; Schmoor, C.; Schmid, M.; Germing, U.; Kuendgen, A.; Rethwisch, V.; Ganser, A.; Platzbecker, U.; et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica 2012, 97, 393–401. [Google Scholar] [CrossRef]
- Kantarjian, H.; Oki, Y.; Garcia-Manero, G.; Huang, X.; O’Brien, S.; Cortes, J.; Faderl, S.; Bueso-Ramos, C.; Ravandi, F.; Estrov, Z. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2007, 109, 52–57. [Google Scholar] [CrossRef]
- Blum, W.; Garzon, R.; Klisovic, R.B.; Schwind, S.; Walker, A.; Geyer, S.; Liu, S.; Havelange, V.; Becker, H.; Schaaf, L.; et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc. Natl. Acad. Sci. USA 2010, 107, 7473–7478. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, E.K.; Feldman, E.J.; Christos, P.J.; Rohan, S.D.; Lagassa, C.B.; Ippoliti, C.; Scandura, J.M.; Carlson, K.; Roboz, G.J. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk. Lymphoma 2013, 54, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Baer, M.R.; Slack, J.L.; Buckstein, R.; Godley, L.A.; Garcia-Manero, G.; Albitar, M.; Larsen, J.S.; Arora, S.; Cullen, M.T.; et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: The alternative dosing for outpatient treatment (ADOPT) trial. J. Clin. Oncol. 2009, 27, 3842–3848. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.P.; Chou, W.C.; Buckstein, R.; Cermak, J.; et al. Multicenter, randomized, open-label, phase III trial of decitabine vs. patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Prebet, T.; Sun, Z.; Figueroa, M.E.; Ketterling, R.; Melnick, A.; Greenberg, P.L.; Herman, J.; Juckett, M.; Smith, M.R.; Malick, L.; et al. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: Results of the US Leukemia Intergroup trial E1905. J. Clin. Oncol. 2014, 32, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, B.; Ossenkoppele, G.J.; van Putten, W.; Schouten, H.C.; Graux, C.; Ferrant, A.; Sonneveld, P.; Maertens, J.; Jongen-Lavrencic, M.; von Lilienfeld-Toal, M.; et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Grövdal, M.; Karimi, M.; Khan, R.; Aggerholm, A.; Antunovic, P.; Astermark, J.; Bernell, P.; Engström, L.M.; Kjeldsen, L.; Linder, O.; et al. Maintenance treatment with azacytidine for patients with high-risk myelodysplastic syndromes (MDS) or acute myeloid leukaemia following MDS in complete remission after induction chemotherapy. Br. J. Haematol. 2010, 150, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Boumber, Y.; Kantarjian, H.; Jorgensen, J.; Wen, S.; Faderl, S.; Castoro, R.; Autry, J.; Garcia-Manero, G.; Borthakur, G.; Jabbour, E.; et al. A randomized study of decitabine vs. conventional care for maintenance therapy in patients with acute myeloid leukemia in complete remission. Leukemia 2012, 26, 2428–2431. [Google Scholar] [CrossRef] [PubMed]
- Lübbert, M.; Bertz, H.; Müller, M.J.; Finke, J. When azanucleoside treatment can be curative: Nonintensive bridging strategy before allografting in older patients with myelodysplastic syndrome/acute myeloid leukemia. J. Clin. Oncol. 2013, 31, 822–823. [Google Scholar] [CrossRef] [PubMed]
- De Padua Silva, L.; de Lima, M.; Kantarjian, H.; Faderl, S.; Kebriaei, P.; Giralt, S.; Davisson, J.; Garcia-Manero, G.; Champlin, R.; Issa, J.P.; et al. Feasibility of allo-SCT after hypomethylating therapy with decitabine for myelodysplastic syndrome. Bone Marrow Transpl. 2009, 43, 839–843. [Google Scholar] [CrossRef]
- Lübbert, M.; Bertz, H.; Rüter, B.; Marks, R.; Claus, R.; Wäsch, R.; Finke, J. Non-intensive treatment with low-dose 5-aza-2′-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone Marrow Transpl. 2009, 44, 585–588. [Google Scholar] [CrossRef]
- Cogle, C.R.; Imanirad, I.; Wiggins, L.E.; Hsu, J.; Brown, R.; Scornik, J.C.; Wingard, J.R. Hypomethylating agent induction therapy followed by hematopoietic cell transplantation is feasible in patients with myelodysplastic syndromes. Clin. Adv. Hematol. Oncol. 2010, 8, 40–46. [Google Scholar]
- Field, T.; Perkins, J.; Huang, Y.; Kharfan-Dabaja, M.A.; Alsina, M.; Ayala, E.; Fernandez, H.F.; Janssen, W.; Lancet, J.; Perez, L.; et al. 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2010, 45, 255–260. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, J.H.; Park, Y.H.; Lee, J.H.; Kim, S.D.; Choi, Y.; Lee, S.B.; Lee, K.H.; Ahn, S.Y.; Lee, Y.S.; et al. Feasibility of hypomethylating agents followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome. Bone Marrow Transpl. 2012, 47, 374–379. [Google Scholar] [CrossRef]
- Gerds, A.T.; Gooley, T.A.; Estey, E.H.; Appelbaum, F.R.; Deeg, H.J.; Scott, B.L. Pretransplantation therapy with azacitidine vs. induction chemotherapy and posttransplantation outcome in patients with MDS. Biol. Blood Marrow Transpl. 2012, 18, 1211–1218. [Google Scholar] [CrossRef]
- Damaj, G.; Duhamel, A.; Robin, M.; Beguin, Y.; Michallet, M.; Mohty, M.; Vigouroux, S.; Bories, P.; Garnier, A.; el Cheikh, J.; et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: A study by the Société Française de Greffe de Moelle et de Thérapie-Cellulaire and the Groupe-Francophone des Myélodysplasies. J. Clin. Oncol. 2012, 30, 4533–4540. [Google Scholar] [CrossRef]
- Platzbecker, U.; Schetelig, J.; Finke, J.; Trenschel, R.; Scott, B.; Kobbe, G.; Schaefer-Eckart, K.; Bornhäuser, M.; Itzykson, R.; Germing, U. Allogeneic hematopoietic cell transplantation in patients age 60–70 years with de novo high-risk myelodysplastic syndrome or secondary acute myelogenous leukemia: Comparison with patients lacking donors who received azacitidine. Biol. Blood Marrow Transpl. 2012, 18, 1415–1421. [Google Scholar] [CrossRef]
- De lima, M.; Giralt, S.; Thall, P.; de Padua Silva, L.; Jones, R.; Komanduri, K.; Braun, T.; Nguyen, H.; Champlin, R.; Garcia-Manero, G. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: A dose and schedule finding study. Cancer 2010, 116, 5420–5431. [Google Scholar] [PubMed]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltman, F.; Kiani, A.; Klut, I.; Knoth, H.; Röllig, C.; Schetelig, J.; et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Giralt, S.; Kantarjian, H.; Garcia-Manero, G.; Jagasia, M.; Kebriaei, P.; de Padua, L.; Shpall, E.; Champlin, R.; de Lima, M. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer 2009, 115, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Lübbert, M.; Bertz, H.; Wäsch, R.; Marks, R.; Rüter, B.; Claus, R.; Finke, J. Efficacy of a 3-day, low-dose treatment with 5-azacytidine followed by donor lymphocyte infusions in older patients with acute myeloid leukemia or chronic myelomonocytic leukemia relapsed after allografting. Bone Marrow Transpl. 2010, 45, 627–632. [Google Scholar] [CrossRef]
- Schroeder, T.; Czibere, A.; Platzbecker, U.; Bug, U.; Uharek, L.; Luft, T.; Giagounidis, A.; Zohren, F.; Bruns, I.; Wolschke, C.; et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia 2013, 27, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.; Wetzler, M.; Löwenberg, B. Therapeutic advances in acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 487–94. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, B.; Zittoun, R.; Kerkhofs, H.; Jehn, U.; Abels, J.; Debusscher, L.; Cauchie, C.; Peetermans, M.; Solbu, G.; Suciu, S. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukaemia: A randomized phase III study of the european organization for research and treatment of cancer leukaemia group. J. Clin. Oncol. 1989, 7, 1268–1274. [Google Scholar] [PubMed]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Mollgard, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Hoglund, M. Age and acute myeloid leukaemia: Real world data on decision to treat and outcomes from the swedish acute leukaemia registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Tilly, H.; Castaigne, S.; Bordessoule, D.; Casassus, P.; le Prise, P.Y.; Tertian, G.; Desablens, B.; Henry-Amar, M.; Degos, L. Low-dose cytarabine vs. intensive chemotherapy in the treatment of acute nonlymphocytic leukaemia in the elderly. J. Clin. Oncol. 1990, 8, 272–279. [Google Scholar] [PubMed]
- Burnett, A.K.; Milligan, D.; Prentice, A.G.; Goldstone, A.H.; McMullin, M.F.; Hills, R.K.; Wheatley, K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukaemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 2007, 109, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Cardama, A.; Ravandi, F.; Liu-Dumlao, T.; Brandt, M.; Faderl, S.; Pierce, S.; Borthakur, G.; Garcia-Manero, G.; Cortes, J.; Kantarjian, H. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012, 120, 4850–4855. [Google Scholar] [CrossRef] [PubMed]
- Van der Helm, L.H.; Scheepers, E.R.; Veeger, N.J.; Daenen, S.M.; Mulder, A.B.; van den Berg, E.; Vellenga, E.; Huls, G. Azacitidine might be beneficial in a subgroup of older AML patients compared to intensive chemotherapy: A single centre retrospective study of 227 consecutive patients. J. Hematol. Oncol. 2013, 16. [Google Scholar] [CrossRef]
- Van der Helm, L.; Alhan, C.; Wijermans, P.W.; van Marwijk Kooy, M.; Schaafsma, R.; Biemond, B.J.; Beeker, A.; Hoogendoorn, M.; van Rees, B.; de Weerdt, O.; et al. Platelet doubling after the first azacitidine cycle is a promising predictor for response in MDS, CMML and AML patients in the Dutch azacitidine compassionate named patient program. Br. J. Haematol. 2011, 155, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Klepin, H.D.; Rao, A.V.; Pardee, T.S. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J. Clin. Oncol. 2014. [Google Scholar] [CrossRef]
- Giles, F.J.; Borthakur, G.; Ravandi, F.; Faderl, S.; Verstovsek, S.; Thomas, D.; Wierda, W.; Ferrajoli, A.; Kornblau, S.; Pierce, S.; et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br. J. Haematol. 2007, 136, 624–627. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruijsen, M.; Lübbert, M.; Wijermans, P.; Huls, G. Clinical Results of Hypomethylating Agents in AML Treatment. J. Clin. Med. 2015, 4, 1-17. https://doi.org/10.3390/jcm4010001
Cruijsen M, Lübbert M, Wijermans P, Huls G. Clinical Results of Hypomethylating Agents in AML Treatment. Journal of Clinical Medicine. 2015; 4(1):1-17. https://doi.org/10.3390/jcm4010001
Chicago/Turabian StyleCruijsen, Marjan, Michael Lübbert, Pierre Wijermans, and Gerwin Huls. 2015. "Clinical Results of Hypomethylating Agents in AML Treatment" Journal of Clinical Medicine 4, no. 1: 1-17. https://doi.org/10.3390/jcm4010001
APA StyleCruijsen, M., Lübbert, M., Wijermans, P., & Huls, G. (2015). Clinical Results of Hypomethylating Agents in AML Treatment. Journal of Clinical Medicine, 4(1), 1-17. https://doi.org/10.3390/jcm4010001





